Removal of Emerging Contaminants as Diclofenac and Caffeine Using Activated Carbon Obtained from Argan Fruit Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Activated Carbon
2.3. Characterization of the Activated Carbon
2.4. Adsorptions Experiments
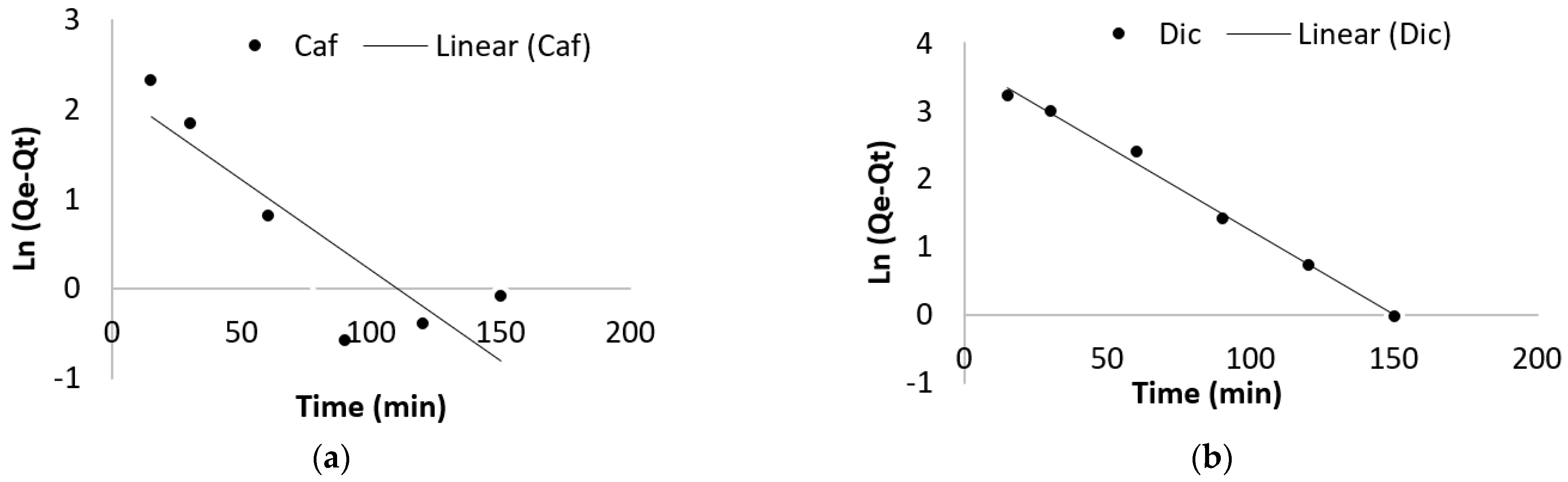
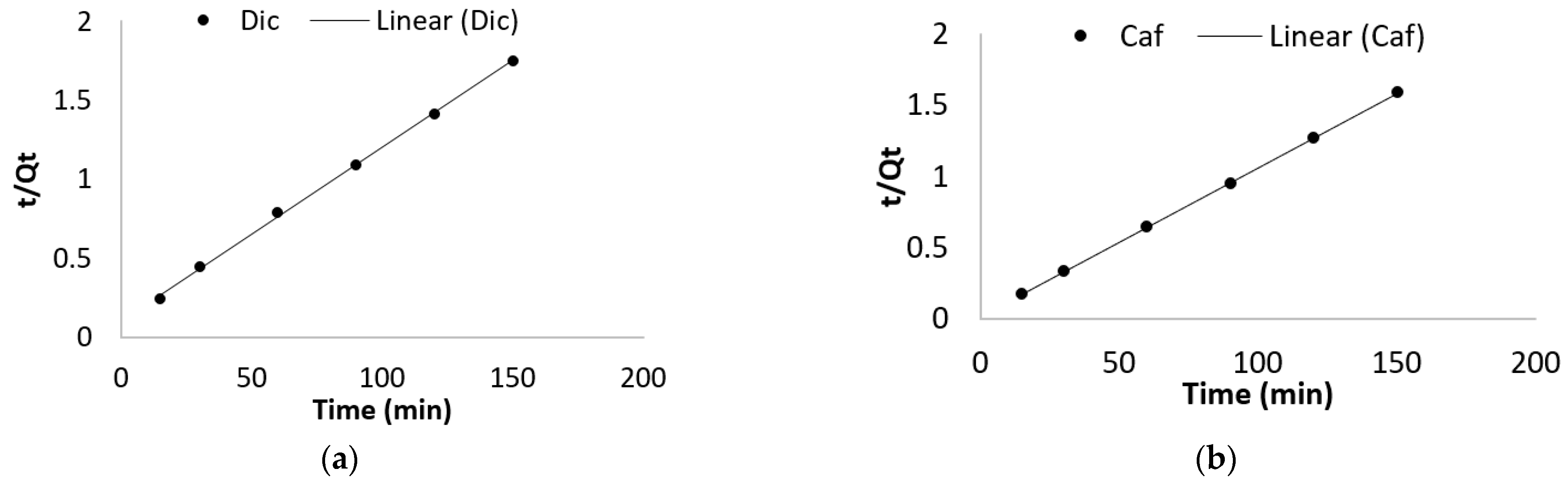
2.4.1. Adsorption Kinetic
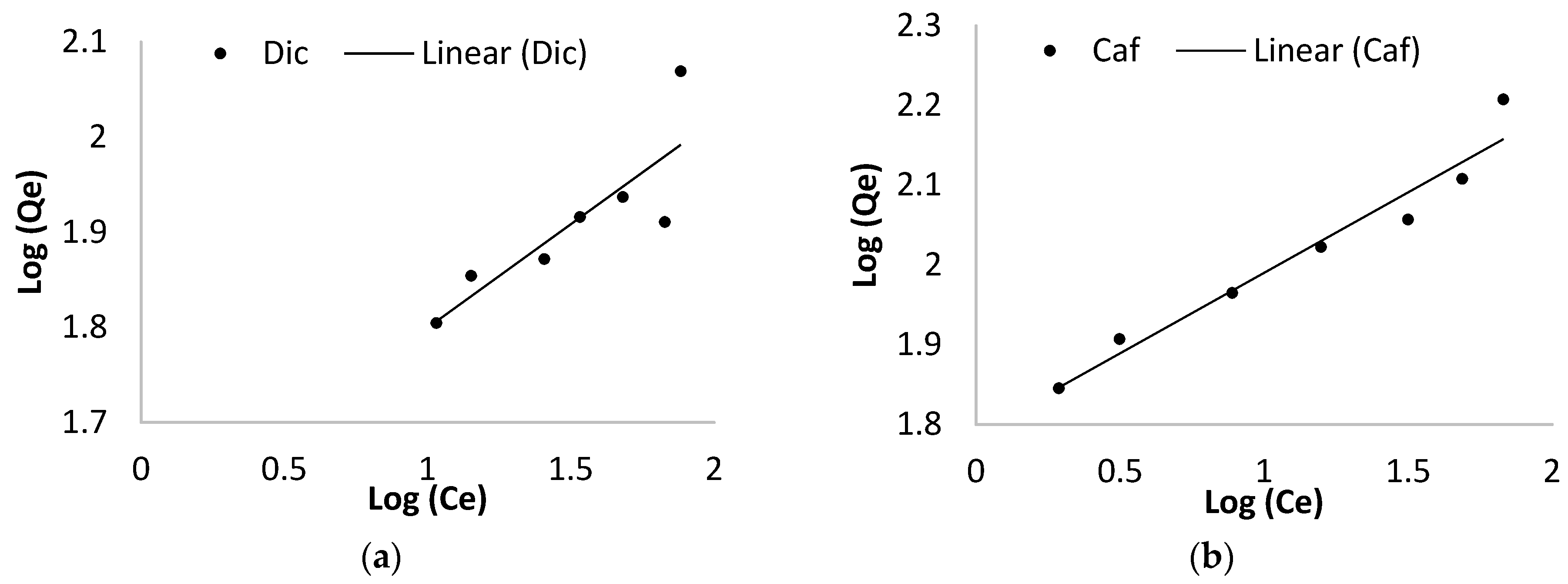
2.4.2. Adsorption Isotherms
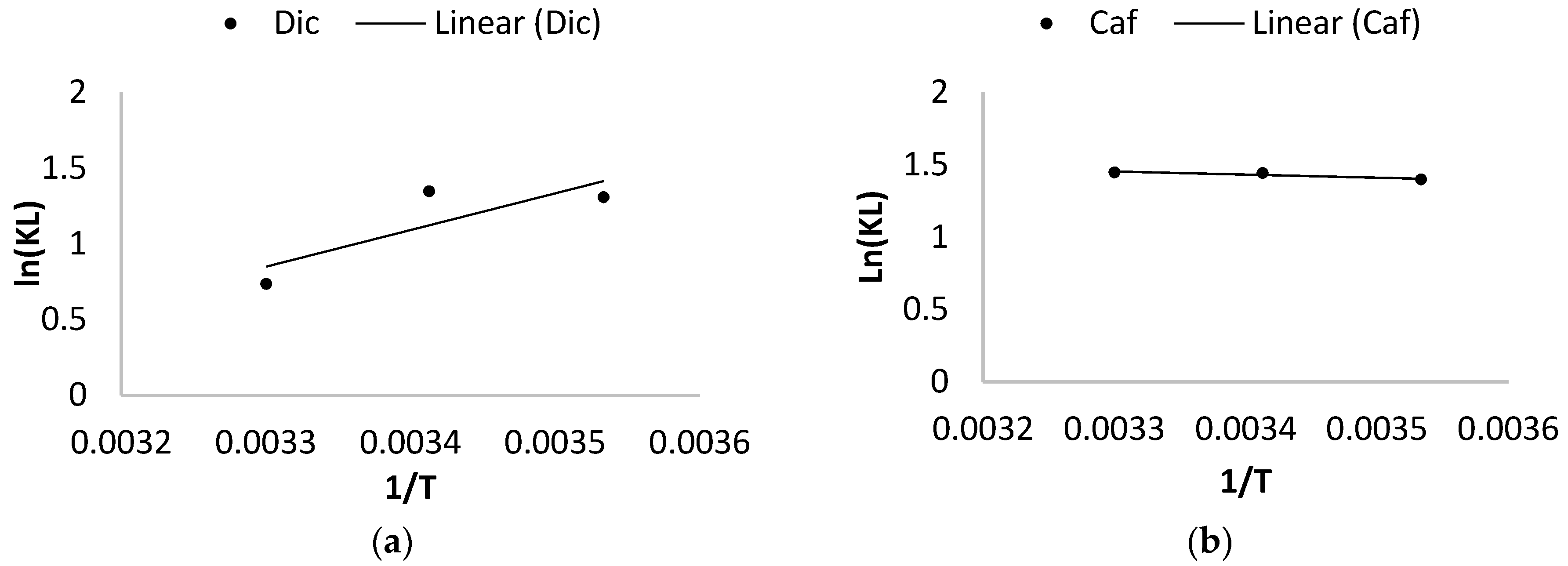
2.4.3. Thermodynamic Study of the Adsorption
3. Results and Discussion
3.1. Characterization of the Activated Carbon
3.2. Adsorption of Emergent Contaminants
3.2.1. Effect of Contact Time and Adsorption Kinetic
3.2.2. Adsorption Isotherm
3.2.3. Effect of Temperature and Thermodynamic Study
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bo, L.; Shengen, Z.; Chang, C.-C. Emerging Pollutants—Part II: Treatment. Water Environ. Res. 2016, 88, 1876–1904. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Khan, E.; Chen, H.; Nguyen, V.T.; Li, Y.; Goh, S.G.; Nguyen, Q.B.; Saeidi, N.; Gin, K.Y.-H. Emerging Contaminants in Wastewater, Stormwater Runoff, and Surface Water: Application as Chemical Markers for Diffuse Sources. Sci. Total Environ. 2019, 676, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Justino, C.; Rocha-Santos, T.; Freitas, A.C.; Duarte, A.C.; Pereira, R. Review of the Ecotoxicological Effects of Emerging Contaminants to Soil Biota. J. Environ. Sci. Health A 2017, 52, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Ribeiro, R.S.; Gomes, H.T.; Sotelo, J.L.; García, J. Synthesis of Carbon Xerogels and Their Application in Adsorption Studies of Caffeine and Diclofenac as Emerging Contaminants. Chem. Eng. Res. Des. 2015, 95, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Fraker, S.L.; Smith, G.R. Direct and Interactive Effects of Ecologically Relevant Concentrations of Organic Wastewater Contaminants on Rana Pipiens Tadpoles. Environ. Toxicol. 2004, 19, 250–256. [Google Scholar] [CrossRef]
- Onaga Medina, F.M.; Aguiar, M.B.; Parolo, M.E.; Avena, M.J. Insights of Competitive Adsorption on Activated Carbon of Binary Caffeine and Diclofenac Solutions. J. Environ. Manag. 2021, 278, 111523. [Google Scholar] [CrossRef]
- Santos-Silva, T.G.; Montagner, C.C.; Martinez, C.B.R. Evaluation of Caffeine Effects on Biochemical and Genotoxic Biomarkers in the Neotropical Freshwater Teleost Prochilodus Lineatus. Environ. Toxicol. Pharmacol. 2018, 58, 237–242. [Google Scholar] [CrossRef]
- Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Equilibrium and Kinetic Studies of Caffeine Adsorption from Aqueous Solutions on Thermally Modified Verde-Lodo Bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Torrellas, S.Á.; García Lovera, R.; Escalona, N.; Sepúlveda, C.; Sotelo, J.L.; García, J. Chemical-Activated Carbons from Peach Stones for the Adsorption of Emerging Contaminants in Aqueous Solutions. Chem. Eng. J. 2015, 279, 788–798. [Google Scholar] [CrossRef]
- Danish, M.; Birnbach, J.; Mohamad Ibrahim, M.N.; Hashim, R.; Majeed, S.; Tay, G.S.; Sapawe, N. Optimization Study of Caffeine Adsorption onto Large Surface Area Wood Activated Carbon through Central Composite Design Approach. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100594. [Google Scholar] [CrossRef]
- Danish, M. Application of Date Stone Activated Carbon for the Removal of Caffeine Molecules from Water. Mater. Today Proc. 2020, 31, 18–22. [Google Scholar] [CrossRef]
- Zanella, H.G.; Spessato, L.; Lopes, G.K.P.; Yokoyama, J.T.C.; Silva, M.C.; Souza, P.S.C.; Ronix, A.; Cazetta, A.L.; Almeida, V.C. Caffeine Adsorption on Activated Biochar Derived from Macrophytes (Eichornia Crassipes). J. Mol. Liq. 2021, 340, 117206. [Google Scholar] [CrossRef]
- da Silva Vasconcelos de Almeida, A.; Vieira, W.T.; Bispo, M.D.; de Melo, S.F.; da Silva, T.L.; Balliano, T.L.; Vieira, M.G.A.; Soletti, J.I. Caffeine Removal Using Activated Biochar from Açaí Seed (Euterpe Oleracea Mart): Experimental Study and Description of Adsorbate Properties Using Density Functional Theory (DFT). J. Environ. Chem. Eng. 2021, 9, 104891. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of Caffeine on Mesoporous Activated Carbon Fibers Prepared from Pineapple Plant Leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef]
- Francoeur, M.; Ferino-Pérez, A.; Yacou, C.; Jean-Marius, C.; Emmanuel, E.; Chérémond, Y.; Jauregui-Haza, U.; Gaspard, S. Activated Carbon Synthetized from Sargassum (Sp) for Adsorption of Caffeine: Understanding the Adsorption Mechanism Using Molecular Modeling. J. Environ. Chem. Eng. 2021, 9, 104795. [Google Scholar] [CrossRef]
- Galhetas, M.; Mestre, A.S.; Pinto, M.L.; Gulyurtlu, I.; Lopes, H.; Carvalho, A.P. Chars from Gasification of Coal and Pine Activated with K2CO3: Acetaminophen and Caffeine Adsorption from Aqueous Solutions. J. Colloid Interface Sci. 2014, 433, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Mengesha, D.N.; Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Ground Coffee Waste-Derived Carbon for Adsorptive Removal of Caffeine: Effect of Surface Chemistry and Porous Structure. Sci. Total Environ. 2021, 151669. [Google Scholar] [CrossRef]
- Melo, L.L.A.; Ide, A.H.; Duarte, J.L.S.; Zanta, C.L.P.S.; Oliveira, L.M.T.M.; Pimentel, W.R.O.; Meili, L. Caffeine Removal Using Elaeis Guineensis Activated Carbon: Adsorption and RSM Studies. Environ. Sci. Pollut. Res. 2020, 27, 27048–27060. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Cimirro, N.F.G.M.; Thue, P.S.; Dias, S.L.P.; Gelesky, M.A.; Dotto, G.L.; dos Reis, G.S.; Pavan, F.A. Conversion of Eragrostis Plana Nees Leaves to Activated Carbon by Microwave-Assisted Pyrolysis for the Removal of Organic Emerging Contaminants from Aqueous Solutions. Environ. Sci. Pollut. Res. 2018, 25, 23315–23327. [Google Scholar] [CrossRef] [Green Version]
- Avcu, T.; Üner, O.; Geçgel, Ü. Adsorptive Removal of Diclofenac Sodium from Aqueous Solution onto Sycamore Ball Activated Carbon—Isotherms, Kinetics, and Thermodynamic Study. Surf. Interfaces 2021, 24, 101097. [Google Scholar] [CrossRef]
- Naghipour, D.; Hoseinzadeh, L.; Taghavi, K.; Jaafari, J. Characterization, Kinetic, Thermodynamic and Isotherm Data for Diclofenac Removal from Aqueous Solution by Activated Carbon Derived from Pine Tree. Data Brief 2018, 18, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- El Naga, A.O.; El Saied, M.; Shaban, S.A.; El Kady, F.Y. Fast Removal of Diclofenac Sodium from Aqueous Solution Using Sugar Cane Bagasse-Derived Activated Carbon. J. Mol. Liq. 2019, 285, 9–19. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.D.T.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Suresh, S.; Garg, A. Tea Waste Derived Activated Carbon for the Adsorption of Sodium Diclofenac from Wastewater: Adsorbent Characteristics, Adsorption Isotherms, Kinetics, and Thermodynamics. Environ. Sci. Pollut. Res. 2018, 25, 32210–32220. [Google Scholar] [CrossRef]
- Bernardo, M.; Rodrigues, S.; Lapa, N.; Matos, I.; Lemos, F.; Batista, M.K.S.; Carvalho, A.P.; Fonseca, I. High Efficacy on Diclofenac Removal by Activated Carbon Produced from Potato Peel Waste. Int. J. Environ. Sci. Technol. 2016, 13, 1989–2000. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of Pharmaceutical Compounds by Activated Carbon Prepared from Agricultural By-Product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Muñoz, M.; Zazo, J.; Casas, J.A.; Garcia, M.M. Synthesis of High Surface Area Carbon Adsorbents Prepared from Pine Sawdust-Onopordum Acanthium L. for Nonsteroidal Anti-Inflammatory Drugs Adsorption. J. Environ. Manag. 2016, 183, 294–305. [Google Scholar] [CrossRef]
- Vedenyapina, M.D.; Stopp, P.; Weichgrebe, D.; Vedenyapin, A.A. Adsorption of Diclofenac Sodium from Aqueous Solutions on Activated Carbon. Solid Fuel Chem. 2016, 50, 46–50. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and Characterization of Activated Hydrochars from Orange Peels as Potential Adsorbents for Emerging Organic Contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Ferreira, L.; Rosales, E.; Danko, A.S.; Sanromán, M.A.; Pazos, M.M. Bacillus Thuringiensis a Promising Bacterium for Degrading Emerging Pollutants. Process Saf. Environ. Prot. 2016, 101, 19–26. [Google Scholar] [CrossRef]
- Barrios, J.A.; Cano, A.; Becerril, J.E.; Jiménez, B. Influence of Solids on the Removal of Emerging Pollutants in Electrooxidation of Municipal Sludge with Boron-Doped Diamond Electrodes. J. Electroanal. Chem. 2016, 776, 148–151. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Rodriguez, E. Elimination of Selected Emerging Contaminants by the Combination of Membrane Filtration and Chemical Oxidation Processes. Water Air Soil Pollut. 2015, 226, 139. [Google Scholar] [CrossRef]
- Esmaeeli, F.; Gorbanian, S.A.; Moazezi, N. Removal of Estradiol Valerate and Progesterone Using Powdered and Granular Activated Carbon from Aqueous Solutions. Int. J. Environ. Res. 2017, 11, 695–705. [Google Scholar] [CrossRef]
- Leite, A.B.; Saucier, C.; Lima, E.C.; dos Reis, G.S.; Umpierres, C.S.; Mello, B.L.; Shirmardi, M.; Dias, S.L.P.; Sampaio, C.H. Activated Carbons from Avocado Seed: Optimisation and Application for Removal of Several Emerging Organic Compounds. Environ. Sci. Pollut. Res. 2018, 25, 7647–7661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.; Lim, Y.; Ngadi, N.; Mat, R.; Hassan, O.; Inuwa, I.M.; Mohamed, N.B.; Low, J.H. Removal of Acetaminophen by Activated Carbon Synthesized from Spent Tea Leaves: Equilibrium, Kinetics and Thermodynamics Studies. Powder Technol. 2018, 338, 878–886. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Chii, Y.Y.; Siddique, B.M. Removal of Cu(II) and Pb(II) Ions from Aqueous Solutions by Adsorption on Sawdust of Meranti Wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Danish, M. Sorption Studies of Zn(II)- and Cd(II)Ions from Aqueous Solution on Treated Sawdust of Sissoo Wood. Holz Als Roh-Und Werkst. 2007, 65, 429–436. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Copper (II), Chromium (III), Nickel (II) and Lead (II) Ions from Aqueous Solutions by Meranti Sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.-M.; Fernandez, B.; Le Cloirec, P. Advantages and Limits of Adsorption Sampling for Physico-Chemical Measurements of Odorous Compounds. Analusis 2000, 28, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.A.; Jothirani, R. A Critical Review on Recent Developments in the Low-Cost Adsorption of Dyes from Wastewater. Desalin. Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, L.; Zhang, X.; Ye, N.; Xing, F. Pyrolytic Characteristics and Kinetic Studies of Three Kinds of Red Algae. Biomass Bioenergy 2011, 35, 1765–1772. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Wang, Z.; Li, Y.; Wang, L.; Ding, L.; Gao, X.; Ma, Y.; Guo, Y. Application Studies of Activated Carbon Derived from Rice Husks Produced by Chemical-Thermal Process—A Review. Adv. Colloid Interface Sci. 2011, 163, 39–52. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural Residues as Precursors for Activated Carbon Production—A Review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Kumar, B.G.; Shivakamy, K.; Miranda, L.R.; Velan, M. Preparation of Steam Activated Carbon from Rubberwood Sawdust (Hevea Brasiliensis) and Its Adsorption Kinetics. J. Hazard. Mater. 2006, 136, 922–929. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Rodríguez-Reinoso, F. Role of Chemical Activation in the Development of Carbon Porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated Carbon Modifications to Enhance Its Water Treatment Applications. An Overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent Progress in the Synthesis of Porous Carbon Materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Husseiny, A.A.; Rao, R.M.; Goktepe, I. The Use of Nutshell Carbons in Drinking Water Filters for Removal of Trace Metals. Water Res. 2004, 38, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, M.; Di Blasi, C. Pyrolysis Kinetics of Wheat and Corn Straw. J. Anal. Appl. Pyrolysis 1998, 44, 181–192. [Google Scholar] [CrossRef]
- Minkova, V.; Razvigorova, M.; Bjornbom, E.; Zanzi, R.; Budinova, T.; Petrov, N. Effect of Water Vapour and Biomass Nature on the Yield and Quality of the Pyrolysis Products from Biomass. Fuel Processing Technol. 2001, 70, 53–61. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Gasification of Biomass Chars in Steam–Nitrogen Mixture. Energy Convers. Manag. 2006, 47, 1004–1013. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of Pyrolysis Conditions on the Structure and Gasification Reactivity of Biomass Chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of Granular Activated Carbon from Fruit Stones and Nutshells and Evaluation of Their Physical, Chemical and Adsorption Properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chang, C.Y.; Lee, S.L. Preparation and Characterization of Activated Carbons from Corn Cob. Carbon 1997, 35, 1198–1200. [Google Scholar] [CrossRef]
- Savova, D.; Apak, E.; Ekinci, E.; Yardim, F.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass Conversion to Carbon Adsorbents and Gas. Biomass Bioenergy 2001, 21, 133–142. [Google Scholar] [CrossRef]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of Activated Carbon from Peanut Hulls in Relation to Conditions of Preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of Pyrolysis Conditions on the Properties of Activated Carbons Prepared from Pistachio-Nut Shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Production of Granular Activated Carbons from Select Agricultural By-Products and Evaluation of Their Physical, Chemical and Adsorption. Bioresour. Technol. 2000, 71, 113–123. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Farissi, H.E.; Zerrouk, M.H.; Cacciola, F.; Bakkali, C.; Brigui, J.; Lovillo, M.P.; da Silva, J.C.G. Dye Removal from Colored Textile Wastewater Using Seeds and Biochar of Barley (Hordeum vulgare L.). Appl. Sci. 2021, 11, 5125. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of Activated Carbon from Forest and Agricultural Residues through CO2 Activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Khallouki, F.; Haubner, R.; Ricarte, I.; Erben, G.; Klika, K.; Ulrich, C.M.; Owen, R.W. Identification of Polyphenolic Compounds in the Flesh of Argan (Morocco) Fruits. Food Chem. 2015, 179, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Guillaume, D.; Gharby, S.; Haddad, A.; Harhar, H.; Charrouf, Z. Effect of Processing on the Quality of Edible Argan Oil. Food Chem. 2010, 120, 426–432. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of Hard Carbon from Argan Shells for Na-Ion Batteries. J. Mater. Chem. A 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Chafik, T. Matériaux Carbonés Nanoporeux Préparés à Partir de La Coque Du Fruit d’argan. International Patent Application No. PCT/MA2011/000009. WO Patent 2012050411A1, 19 April 2012. [Google Scholar]
- Edebali, S. Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; ISBN 978-1-78984-819-9. [Google Scholar]
- Kumar, P.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of Dye from Aqueous Solution by Cashew Nut Shell: Studies on Equilibrium Isotherm, Kinetics and Thermodynamics of Interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Yuh-Shan, H. Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. An Overview of Dye Removal via Activated Carbon Adsorption Process. Desalination Water Treat. 2010, 19, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption Isotherm Models for Basic Dye Adsorption by Peat in Single and Binary Component Systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, e3039817. [Google Scholar] [CrossRef]
- Kecili, R.; Hussain, C.M. Chapter 4—Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. ISBN 978-0-12-812792-6. [Google Scholar]
- Bulut, Y.; Aydın, H. A Kinetics and Thermodynamics Study of Methylene Blue Adsorption on Wheat Shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Gök, Ö.; Özcan, A.; Erdem, B.; Özcan, A.S. Prediction of the Kinetics, Equilibrium and Thermodynamic Parameters of Adsorption of Copper(II) Ions onto 8-Hydroxy Quinoline Immobilized Bentonite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 174–185. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.J.; Wong, J.W.C. Thermodynamic Parameters for Adsorption Equilibrium of Heavy Metals and Dyes from Wastewater with Low-Cost Adsorbents. J. Colloid Interface Sci. 2005, 291, 588–592. [Google Scholar] [CrossRef]
- Liu, X.; Lee, D.-J. Thermodynamic Parameters for Adsorption Equilibrium of Heavy Metals and Dyes from Wastewaters. Bioresour. Technol. 2014, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Kyzas, G.Z. Are the Thermodynamic Parameters Correctly Estimated in Liquid-Phase Adsorption Phenomena? J. Mol. Liq. 2016, 218, 174–185. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The Effect of the Biomass Components Lignin, Cellulose and Hemicellulose on TGA and Fixed Bed Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption Isotherms and Kinetic Modeling of Methylene Blue Dye onto a Carbonaceous Hydrochar Adsorbent Derived from Coffee Husk Waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fund. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Zbair, M.; Ainassaari, K.; Drif, A.; Ojala, S.; Bottlinger, M.; Pirilä, M.; Bensitel, M.; Brahmi, R.; Keiski, R.L. Toward new benchmark adsorbents: Preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ. Sci. Pollut. Res. 2018, 25, 1869–1882. [Google Scholar] [CrossRef] [Green Version]
- Mokhati, A.; Benturki, O.; Bernardo, M.; Kecira, Z.; Matos, I.; Lapa, N.; Ventura, M.; Soares, O.; Rego, A.B.D.; Fonseca, I. Nanoporous carbons prepared from argan nutshells as potential removal agents of diclofenac and paroxetine. J. Mol. Liq. 2021, 326, 115368. [Google Scholar] [CrossRef]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Hydrothermal Carbonization of Argan Nut Shell: Functional Mesoporous Carbon with Excellent Performance in the Adsorption of Bisphenol A and Diuron. Waste Biomass Valor. 2020, 11, 1565–1584. [Google Scholar] [CrossRef] [Green Version]
- El Khomri, M.; El Messaoudi, N.; Dbik, A.; Bentahar, S.; Lacherai, A.; Faska, N.; Jada, A. Regeneration of Argan Nutshell and Almond Shell Using HNO3 for Their Reusability to Remove Cationic Dye from Aqueous Solution. Chem. Eng. Commun. 2021, 1963960. [Google Scholar] [CrossRef]

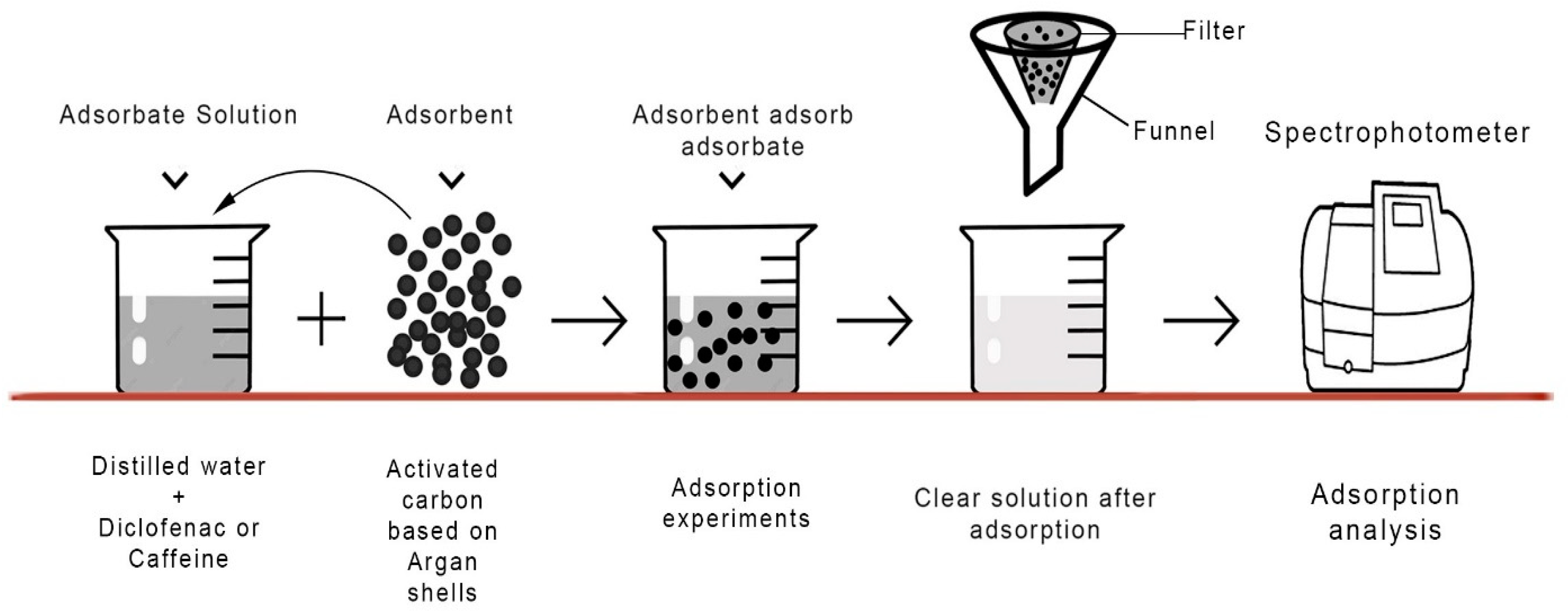
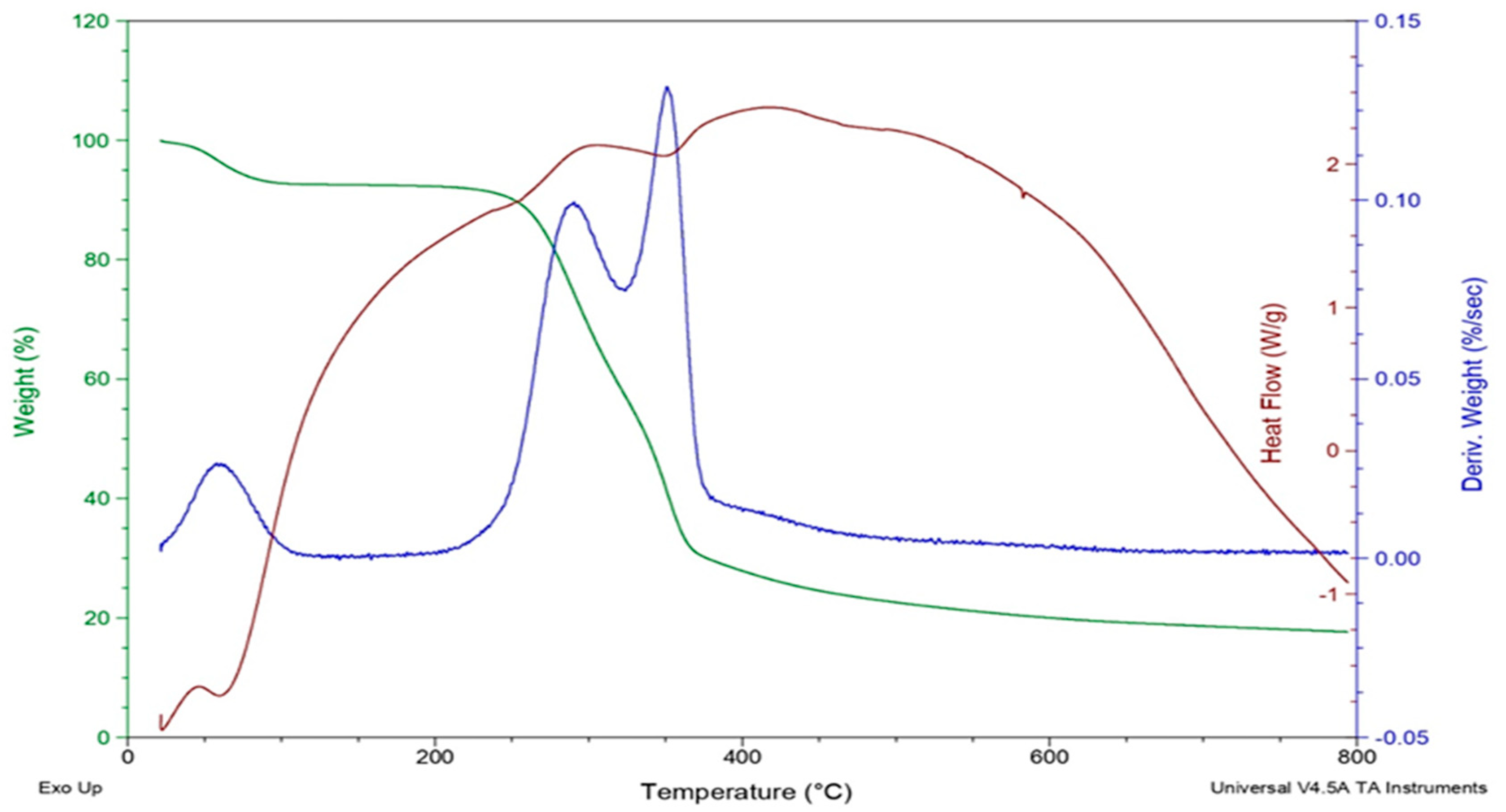

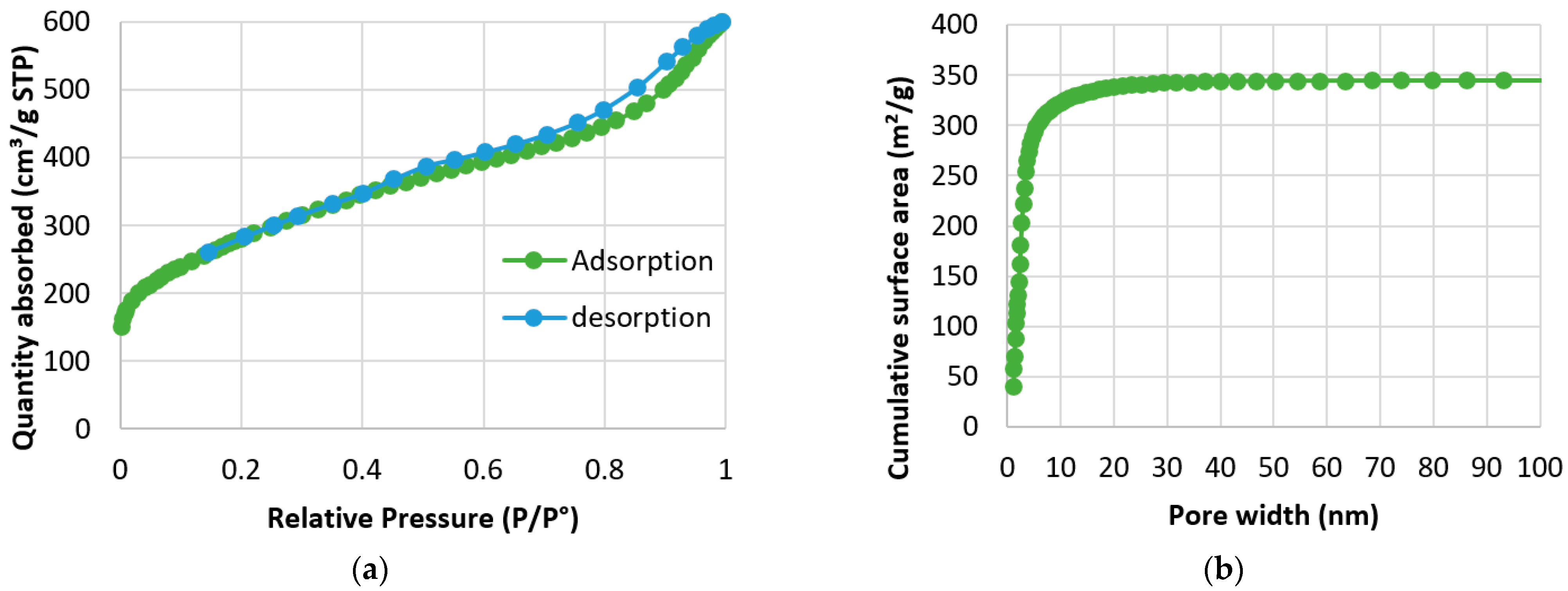
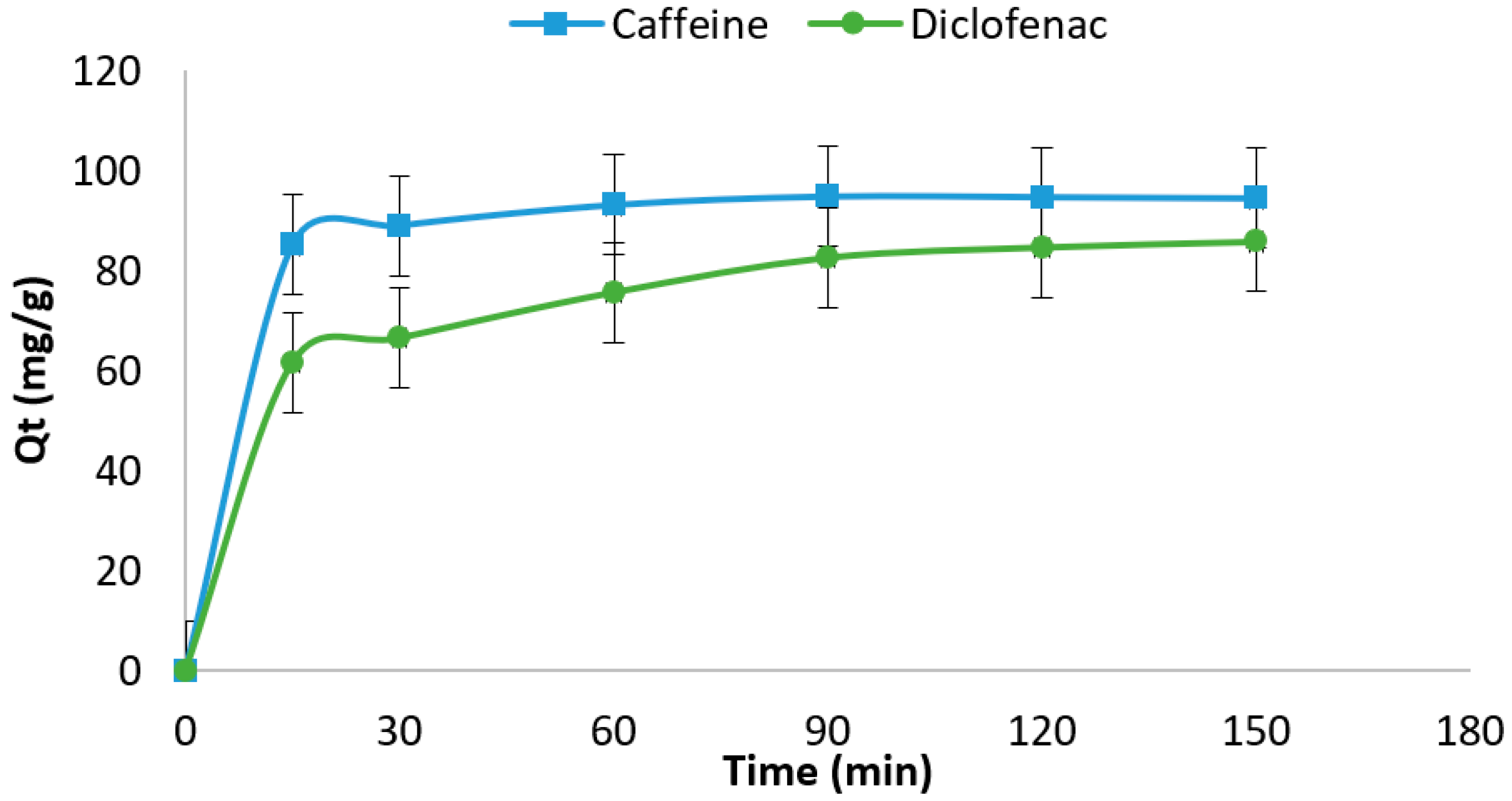

| Absorbate | Absorbent | Initial Concentration | Absorbent Dosage | Time | Adsorption Capacity | References |
|---|---|---|---|---|---|---|
| Caffeine | Peach stones | 100 mg | 0.12 g | 2 h | 126 mg/g | [9] |
| Acacia mangium wood | 100 mg | 3 g | 61 min | 30.9 mg/g | [10] | |
| Date stone | 100 mg | 1 g | 80 min | 28 mg/g | [11] | |
| Macrophytes | 150 mg | 1 g | 1 h | 117.8 mg/g | [12] | |
| Açaí seed | 300 mg | 1 g | 3 h | 176.8 mg/g | [13] | |
| Pineapple Plant leaves | 500 mg | 1 g | 4 h | 152 mg/g | [14] | |
| Sargassum | 20 mg | 0.6 g | 90 min | 221.6 mg/g | [15] | |
| Pine Wood | 120 mg | 0.3 g | 5 h | 362 mg/g | [16] | |
| Coffee waste | 25 mg | 0.1 g | 30 min | 274.2 mg/g | [17] | |
| Elaeis guineensis | 20 mg | 0.2 g | 5 h | 13.5 mg/g | [18] | |
| Eragrostis Plana Nees leaves | 200 mg | 0.07 g | 1 h | 235.5 mg/g | [19] | |
| Argan nutshells | 100 mg | 1 g | 90 min | 210.65 mg/g | This work | |
| Diclofenac | Sycamore ball | 150 mg | 0.2 g | 2 h | 178.9 mg/g | [20] |
| Pine tree | 100 mg | 0.8 g | 2 h | 54.67 mg/g | [21] | |
| Sugar cane bagasse | 50 mg | 0.4 g | 15 min | 315 mg/g | [22] | |
| Cocoa shell | 150 mg | 1 g | 223 min | 63.47 mg/g | [23] | |
| Tea waste | 30 mg | 0.3 g | 8 h | 62.5 mg/g | [24] | |
| Potato peel waste | 50 mg | 0.4 g | 24 h | 68.5 mg/g | [25] | |
| Olive-waste cakes | 50 mg | 0.1 g | 26 h | 56.2 mg/g | [26] | |
| Pine sawdust-Onopordum acanthium | 100 mg | 2.4 g | 1 h | 263.7 mg/g | [27] | |
| Coconut shell | 200 mg | 0.5 g | 24 h | 103 mg/g | [28] | |
| Peach stones | 100 mg | 0.12 g | 2 h | 200 mg/g | [9] | |
| Orange peels | 0.5 mM | 0.5 g | 24 h | 52.2 mg/g | [29] | |
| Argan nutshells | 100 mg | 1 g | 90 min | 126.16 mg/g | Present work |
| Emerging Contaminant | Mass Molar (g/Mol) | PKa | Size (nm) | Chemical Structure |
|---|---|---|---|---|
| Caffeine | 194.2 | 0.82 | 0.98–0.87 | 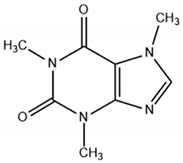 |
| Diclofenac | 318.1 | 4.15 | 0.97–0.96 | 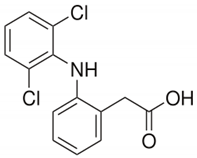 |
| Absorbent | BET Surface Area (m2/g) | Dubinin-Radushkevich Surface Area (m2/g) | Dubinin-Astakhov Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| AC obtained from argan | 1007.76 | 1063.70 | 1042.50 | 0.85 | 3.38 |
| Pseudo First Order | Pseudo Second Order | |||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | K1 | R2 | Qe (mg/g) | K2 | R2 | |
| Dic | 41.01 | −0.00016 | 0.991 | 91.16 | 77576.79 | 0.999 |
| Caf | 9.28 | −0.00016 | 0.872 | 95.99 | 463867.18 | 0.999 |
| Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Qm(mg/g) | Kl (L/mg) | R2 | Rl | 1/n | Kf (L/g) | R2 | |
| Dic | 126.16 | 0.24 | 0.99 | 0.17 | 1.50 | 38.19 | 0.85 |
| Caf | 210.65 | 0.05 | 0.99 | 0.27 | 1.08 | 61.43 | 0.97 |
| Adsorbent | Contaminants | BET Surface Area (m2/g) | Adsorption Capacity (mg/g)/Removal Efficiency (%) | References |
|---|---|---|---|---|
| AC based on Argan nutshells | Dic | 1007 | 126 | Present work |
| Caf | 210 | |||
| AC-HP | BPA | 1372 | 1250 | [92] |
| ACH | DCF | 1542 | 149 | [93] |
| PARX | 168 | |||
| ANS | BPA | 42 | 1162 | [94] |
| ANS | CV | - | 98.21% | [95] |
| Contaminants | T (°C) | ΔG° (Kj/mol) | ΔH° (Kj/mol) | ΔS° (Kj/mol/k) | R2 |
|---|---|---|---|---|---|
| Dic | 10 | −3.28 | −20.11 | −59.32 | 0.82 |
| 20 | −3.07 | ||||
| 30 | −1.85 | ||||
| Caf | 10 | −3.29 | 1.77 | 17.95 | 0.92 |
| 20 | −3.51 | ||||
| 30 | −3.64 |
| Type of Analysis | Parameter Study | Type of Sample | Mean | Std. Error | 95% Confidence Interval | Test ANOVA | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | F | Sig. | |||||
| Effect of adsorbent dose on adsorption yield of Caffeine and Diclofenac | Adsorption yield, Caffeine (%) | AC | 72.448 | 10.416 | 46.960 | 97.937 | 0.001 | 0.000 S |
| Adsorption yield, Diclofenac (%) | AC | 60.466 | 9.654 | 36.343 | 84.089 | 0.002 | 0.000 S | |
| Effect of Concentration on the adsorption capacity of Caffeine and Diclofenac | Adsorption capacity, Caffeine (mg/g) | AC | 79.509 | 15.820 | 11.438 | 147.579 | 0.002 | 0.000 S |
| Adsorption capacity, Diclofenac (mg/g) | AC | 80.226 | 12.080 | 28.247 | 132.206 | 0.001 | 0.000 S | |
| Effect of contact time on adsorption capacity of Caffeine and Diclofenac | Adsorption capacity, Caffeine (mg/g) | AC | 91.839 | 1.619 | 87.675 | 96.002 | 0.003 | 0.001 S |
| Adsorption capacity, Diclofenac (mg/g) | AC | 76.133 | 4.123 | 65.534 | 86.733 | 0.002 | 0.000 S | |
| Effect of temperature on adsorption of Caffeine and Diclofenac | Adsorption capacity, Caffeine (mg/g) | AC | 95.869 | 5.743 | 71.548 | 120.583 | 0.001 | 0.001 S |
| Adsorption capacity, Caffeine (mg/g) | AC | 83.449 | 4.569 | 63.786 | 103.112 | 0.003 | 0.000 S | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhcain, B.; Carrillo-Peña, D.; El Mansouri, F.; Ez Zoubi, Y.; Mateos, R.; Morán, A.; Quiroga, J.M.; Zerrouk, M.H. Removal of Emerging Contaminants as Diclofenac and Caffeine Using Activated Carbon Obtained from Argan Fruit Shells. Appl. Sci. 2022, 12, 2922. https://doi.org/10.3390/app12062922
Bouhcain B, Carrillo-Peña D, El Mansouri F, Ez Zoubi Y, Mateos R, Morán A, Quiroga JM, Zerrouk MH. Removal of Emerging Contaminants as Diclofenac and Caffeine Using Activated Carbon Obtained from Argan Fruit Shells. Applied Sciences. 2022; 12(6):2922. https://doi.org/10.3390/app12062922
Chicago/Turabian StyleBouhcain, Badr, Daniela Carrillo-Peña, Fouad El Mansouri, Yassine Ez Zoubi, Raúl Mateos, Antonio Morán, José María Quiroga, and Mohammed Hassani Zerrouk. 2022. "Removal of Emerging Contaminants as Diclofenac and Caffeine Using Activated Carbon Obtained from Argan Fruit Shells" Applied Sciences 12, no. 6: 2922. https://doi.org/10.3390/app12062922
APA StyleBouhcain, B., Carrillo-Peña, D., El Mansouri, F., Ez Zoubi, Y., Mateos, R., Morán, A., Quiroga, J. M., & Zerrouk, M. H. (2022). Removal of Emerging Contaminants as Diclofenac and Caffeine Using Activated Carbon Obtained from Argan Fruit Shells. Applied Sciences, 12(6), 2922. https://doi.org/10.3390/app12062922











