Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plasma Device
2.3. Treatment of Rice Stem Cells with Plasma
2.4. Reactive Species Measurement in Gas Phase
2.5. Extraction of Rice Stem Cells
2.6. Assay for the Amount of Total Polyphenol
2.7. Total RNA Isolation and qRT-PCR
2.8. Gas Chromatography–Mass Spectrometery (GC-MS) Analysis
2.9. Cell Culture
2.10. Cell Viability Assay
2.11. Statistical Analysis
3. Results and Discussion
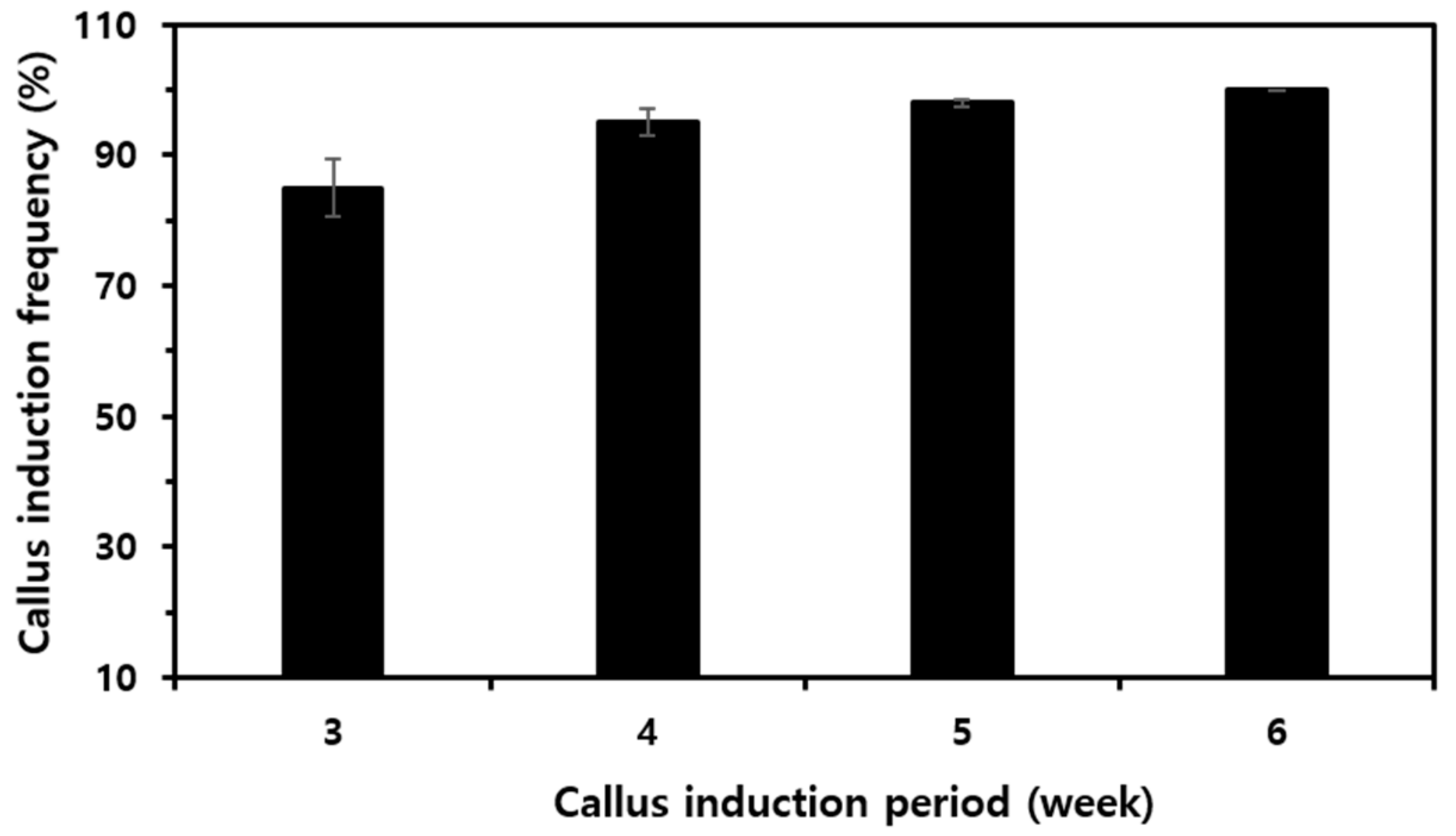
3.1. Preparation of Stem Cells from Rice Seeds
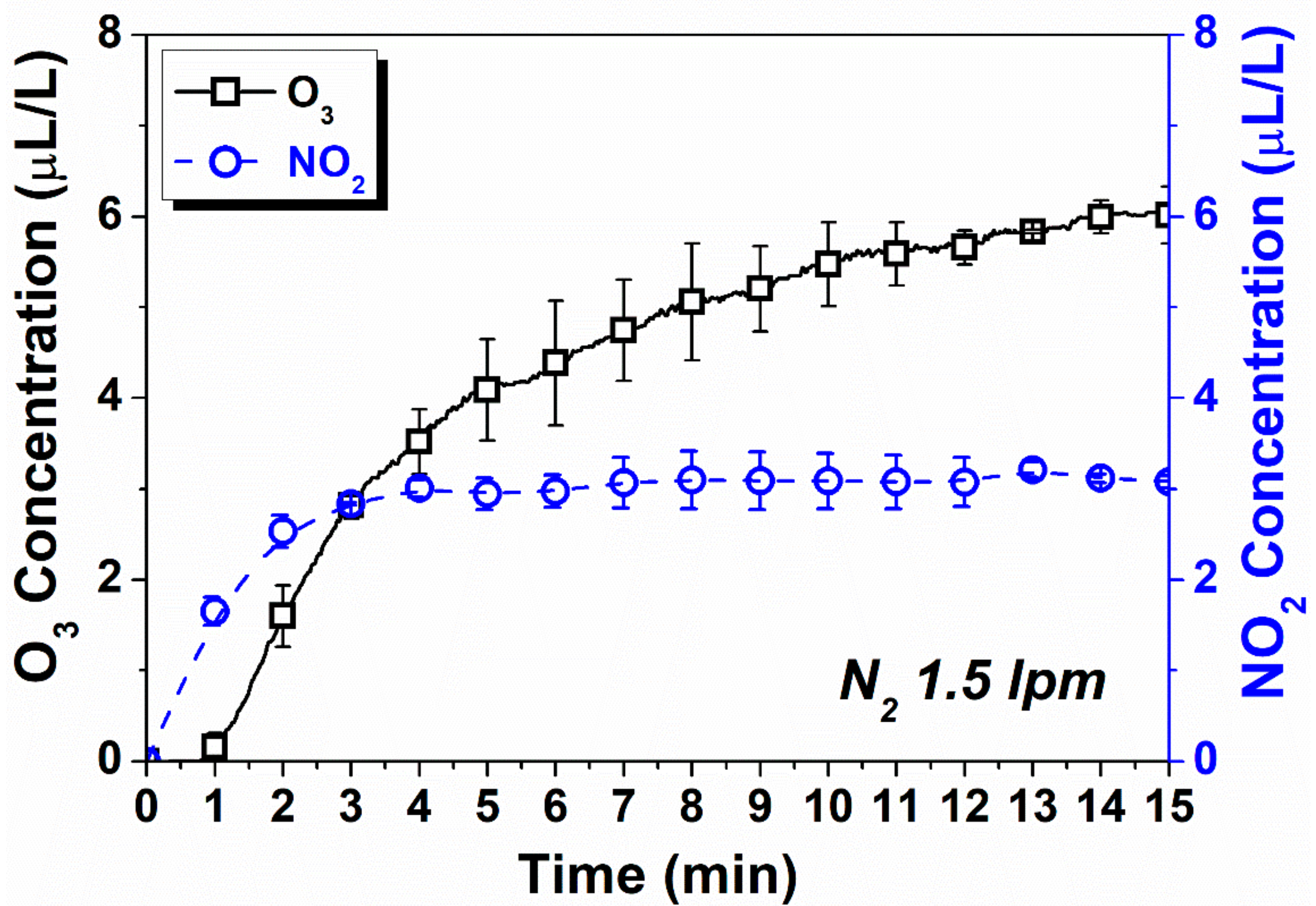
3.2. Ozone and NOx Analysis
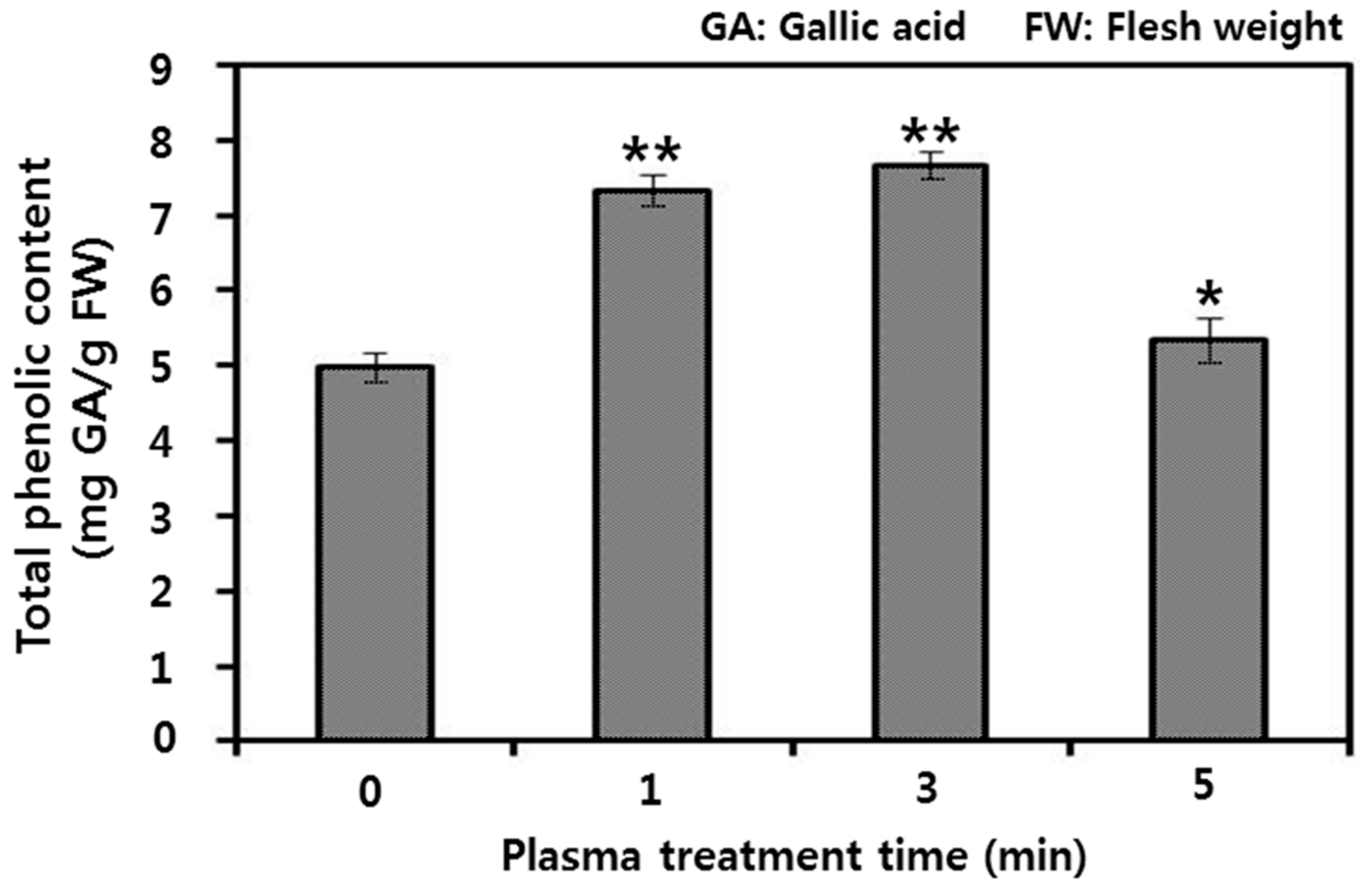
3.3. Antioxidant Activities of Rice Stem Cell Extracts
3.4. GC-MS Analysis of Rice Stem Cell Extracts
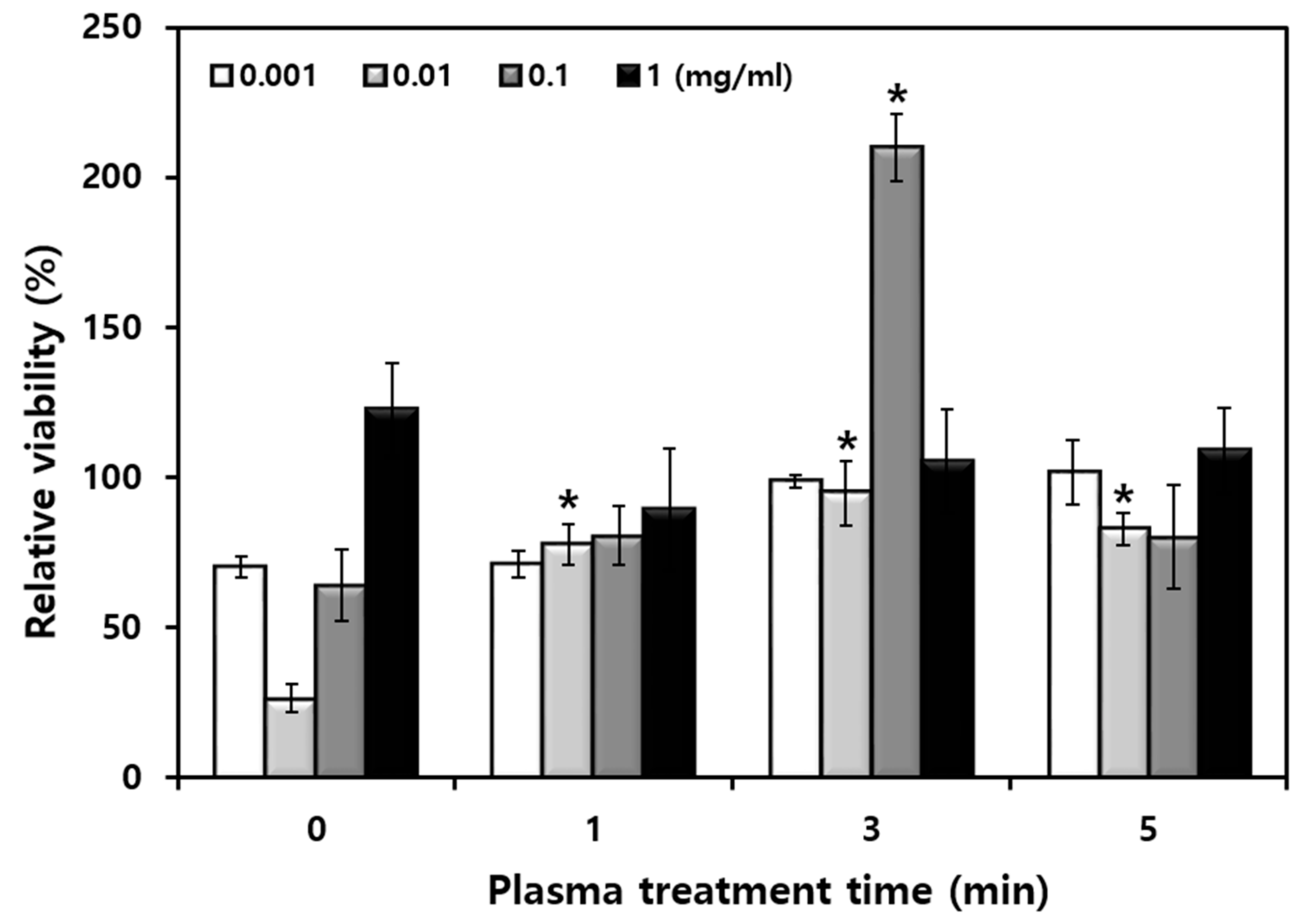
3.5. Effects of Rice Stem Cell Extracts against Oxidative Damage on HFB
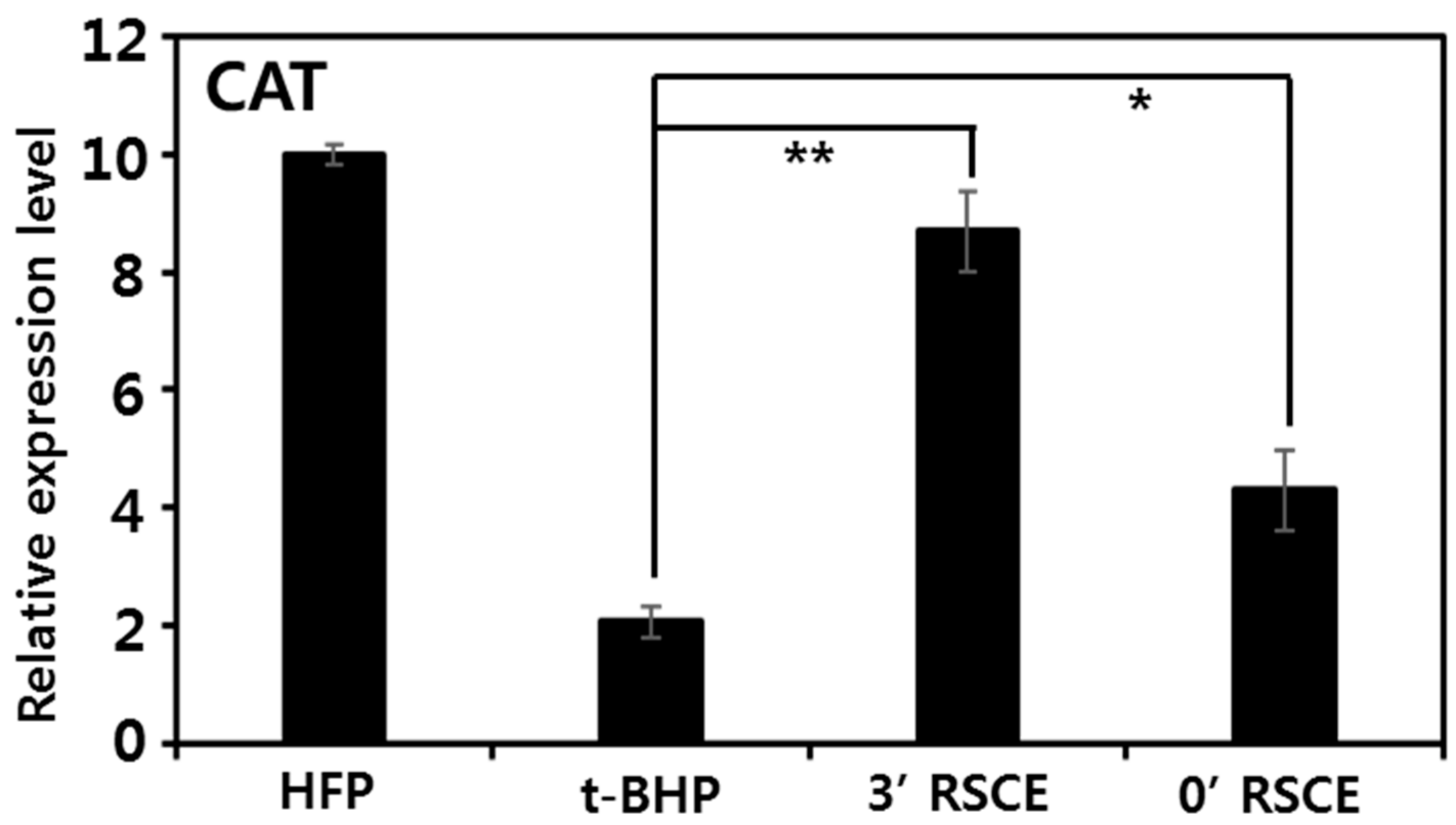
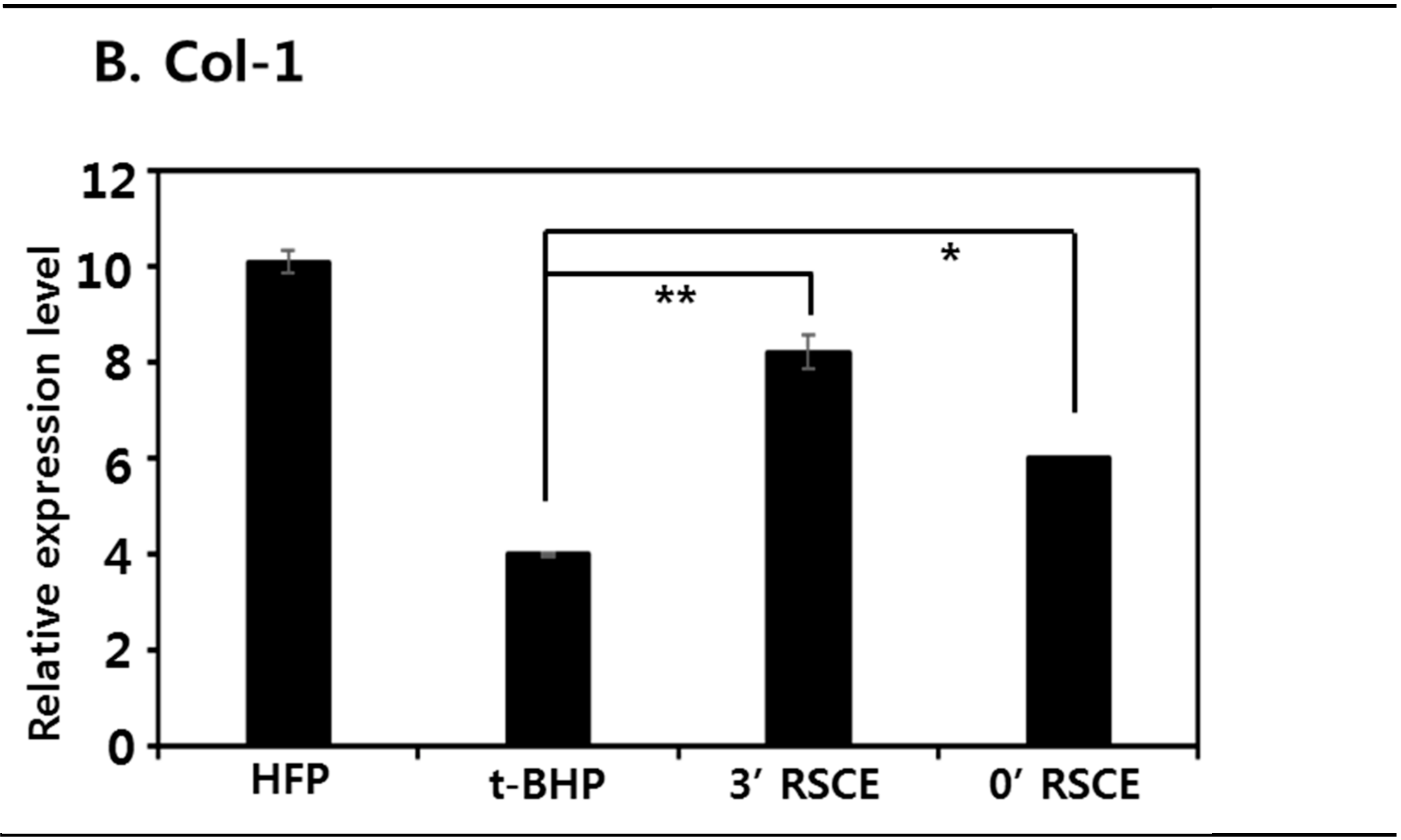
3.6. Antiaging Effects of Rice Stem Cell Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.M.; Yoon, J.Y.; Lim, K.M.; Hahn, H.J.; Kim, Y.R.; Ahn, K.J.; An, S.K. Effects of the complex containing Centella asiaticaand folic acid-fermented extracts, acetyl glutamine, and nicotinic acid adenine dinucleotide phosphate on the inhibition of senescence and melanogenesis, promotion of collagen expression, cellular regeneration, and keratinocyte differentiation, and anti-inflammation. Korean J. Aesthet. Cosmetol. 2013, 11, 675–684. [Google Scholar]
- Yasui, K.; Sakurai, H. Chemiluminescent detection and imaging of reactive oxygen species in live mouse skin exposed to UVA. Biochem. Biophys. Res. Commun. 2000, 269, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S. Walnut husk ethanol extract possess antioxidant activity and inhibitory effect of matrix metalloproteinase-1 expression induced by tumor necrosis factor alpha in human keratinocyte. Korean J. Aesthet. Cosmetol. 2013, 11, 715–719. [Google Scholar]
- Kim, D.S.; Jeon, B.K.; Mun, Y.J.; Kim, Y.M.; Lee, Y.E.; Woo, W.H. Effect of Dioscorea aimadoimo on anti-aging and skin moisture capacity. J. Orient. Physiol. Pathol. 2011, 25, 425–430. [Google Scholar]
- Mohammad, A.E.; Ali, N.; Sarvin, M.A.; Hossein, K.; Seyedeh, M.G.; Sobhan, M.D. Enhanced catalytic and antibacterial efficiency of biosynthesized Convolvulus fruticosus extract capped gold nanoparticles (CFE@AuNPs). J. Photochem. Photobiol. B Biol. 2020, 209, 111949. [Google Scholar] [CrossRef]
- Pardis, M.; Mehdi, S.A.; Sobhan, M.D.; Ahmad, B.R.; Seyedeh, M.G. PEG-Citrate dendrimer second generation: Is this a good carrier for imaging agents In Vitro and In Vivo? IET Nanobiotechnol. 2019, 13, 560–564. [Google Scholar] [CrossRef]
- Lucia, S.; Lucia, M.; Evelyn, O.; Massimo, L.; Luisa, F.; Luca, P.; Davide, P.; Miriam, C. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437–102460. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Future Sci. 2017, 3, FSO226. [Google Scholar] [CrossRef] [Green Version]
- Vasil, I.K.; Vasil, V. Totipotency and embryogenesis in plant cell and tissue cultures. In Vitro 1972, 8, 117–125. [Google Scholar] [CrossRef]
- Fay, M.F. Conservation of rare and endangered plants using in vitro methods. In Vitro Cell. Dev. Biol.-Plant 1992, 28, 1–4. [Google Scholar] [CrossRef]
- Irvani, N.; Solouki, M.; Omidi, M.; Zare, A.; Shahnazi, S.J.P.C. Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tissue Organ Cult. 2010, 100, 293–299. [Google Scholar] [CrossRef]
- Oh, S.T.; Jung, H.S.; Cho, M.J.; Song, M.Y.; Moh, S.H.; Seo, H.H. Effect of Artemisia annua Linne callus induced by plant cell culture technology on wound healing. J. Korea Acad.-Ind. Coop. Soc. 2014, 15, 5628–5636. [Google Scholar] [CrossRef] [Green Version]
- Saewan, N.; Vichit, W.; Prinyarux, T. Anti-aging efficacy of thai red rice callus cosmetic product. J. Appl. Sci. 2018, 17, 63–72. [Google Scholar] [CrossRef]
- Mousumee, K.; Soyeon, P.; Hyeon-Jin, K.; Kui-Jae, L.; Dea-Heon, K.; So-Hyeon, B.; Seong-Tshool, H. The Resveratrol Rice DJ526 Callus Significantly Increases the Lifespan of Drosophila (Resveratrol Rice DJ526 Callus for Longevity). Nutrients 2019, 11, 983. [Google Scholar] [CrossRef] [Green Version]
- Widowati, W.; Fauziah, N.; Herdiman, H.; Afni, M.; Afifah, E.; Kusuma, H.S.W.; Nufus, H.; Arumwardana, S.; Rihibiha, D.D. Antioxidant and anti-aging assays of Oryza Sativa extracts, vanillin and coumaric Acid. J. Nat. Remedies 2016, 16, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’asta, C.; Galaverna, G.; Scazzina, F.; Nicoletta, P. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef]
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625. [Google Scholar] [CrossRef]
- Dezest, M.; Chavatte, L.; Bourdens, M.; Quinton, D.; Camus, M.; Garrigues, L.; Descargues, P.; Arbault, S.; Burlet-Schiltz, O.; Casteilla, L.; et al. Mechanistic insights into the impact of Cold Atmospheric Pressure Plasma on human epithelial cell lines. Sci. Rep. 2017, 7, 41163. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Sherman, J.; Murphy, M.; Ratovitski, E.; Canady, J.; Keidar, M. The effect of tuning cold plasma composition on glioblastoma cell viability. PLoS ONE 2014, 9, e98652. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.C. The N6 medium and its applications to anther culture of cereal crops. In Proceedings of the Symposium on Plant Tissue Culture, Beijing, China, 25–30 May 1978; Science Press: Beijing, China, 1978; pp. 45–50. [Google Scholar]
- Tabei, Y. (Ed.) Transformation Protocol. In Plants; Kagaku-Doujin: Tokyo, Japan, 2012. (In Japanese) [Google Scholar]
- Islam, M.M.; Ahmed, M.; Mahaldar, D. In vitro callus induction and plant regeneration in seed explants of rice (Oryza sativa L.). Res. J. Agric. Biol. Sci. 2005, 1, 72–75. [Google Scholar]
- Shahsavari, E.; Maheran, A.A.; Akmar, A.S.N.; Hanafi, M.M. The effect of plant growth regulators on optimization of tissue culture system in Malaysian upland rice. Afr. J. Biotechnol. 2010, 9, 2089–2094. [Google Scholar]
- Ji, S.H.; Kim, J.S.; Lee, C.H.; Seo, H.S.; Chun, S.C.; Oh, J.S.; Choi, E.H.; Park, G.S. Enhancement of vitality and activity of a plant growth promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Ikawa, M.; Schafer, T.; Dollard, C.; Sasner, J. Utilization of Folin-Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Ma, X.B.; Yang, J. An optimized preparation method to obtain high-quality RNA from dry sunflower seeds. Genet. Mol. Res. 2011, 10, 160–168. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Burton, M.; Chen, Q.M.; Gonos, E.S.; Frippiat, C.; Mazarati, J.B.; Eliaers, F.; Remacle, J.; Toussaint, O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic. Biol. Med. 2000, 28, 361–373. [Google Scholar] [CrossRef]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotkova, H.; Garnol, T.; Drahota, A.; Cervinkova, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem. J. 1989, 257, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Crane, D.; Haussinger, D.; Graf, P.; Sies, H. Decreased flux through pyruvate dehydrogenase by thiol oxidation during t-butyl hydroperoxide metabolism in perfused rat liver. Hoppe-Seyler’s Z. Physiol. Chem. Bd. 1983, 364, 977–987. [Google Scholar] [CrossRef]
- Sohal, R.S.; Allen, R.G.; Nations, C. Oxygen free radicals play a role in cellular differentiation: An hypothesis. J. Free Radic. Biol. 1986, 2, 175–181. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.J. ROS and RNS: Key signaling molecules in plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef] [Green Version]
- Burdon, R.H.; Gill, V.; Rice-Evans, C. Cell proliferation and oxidative stress. Free Radic. Res. Commun. 1989, 7, 149–159. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459–13475. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Fussell, J.C.; Kelly, F.J. Oxidative contribution of air pollution to extrinsic skin ageing. Free Radic. Biol. Med. 2020, 151, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Overexpression of neprilysin plays as essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Total (ddH2O) | 1 L |
|---|---|
| Sucrose | 30 g |
| Casamino acid | 0.3 g |
| Proline | 2.878 g |
| CHU N6 | 3.956 g |
| Myo-inosiitol | 0.1 g |
| 2.4-D (mg/mL) | 2 mL |
| pH | 5.8 |
| Gellan Gum | 4 g |
| Primer | Temperature (°C) | Sequence (5′→3′) |
|---|---|---|
| CAT | 60 | AACTGTCCCTACCGTGCTCG |
| ATTGGCAGTGTTGAATCTCCGC | ||
| Col-1 | 60 | GAGGGCCAAGACGAAGACATC |
| CAGATCACGTCATCGCACAAC | ||
| MMP-9 | 60 | ACTCGGGTGGCAGAGATGC |
| AGGTGATGTTGTGGTGGTGC | ||
| GAPDH | 60 | ATGAGAAGTATGACAACAGCC |
| AGTCCTTCCACGATACCAAA |
| Retention Time (min) | Metabolites 1 | Peak Height Changed 2 |
|---|---|---|
| 34.805 | Fructose | 3 > 1 > C = 5 |
| 36.690 | Galactopyranoside | 3 > 1 > C > 5 |
| 47.830 | Melibiose | 3 > C = 1 = 5 |
| 49.325 | Glycoside | 3 > 1 > C > 5 |
| 49.620 | Sucrose | 3 > 1 > C > 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.H.; Akter, M.; Ko, E.Y.; Choi, E.H.; Keum, Y.S.; Han, I. Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment. Appl. Sci. 2022, 12, 2903. https://doi.org/10.3390/app12062903
Ji SH, Akter M, Ko EY, Choi EH, Keum YS, Han I. Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment. Applied Sciences. 2022; 12(6):2903. https://doi.org/10.3390/app12062903
Chicago/Turabian StyleJi, Sang Hye, Mahmuda Akter, Eun Young Ko, Eun Ha Choi, Young Soo Keum, and Ihn Han. 2022. "Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment" Applied Sciences 12, no. 6: 2903. https://doi.org/10.3390/app12062903
APA StyleJi, S. H., Akter, M., Ko, E. Y., Choi, E. H., Keum, Y. S., & Han, I. (2022). Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment. Applied Sciences, 12(6), 2903. https://doi.org/10.3390/app12062903








