A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Preparing Feed Additives by Cultivation of the Yeast Strain
2.3. Determination of the Chemical Composition of the Swine Blood, Feed Additives Derived from It, and the Diets for the Mice
2.4. Measurement of Phytase Activity
2.5. Diets for the Mice
2.6. Animals
2.7. Measuring ADG and Statistical Analysis
3. Results
3.1. Manufacturing Feed Additives by Using Y. lipolytica Strains
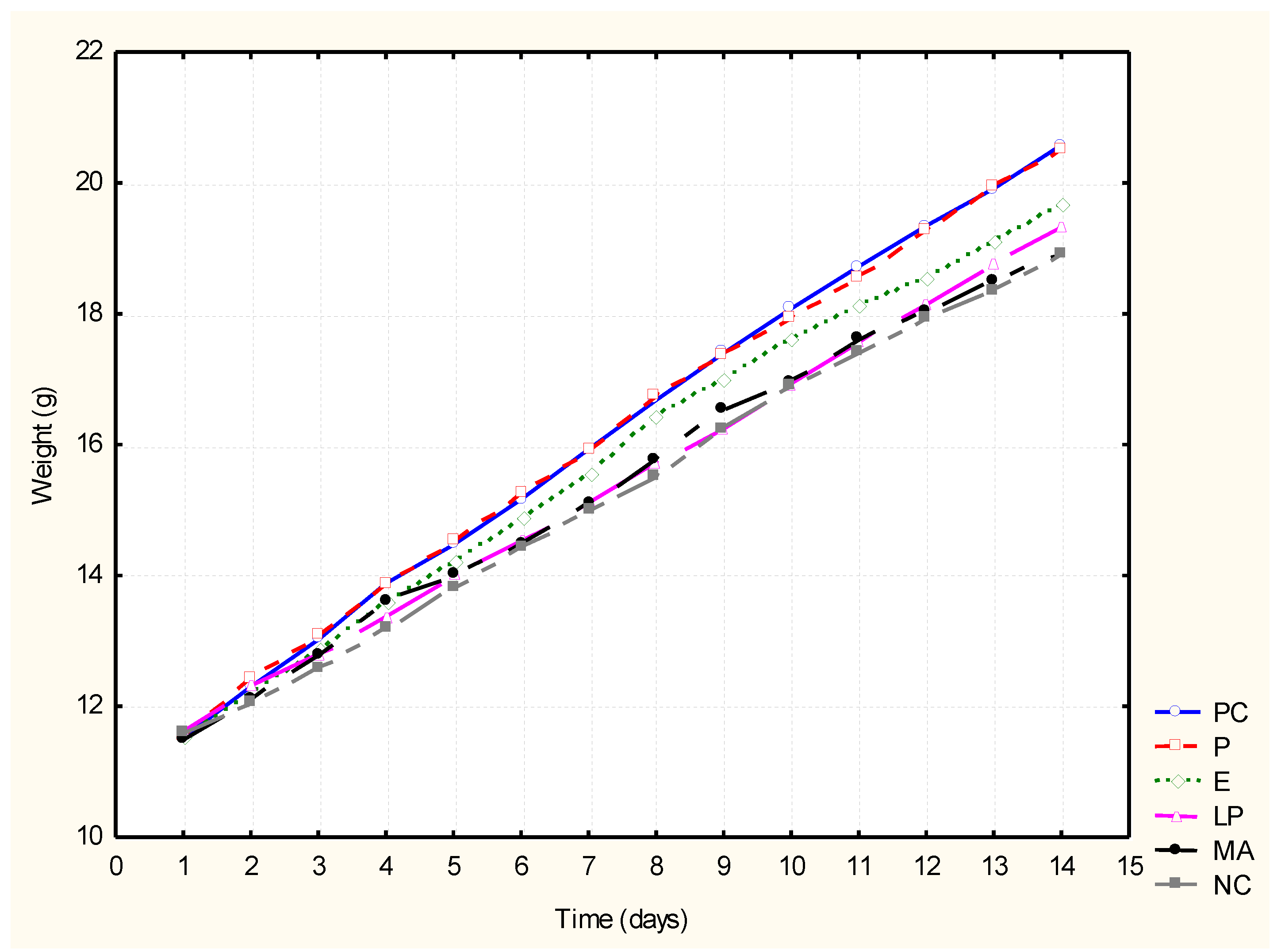
3.2. Testing the Impact of the Feed Additives on ADG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeola, O.; Cowieson, A.J. BOARD-INVITED REVIEW: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. The chemistry and biochemistry of inositol polyphosphates. Rev. Pure Appl. Chem. 1966, 16, 209–215. [Google Scholar]
- Morgan, N.; Walk, C.; Bedford, M.; Burton, E. Contribution of intestinal- and cereal-derived phytase activity on phytate degradation in young broilers. Poult. Sci. 2015, 94, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-48903-4. [Google Scholar]
- Tsai, T.C.; Dove, R.; Bedford, M.R.; Azain, M.J. Effect of phytase on phosphorous balance in 20-kg barrows fed low or adequate phosphorous diets. Anim. Nutr. 2020, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.-C.; Choi, W.-C.; Park, S.; Kim, Y.-O.; Oh, T.-K. Biochemical properties and substrate specificities of alkaline and histidine acid phytases. Appl. Microbiol. Biotechnol. 2004, 63, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Piddington, C.S.; Houston, C.S.; Paloheimo, M.; Cantrell, M.; Miettinen-Oinonen, A.; Nevalainen, H.; Rambosek, J. The cloning and sequencing of the genes encoding phytase (phy) and pH 2.5-optimum acid phosphatase (aph) from Aspergillus niger var. awamori. Gene 1993, 133, 55–62. [Google Scholar] [CrossRef]
- Pasamontes, L.; Haiker, M.; Wyss, M.; Tessier, M.; van Loon, A.P. Gene cloning, purification, and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Appl. Environ. Microbiol. 1997, 63, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.B.; Vogel, K.; Weimann, B.J.; Pasamontes, L.; van Loon, A.P.G.M.Y. The phytase subfamily of histidine acid phosphatases: Isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 1997, 143, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.S.; Tarafdar, J. Phytase and phosphatase producing fungi in and and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol. Biochem. 2003, 35, 745–751. [Google Scholar] [CrossRef]
- Pasamontes, L.; Haiker, M.; Henriquez-Huecas, M.; Mitchell, D.B.; van Loon, A.P. Cloning of the phytases from Emericella nidulans and the thermophilic fungus Talaromyces thermophilus. Biochim. Biophys. Acta 1997, 1353, 217–223. [Google Scholar] [CrossRef]
- Ullah, A.H. Aspergillus ficuum phytase: Partial primary structure, substrate selectivity, and kinetic characterization. Prep. Biochem. 1988, 18, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Brugger, R.; Kronenberger, A.; Rémy, R.; Fimbel, R.; Oesterhelt, G.; Lehmann, M.; van Loon, A.P. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): Catalytic properties. Appl. Environ. Microbiol. 1999, 65, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Dassa, E.; Boquet, P.L. Identification of the gene appA for the acid phosphatase (pH optimum 2.5) of Escherichia coli. Mol. Gen. Genet. MGG 1985, 200, 68. [Google Scholar] [CrossRef] [PubMed]
- Dassa, J.; Marck, C.; Boquet, P.L. The complete nucleotide sequence of the Escherichia coli gene appA reveals significant homology between pH 2.5 acid phosphatase and glucose-1-phosphatase. J. Bacteriol. 1990, 172, 5497–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinin, N.V.; Serkina, A.V.; Gelfand, M.S.; Shevelev, A.B.; Sineoky, S.P. Gene Cloning, Expression and characterization of novel phytase from Obesumbacterium proteus. FEMS Microbiol. Lett. 2004, 236, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, R.; Krentler, C.; Schmidt, B.; Schröder, W.; Lorkowski, G.; Culley, J.; Mersmann, G.; Geier, C.; Waheed, A.; Gottschalk, S. Human lysosomal acid phosphatase: Cloning, expression and chromosomal assignment. EMBO J. 1988, 7, 2343–2350. [Google Scholar] [CrossRef]
- Geier, C.; Figura, K.V.; Pohlmann, R. Molecular cloning of the mouse lysosomal acid phosphatase. Biol. Chem. Hoppe-Seyler 1991, 372, 301–304. [Google Scholar] [CrossRef]
- Van Etten, R.L.; Davidson, R.; Stevis, P.E.; MacArthur, H.; Moore, D.L. Covalent structure, disulfide bonding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. J. Biol. Chem. 1991, 266, 2313–2319. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Callegari, M.A.; Dias, C.P.; Bridi, A.M.; Pierozan, C.R.; Foppa, L.; Martins, C.C.d.S.; Dias, F.T.F.; Passos, A.; Hermes, R. Increasing doses of phytase from Citrobacter braakii in diets with reduced inorganic phosphorus and calcium improve growth performance and lean meat of growing and finishing pigs. PLoS ONE 2019, 14, e0217490. [Google Scholar] [CrossRef] [PubMed]
- Srikanthithasan, K.; Macelline, S.P.; Wickramasuriya, S.S.; Tharangani, H.; Jayasena, D.D.; Heo, J.-M. Effects of adding phytase from Aspergillus niger to a low phosphorus diet on growth performance, tibia characteristics, phosphorus excretion, and meat quality of broilers 35 days after hatching. J. Poult. Sci. 2020, 57, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Gordeeva, T.; Borshchevskaya, L.; Kalinina, A.N.; Sineoky, S.; Voronin, S.P.; Kashirskaya, M.D. Expression and characteristics of phytases from Obesumbacterium proteus in Pichia pastoris yeast. Biotekhnologiya 2018, 34, 18–25. [Google Scholar] [CrossRef]
- Gordeeva, T.; Borshchevskaya, L.; Kalinina, A.N.; Sineoky, S.; Kashirskaya, M.D.; Voronin, S.P. Increase in thermal stability of phytase from Citrobacter freundii by site-directed saturation mutagenesis. Biotekhnologiya 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Gordeyeva, T.L.; Borshchevskaya, L.N.; Kalinina, A.N.; Sineokiy, S.P.; Voronin, S.P.; Kashirskaya, M.D. Rekombinantnyy produtsent kormovogo fermenta fitazy na osnove drozhzhey Pichia pastoris. Aktual’naya Biotekhnol. 2018, 3, 117. [Google Scholar]
- Isakova, E.P.; Serdyuk, E.G.; Gessler, N.N.; Trubnikova, E.V.; Biryukova, Y.K.; Epova, E.Y.; Deryabina, Y.I.; Nikolaev, A.V. A new recombinant strain of Yarrowia lipolytica producing encapsulated phytase from Obesumbacterium proteus. Dokl. Biochem. Biophys. 2018, 481, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Savichev, A.T.; Sorokin, S.E. Rentgenofluorestsentnyy energodispersionnyy analiz zol’nykh elementov v rasteniyakh. Agrokhimiya 2001, 12, 61–67. [Google Scholar]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Norton, J.D.; Yang, S.P.; Diffley, P. Influence of source and quantity of protein on the development of immunity and resistance to African trypanosomiasis. Infect. Immun. 1986, 51, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Czech, A.; Smolczyk, A.; Grela, E.R.; Kiesz, M. Effect of dietary supplementation with Yarrowia lipolytica or Saccharomyces cerevisiae yeast and probiotic additives on growth performance, basic nutrients digestibility and biochemical blood profile in piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1720–1730. [Google Scholar] [CrossRef]
- Ziarat, M.; Kermanshahi, H.; Mogaddam, H.; Majidzadeh Heravi, R. Performance of an Escherichia coli phytase expressed in Lactococcus lactis on nutrient retention, bone traits and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2020, 104, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; El-Naggar, A.H.; Kornaros, M.; Sun, J. Valorizing lignin-like dyes and textile dyeing wastewater by a newly constructed lipid-producing and lignin modifying oleaginous yeast consortium valued for biodiesel and bioremediation. J. Hazard. Mater. 2021, 403, 123575. [Google Scholar] [CrossRef]
- Quarterman, J.C.; Slininger, P.J.; Hector, R.E.; Dien, B.S. Engineering Candida phangngensis-an oleaginous yeast from the Yarrowia clade-for enhanced detoxification of lignocellulose-derived inhibitors and lipid overproduction. FEMS Yeast Res. 2018, 18, foy102. [Google Scholar] [CrossRef] [PubMed]
- Iwama, R.; Kobayashi, S.; Ishimaru, C.; Ohta, A.; Horiuchi, H.; Fukuda, R. Functional roles and substrate specificities of twelve cytochromes P450 belonging to CYP52 family in n-alkane assimilating yeast Yarrowia lipolytica. Fungal Genet. Biol. FG B 2016, 91, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.D.; Fernandes, K.M.; Dias, R.S.; Rocha, A.S.; de Oliveira, L.L.; Neves, C.A.; de Paula, S.O.; Mantovani, H.C. Effects of the oral administration of viable and heat-killed Streptococcus bovis HC5 cells to pre-sensitized BALB/c mice. PLoS ONE 2012, 7, e48313. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Acton, A.J.; Considine, R.V. The Angiogenic inhibitor TNP-470 decreases caloric intake and weight gain in high-fat fed mice. Obesity Silver Spring MD 2012, 20, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
| Nutritional Parameters, % | PC | NC | P |
|---|---|---|---|
| Positive Control | Negative Control | NC Supplemented with 2% Na2HPO4·2H2O | |
| Moisture | 16.2 | 15.2 | 14.7 |
| Total phosphorus | 0.54 | 0.45 | 1.45 |
| Accessible phosphorus | 0.21 | 0.15 | 1.15 |
| Na | 0.01 | 0.02 | 0.02 |
| Mg | 0.29 | 0.27 | 0.26 |
| Al | 0.05 | 0.1 | 0.1 |
| Si | 0.62 | 0.29 | 0.28 |
| S | 0.16 | 0.14 | 0.14 |
| Cl | 0.05 | 0.05 | 0.05 |
| K | 0.68 | 0.57 | 0.55 |
| Ca | 0.11 | 0.09 | 0.09 |
| Group | Day 5 | Day 10 | Day 14 | ||||||||||||
| MeanADG±SEMofADG | %ADGRelativeto | p | MeanADG±SEMofADG | %ADGRelativeto | p | MeanADG±SEMofADG | %ADGRelativeto | p | |||||||
| PC | NC | PC | NC | PC | NC | PC | NC | PC | NC | PC | NC | ||||
| PC | 0.59 ± 0.29 | - | 34.09 | - | p < 0.05 | 0.66 ± 0.14 | 24.73 | - | p < 0.05 | 0.65 ± 0.25 | 24.47 | - | p < 0.05 | ||
| NC | 0.44 ± 0.19 | −25.42 | - | p < 0.05 | - | 0.53 ± 0.26 | −19.83 | - | p < 0.05 | - | 0.52 ± 0.29 | −19.65 | - | p < 0.05 | - |
| E | 0.53 ± 0.20 | −9.15 | 21.81 | p > 0.05 | p > 0.05 | 0.60 ± 0.18 | −8.18 | 14.52 | p < 0.05 | p > 0.05 | 0.58 ± 0.18 | −10.33 | 11.61 | p < 0.05 | p > 0.05 |
| MA | 0.51 ± 0.09 | −13.55 | 15.91 | p > 0.05 | p > 0.05 | 0.54 ± 0.26 | −17.41 | 2.26 | p < 0.05 | p < 0.05 | 0.52 ± 0.31 | −18.67 | 1.23 | p < 0.05 | p < 0.05 |
| P | 0.60 ± 0.19 | 1.69 | 36.36 | p > 0.05 | p < 0.05 | 0.63 ± 0.30 | −3.64 | 20.18 | p > 0.05 | p < 0.05 | 0.64 ± 0.35 | −1.65 | 22.40 | p > 0.05 | p < 0.05 |
| LP | 0.48 ± 0.20 | −17.96 | 10.00 | p > 0.05 | p > 0.05 | 0.52 ± 0.17 | −20.28 | −0.56 | p < 0.05 | p > 0.05 | 0.55 ± 0.17 | −15.48 | 5.19 | p < 0.05 | p > 0.05 |
| Group | Day5 | Day10 | Day14 | ||||||||||||
| MeanADG±SEMofADG | %ADGRelativeto | p | MeanADG±SEMofADG | %ADGRelativeto | p | MeanADG±SEMofADG | %ADGRelativeto | p | |||||||
| PC | NC | PC | NC | PC | NC | PC | NC | PC | NC | PC | NC | ||||
| PC | 0.59 ± 0.29 | - | 34.09 | - | 0.01 | 0.66 ± 0.14 | 24.73 | - | 0.001 | 0.65 ± 0.25 | 24.47 | - | 0.001 | ||
| NC | 0.44 ± 0.19 | −25.42 | - | 0.01 | - | 0.53 ± 0.26 | −19.83 | - | 0.001 | - | 0.52 ± 0.29 | −19.65 | - | 0.001 | - |
| E | 0.53 ± 0.20 | −9.15 | 21.81 | 0.19 | 0.12 | 0.60 ± 0.18 | −8.18 | 14.52 | 0.003 | 0.97 | 0.58 ± 0.18 | −10.33 | 11.61 | 0.001 | 0.73 |
| MA | 0.51 ± 0.09 | −13.55 | 15.91 | 0.73 | 0.07 | 0.54 ± 0.26 | −17.41 | 2.26 | 0.01 | 0.03 | 0.52 ± 0.31 | −18.67 | 1.23 | 0.002 | 0.007 |
| P | 0.60 ± 0.19 | 1.69 | 36.36 | 0.35 | 0.009 | 0.63 ± 0.30 | −3.64 | 20.18 | 0.66 | 0.01 | 0.64 ± 0.35 | −1.65 | 22.40 | 0.78 | 0.001 |
| LP | 0.48 ± 0.20 | −17.96 | 10.00 | 0.10 | 0.43 | 0.52 ± 0.17 | −20.28 | −0.56 | 0.001 | 0.73 | 0.55 ± 0.17 | −15.48 | 5.19 | 0.001 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilova, M.A.; Epova, E.Y.; Trubnikova, E.V.; Shevelev, A.B. A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste. Appl. Sci. 2022, 12, 3094. https://doi.org/10.3390/app12063094
Danilova MA, Epova EY, Trubnikova EV, Shevelev AB. A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste. Applied Sciences. 2022; 12(6):3094. https://doi.org/10.3390/app12063094
Chicago/Turabian StyleDanilova, Maria A., Ekaterina Yu. Epova, Elena V. Trubnikova, and Alexei B. Shevelev. 2022. "A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste" Applied Sciences 12, no. 6: 3094. https://doi.org/10.3390/app12063094
APA StyleDanilova, M. A., Epova, E. Y., Trubnikova, E. V., & Shevelev, A. B. (2022). A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste. Applied Sciences, 12(6), 3094. https://doi.org/10.3390/app12063094





