Abstract
Alopecia is a chronic inflammatory skin disease with various causes. Lespedeza bicolor extract (LBE) has been reported to have anti-inflammatory and antioxidative effects. In this study, the activity and mechanisms of LBE as a hair growth agent were investigated. Effects of cell proliferation, cytotoxicity, and cell cycle regulation of LBE and its active component protocatechuic acid (PCA) were evaluated in human dermal papilla cells (DPCs). Hair regeneration effects of LBE in 6-week-old C57BL/6 male mice were also determined using positive control 5% minoxidil. The dose-dependent proliferation of DPCs was estimated in response to LBE treatment (0.8–20 µg/mL). Additionally, significant extension of the anagen phase during the hair cell cycle upon LBE treatment was observed histologically and morphologically. Cell cycle arrest gene expression was determined by quantitative real-time polymerase chain reaction. Lespedeza bicolor could be a potent treatment against alopecia through enhancing DPC proliferation and hair regrowth via anagen phase arrest.
1. Introduction
Alopecia, also known as hair loss, is a dermatological disorder with a global distribution. Alopecia has many causes, including genetic factors, androgenic alopecia, stress, nutritional deficiencies, aging, and chronic inflammatory dermatological diseases. The hair growth cycle moves through several phases, including degeneration (catagen phase), rest (telogen phase), and regeneration (anagen phase) in adult hair follicles (HF) for extended periods [1,2,3,4]. Because HFs are composed of dermal papilla cells (DPCs) and skin sheaths of human hair, they have an essential function of controlling hair growth throughout pathogenesis of specific conditions, including hair loss, as well as during the normal hair cycle [5]. The size and number of DPCs are closely related to the hair cycle, and the number of DPCs is relatively high in the regeneration phase [6]. Hair loss is often caused by a chronic scalp inflammation reaction and, as a result, the anagen phase of HF is shortened [7], and all or part of the hair of the head or body may be lost [8]. Currently, androgen receptor antagonists, androgen-independent hair growth stimulants, and 5α-reductase inhibitors, among others, are used as therapeutic agents for preventing hair loss and promoting hair growth [9]. However, due to unpredictable effects and side effects such as decreased sexual desire, their use may be restricted or made only temporarily available [10,11,12,13]. Therefore, research into new drugs to promote hair growth is necessary. Lespedeza bicolor is a legume that grows wild throughout Asia and has recently been cultivated for ornamental and medicinal purposes [14]. Lespedeza bicolor has been used in folk medicine to treat nephritis, azotemia, hyperpigmentation, diuresis, diabetes, and inflammation [14,15,16,17,18]. Additionally, L. bicolor ethanol extract possesses antioxidant, anti-inflammatory, estrogenic, antityrosinase, antifungal, and antimicrobial activities [14,17,19]. Lespedeza bicolor contains isoflavones such as genistein and daidzein, flavonoids such as catechin, quercetin, rutin, luteolin, and naringin, and flavonoid-based metabolites such as protocatechuic acid (PCA) [15]. PCA is one of the most effective components of many natural products and is the main metabolite of anthocyanins. PCA has been reported to have various pharmacological functions such as antioxidant, anti-inflammatory, antiviral, antiaging, and antiosteoporosis effects, as well as being involved in stem cell proliferation and differentiation [20,21,22]. In this study, the effects of LBE on DPC proliferation and cell cycle were investigated. Additionally, hair regeneration promotion effects by LBE were observed in C57BL/6 mice.
2. Materials and Methods
2.1. Materials and Reagents
Lespedeza bicolor was obtained from Ongbok Mountain, Chungbuk, Korea. A voucher specimen (BMRI-18) was deposited in the laboratory of one of the corresponding authors (S.C. Kang). The dried Lespedeza bicolor leaves (100 g) were extracted with 80% ethanol or water (1:10 ratio) at room temperature for 24 h, consecutively. The solvent extract was filtered using a vacuum filter and concentrated using a vacuum evaporator. A total of 19 g of residue was obtained and stored at −20 °C until use. Minoxidil, MTT, and PCA were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Cells
DPC was purchased as primary cells from ScienCell (Carlsbad, CA, USA). Mesenchymal stem cell medium (MSCM), mesangial cell growth factor supplement (MCGS), and fetal bovine serum (FBS) were purchased from ScienCell. DPCs were cultured using MSCM with 2.5% FBS, 1% MCGS, and 1% penicillin/streptomycin in a 5% CO2 incubator at 37 °C.
2.3. Cell Viability and Proliferation Assay
DPCs (1 × 104 cells/mL) were divided into 96-well plates and incubated at 37 °C overnight. LBE was treated with various concentrations (0.8, 4, 20, 100, and 500 μg/mL), cultured for 24 h or 72 h, and then cultured for 3 h after adding MTT solution (5 mg/mL) to each well. The formazan was dissolved in dimethyl sulfoxide to measure absorbance at 540 nm using a plate reader (Tecan, Switzerland).
2.4. Western Blot Analysis
Each sample-treated DPC was lysed with Pierce RIPA lysis and extraction buffer (Thermo Fisher Scientific, Whaltham, MA, USA) at room temperature for 20 min. Protein was quantified to 30 μg using a bovine serum albumin (BSA) assay. Proteins of the same concentration were decomposed into 12% SDS-PAGE. The membrane was blocked for 1.5 h at room temperature using skim milk buffer and was incubated with appropriate dilutions of primary antibody. Next, the secondary antibody was reacted at room temperature for 1 h. Protein was detected using EZ-western Lumi Pico reagents (DoGenBio, Seoul, Korea). The detected band was quantified using an image analyzing program (Java, Santa Clara, CA, USA).
2.5. In Vivo Experiments
Six-week-old male C57BL/6 mice (a total of 40 mice) were purchased from SLC (Shizuoka, Japan) and were fed a commercial pellet diet and fresh water ad libitum. Mice were housed in a controlled environment with humidity (55 ± 5%), ambient temperature (22 ± 2 °C), and a 12 h light–dark cycle. Animal testing was performed in accordance with Semyung University’s current Code of Ethics for Animal Care and Use (SMECAE 091101). At autopsy, mice were anesthetized by intraperitoneal injection with a mixture of zoletil (Virbac, Carros, France) and rumpun (Bayer, Leverkusen, Germany). The back hairs of mice were removed using a wax–rosin mixture according to Chase’s method (1954) [23], and the mice were divided into 4 groups of 10 individuals: normal control (vehicle control), 5% minoxidil, LBE 1 mg/mL, and LBE 10 mg/mL. All experimental groups were treated with 100 μL of each test substance daily for 18 days on the dorsal skin. The mice’s back and hairs were observed on the 1, 4, 7, 14, and 18th days and separated to investigate histological features. Individual skin tissues were manufactured to a thickness of 4 μm using a Leica ASP300 tissue processor (Leica Microsystems, Wetzlar, Germany) and then stained with hematoxylin & eosin. Afterward, histological morphologies were observed using optical microscopy (Olympus CX 31; Tokyo, Japan).
2.6. HPLC Analysis
To characterize the extract, the content of PCA, a major component of L. bicolor, was measured in the LBE using HPLC (Shimadzu, LC-20AD, PDA detector equipped with Skypack 5 μm C18 column (150 4.6 mm)). Quantitative analysis was performed for 10 min using an isocratic elution (acetonitrile/0.1% trifluoroacetic acid in water = 70:30) with PDA detection (280 nm). The flow rate was 1 mL/min, and 10-μL aliquots of samples were injected for analysis.
2.7. Cell Cycle Analysis
Cell cycles were analyzed using a Tali® image-based cytometer (Thermo Fisher Scientific) according to the manufacturer’s protocol. DPCs were harvested after treating with LBE, PCA, and minoxidil for 60 h. The harvested DPCs were immobilized overnight with cold 70% ethanol at −20 °C. Next, the DPCs were washed with cold PBS and reacted with the Tali® Cell Cycle Solution at room temperature for 30 min while the light was blocked.
2.8. Quantitative RT-PCR
Total RNA was purified using RNAisoPlus reagent (Takara, Kusatsu, Japan). Purified total RNA (1 μg) was synthesized using the PrimeScript II first strand cDNA synthesis kit (Takara, Kusatsu, Japan), and the assays were run using SYBR Premix Ex Taq II (Takara, Kusatsu, Japan) for quantitative real-time PCR. Briefly, the reaction mixture (total volume 20 µL) consisted of 10 µL 2× master mix solution, 8 µL DEPC water, 1 µL cDNA template, and 1 µL primers (forward/reverse), followed by heat cycling, preincubation at 95 °C (20 min), 40 cycles were run at 95 °C (20 s), and 58–61 °C (40 s). Quantitative real-time PCR was performed with a QuantStudioTM 5 Real-Time PCR system (Thermo Fisher Scientific) using the primers summarized in Table 1.

Table 1.
Nucleotide sequence of primers and expected size of PCR products.
2.9. Statistical Analysis
Results are presented as the mean ± standard deviation (SD) of three independent experiments. Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). Data were analyzed through Duncan’s multiple comparison tests following a one-way analysis of variance (ANOVA). Statistical significance was accepted for p values < 0.05.
3. Results
3.1. Effects of LBE on Human Hair Dermal Cell Proliferation
Although cytotoxicity was observed in DPCs treated with high concentrations of LBE, this treatment did not induce any morphological changes. LBE treatment (0.8 to 20 µg/mL) significantly increased the cell proliferation rate of DPCs (7.5–29.8%) compared with the control group (Figure 1). The highest cell proliferation was reached at the 20-µg/mL treatment level.
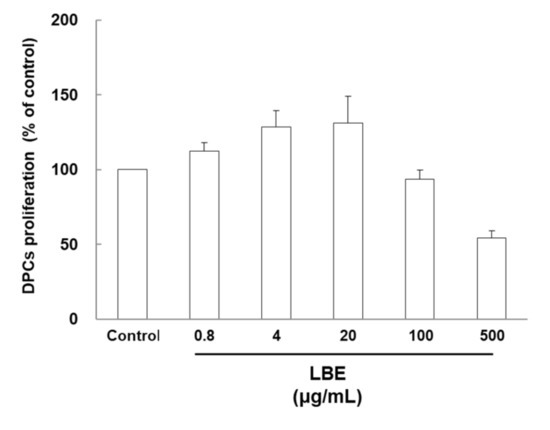
Figure 1.
Cytotoxicity of Lespedeza bicolor extract (LBE) in DPCs. Cells were treated with various concentrations of LBE for 24 h. Representative data of each group are expressed as mean ± SD.
3.2. Effects of LBE on Protein Expressions in DPCs
LBE treatment increased the expression of Bcl-2, ERK, and p38 in a dose-dependent manner in DPCs (Figure 2); however, LBE did not affect Bax expression in DPCs. These results indicate that LBE could promote HF proliferation and hair growth in vitro.
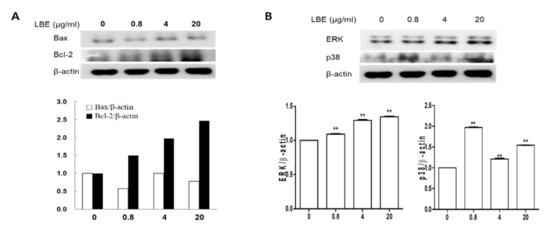
Figure 2.
The effects of LBE on protein expression in DPCs. Western blot analysis of the protein levels of (A) Bax and Bcl-2, (B) ERK, and p38. Representative data for each group are expressed as mean ± SD. ** p < 0.01 compared with the control groups.
3.3. Effects of LBE on Hair Regeneration from In Vivo
The effects of LBE administered subcutaneously during the anagen phase after depilation were evaluated. The hair growth cycle of the C57BL/6 mouse is temporally synchronized. The skin of degenerated mice in the telogen phase is pink, but at the beginning of the anagen phase, the skin turns gray [13].
As shown in Figure 3A, both the LBE and minoxidil groups showed light gray skin from day seven after depilation. In particular, 14 days after depilation, 10 mg/mL of LBE treatment induced darker skin than did the 1 mg/mL LBE treatment. Furthermore, the 10 mg/mL LBE treatment yielded more hair shafts than the minoxidil treatment. However, the skin color of the control group remained pink until the 9th day and turned gray on the 10th day. The hair shaft of the control group was first observed on the 11th day. A mature anagen phase was observed on the 18th day after depilation in all mice (Figure 3B). Additionally, hair weight was more than doubled in the 10 mg/mL LBE group on day 18 compared with the normal and positive control groups. Skin tissue samples for histological analysis were collected on the 18th day after depilation. The 10 mg/mL LBE treatment induced longer and larger HFs than the minoxidil treatment; however, both treatments had catagen-like HF morphology (Figure 3C). These results indicated that LBE might stimulate hair growth via early telogen to mature anagen conversion of HFs (Figure 3).
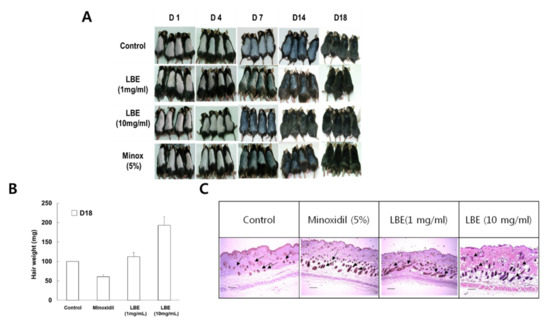
Figure 3.
Hair generative effects of LBE in vivo. (A) After depilation, photos were taken on the 1, 4, 7, 14, and 18th days. Skin darkness turned gray earlier in the LBE treatment group than in the control group; (B) hair weight; (C) effects of LBE on anagen phase extension in C57BL/6 mice. The back skin tissue of C57BL/6 mice was separated 18 days after depilation and stained with hematoxylin & eosin. Representative histology from four animals is shown. Compared with the control (vehicle and positive) groups, the LBE groups had greater hair follicle growth from the subcutaneous to the epidermis. Scale bars = 0.5 µm.
3.4. HPLC Analysis
PCA has already been reported to inhibit oxidative damage, so it was speculated that it could also prevent hair loss by preventing oxidative damage to hair follicle cells. Additionally, it has long been known to inhibit oxidative damage of L. bicolor water extract. Therefore, we tried to compare the PCA content of L. bicolor water extract and LBE. It was confirmed that LBE contained 14 times as much as L. bicolor water extract as PCA (Figure 4). PCA was selected as an indicator component of LBE.
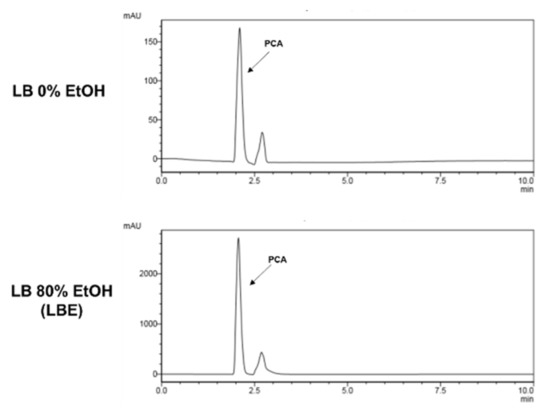
Figure 4.
High-performance liquid chromatography (HPLC) chromatogram of protocatechuic acid (PCA) in LBE.
3.5. Effects of LBE on Cell Cycle Phase in DPCs
Cell proliferation efficacy over time was measured using LBE, PCA, and minoxidil as a positive control. Overall cell proliferation capacity increased at 60 h rather than 36 h after treatment with LBE and PCA. However, minoxidil, a positive control, increased the proliferation capacity of DPCs at 36 h and decreased cell proliferation capacity at 60 h (Figure 5A). Therefore, the optimal times for controlling cell proliferation with LBE and minoxidil were different.
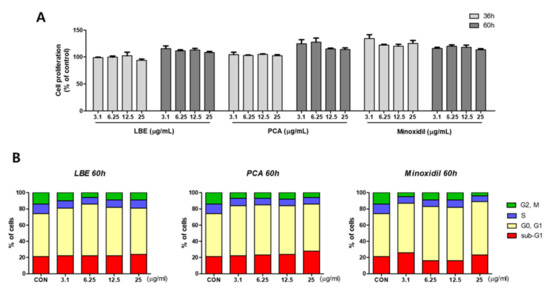
Figure 5.
Effects of LBE, PCA, and minoxidil on hair regeneration in human papilla cells: (A) cell proliferation of LBE, PCA, and minoxidil in human dermal papilla cells. Cells were treated with various concentrations of LBE, PCA, and minoxidil for 36 h and 60 h. Minoxidil was used as a positive control. Representative data of each group are expressed as mean ± SD. (B) The effect of LBE, PCA, and minoxidil on the cell cycle in human dermal papilla cells.: cells were treated with LBE, PCA, and minoxidil at various concentrations for 60 h; cell cycle stages (sub G1, G0/G1, S, G2/M) were measured using a Tali image-based cytometer.
To determine how LBE and PCA affect the cell cycle, we investigated the effects of LBE and PCA on the cell cycle using a Tali cell cycle kit. As displayed in Figure 5B, LBE and PCA arrested cell growth. When DPCs were treated with LBE at a concentration of 6.25 μg/mL, the G0/G1 phase increased by 11% compared with the normal control group. These results showed that LBE treatment increased the number of organelles of DPCs and regulated cell growth, resulting in an increase in the G1 phase. Additionally, because cell proliferation has already progressed after cell division by LBE and the G1 phase has increased, the actual cell proliferation phase, the G2/M phase, decreased compared with the normal control.
3.6. Effects of LBE on Cell Cycle Related Biomarkers in DPCs
To determine how LBE and PCA affect cell cycle-related biomarkers, we investigated the effects of LBE and PCA using quantitative real-time PCR. As displayed in Figure 6, LBE increased expression of Bcl-2, Bax-α, CCNB1, p38, ERK1, ERK2, and p21 and decreased expression of p53 and p57. PCA decreased all biomarker gene expression, minoxidil increased expression of Bax-α, ERK2, p21, and p53 and suppressed CCNB1 expression. Therefore, p21, p38, and ERK were regulated in the proliferation of DPCs due to the regulation of cell cycle arrest by LBE because they are genes related to cell cycle arrest.
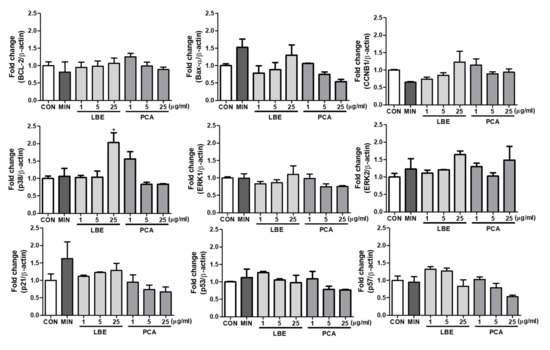
Figure 6.
qRT-PCR analysis of genes related to cell cycle arrest (ERK, p21, p53), differentiation (p38), mitosis (CCNB1), proliferation (p57), and apoptosis (Bax, Bcl-2) after 60 h of treatment of human dermal papilla cells with LBE, PCA, and minoxidil. Representative data from each group are expressed as means ± SDs (n = 3). * p < 0.05 compared with the control groups.
4. Discussion
Alopecia, which has various causes or interactions, including aging, endocrine imbalances, and genetic factors, is a common hair disease, especially in adult males [2]. It is not clear whether the proliferation rate or apoptotic change of DPCs increase could affect alopecia. For example, the front of patients with androgenetic alopecia (bald) has a low proliferation rate, inducing HF miniaturization, which could be caused by an increase in DNA damage and imbalance in the cell’s recovery capacity [24]. HF growth cycles include three consecutive phases, namely, growth, regression, and relative quiescence. HFs are mini organs with complex structures and uncertain functions that develop in dynamic and alternately changing environments of various molecular signals.
Minoxidil, which is widely used as a treatment for alopecia, has been used for more than 30 years to promote hair growth, but it has side effects such as decreased sexual function. Additionally, minoxidil has a concentration-dependent effect on the differentiation and proliferation of normal human keratinocytes [25] and induces significant DPC proliferation [26].
This study clearly demonstrates that LBE can induce DPC proliferation and promote hair regrowth in C57BL/6 mice. The results showed that LBE treatment enhanced the proliferation rate of DPCs at 0.8–20 μg/mL, whereas its cytotoxic effects on DPCs appeared at higher concentrations (100–500 μg/mL), indicating its high safety profile. Therefore, the results of this study suggest that 0.8–20 µg/mL of LBE may stimulate DPC proliferation and may be safe for subcutaneous use, especially for the scalp, which has anti-inflammatory properties and for the increase in DPCs [27]. Cytotoxicity to human DPCs appeared at high concentrations (100–500 µg/mL), confirming that the safety margin was high. Therefore, the results of this study demonstrated that LBE can stimulate DPC proliferation and may be safe for subcutaneous use, especially on the scalp. The function of DPCs is regulated by the altered shape of hair in the anagen and telogen phases. According to Muller-Rover et al. [28], in mouse experiments, on day nine after hair removal, all HF entered the final stage of the growth phase of the hair cycle. After hair loss, spontaneous regression begins to appear around the 17th day and enters the resting phase (telogen) around the 20th day. HF circulation in young mice follows a rather precise time scale [29]. Therefore, the hair growth-promoting effect of LBE was observed using 6-week-old mice. A similar pattern to that of hair circulation due to hair loss was identified. Topical application of 10 mg/mL LBE can control the early anagen phase and extend the mature anagen phase better than minoxidil as a positive control.
Our previous study showed that ginseng berry increased DPCs by regulating the expression of Bcl-2, p38, and ERK, which was strongly correlated with promoting hair regeneration in vivo [30]. Previous studies showed that topical administration of LBE could act as a potent hair cycle regulator by promoting DPC proliferation. In this study, the G1 phase increased, and the S and G2/M phases decreased after LBE treatment for 60 h, regardless of whether LBE treatment affected the cell cycle of human hair DPC. These results indicate the potential for cell cycle arrest regulation by LBE. Thereafter, cell cycle-related gene expression regulation was confirmed through LBE, and LBE regulated the expression of p21, p38, and ERK genes involved in cell cycle arrest. Therefore, LBE is thought to increase the G1 phase and regulate DPC proliferation by regulating the expression of p38 and ERK at the mRNA and protein levels.
5. Conclusions
In this study, LBE and its active ingredient, PCA, were used to investigate the potential use of LBE as a hair growth promotion agent. Our results provide useful information on a previously unexplored aspect to demonstrate the practical importance of LBE for treating alopecia. Although this study is limited to nonclinical observations, it shows that LBE promotes hair growth by increasing the duration of the G1 phase in DPCs. Nevertheless, it is necessary to determine whether LBE can be used as a therapeutic agent through clinical trials.
Author Contributions
Conceptualization, S.P. and S.-C.K.; methodology, S.-Y.S., J.-E.K., S.K. and Y.-G.L.; validation, S.-Y.S. and Y.-G.L.; investigation, S.-Y.S., J.-E.K., S.K. and Y.-G.L.; data curation, S.P. and S.-C.K.; writing—original draft preparation, S.-Y.S., J.-E.K.; writing—review and editing, J.-E.K., Y.-G.L., S.P. and S.-C.K.; supervision, S.P. and S.-C.K.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Semyung University Research Grant (20180501).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Semyung University’s current Code of Ethics for Animal Care and Use (SMECAE 091101).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tredget, E.E.; Wu, Y. Dynamic signals for hair follicle development and regeneration. Stem Cells Dev. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Myung, P.; Ito, M. Dissecting the bulge in hair regeneration. J. Clin. Investig. 2012, 122, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nuaimi, Y.; Baier, G.; Watson, R.E.; Chuong, C.M.; Paus, R. The cycling hair follicle as an ideal systems biology research model. Exp. Dermatol. 2010, 19, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, K.; Stephenson, T.J.; Messenger, A.G. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.A.; Sinclair, R.; Harrap, S.B. Androgenetic alopecia: Pathogenesis and potential for therapy. Expert Rev. Mol. Med. 2002, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, R.; Patel, M.; Dawson, T.L., Jr.; Yazdabadi, A.; Yip, L.; Perez, A.; Rufaut, N.W. Hair loss in women: Medical and cosmetic approaches to increase scalp hair fullness. Br. J. Dermatol. 2011, 165 (Suppl. 3), 12–18. [Google Scholar] [CrossRef]
- Arca, E.; Acikgoz, G.; Tastan, H.B.; Kose, O.; Kurumlu, Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology 2004, 209, 117–125. [Google Scholar] [CrossRef]
- McClellan, K.J.; Markham, A. Finasteride: A review of its use in male pattern hair loss. Drugs 1999, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Trueb, R.M.; Itin, P.; Itin und Schweizerische Arbeitsgruppe für Trichologie. Photographic documentation of the effectiveness of 1 mg. oral finasteride in treatment of androgenic alopecia in the man in routine general practice in Switzerland. Praxis 2001, 90, 2087–2093. [Google Scholar] [PubMed]
- Paus, R.; Haslam, I.S.; Sharov, A.A.; Botchkarev, V.A. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013, 14, e50–e59. [Google Scholar] [CrossRef]
- Lee, S.J.; Hossaine, M.D.; Park, S.C. A potential anti-inflammation activity and depigmentation effect of Lespedeza bicolor extract and its fractions. Saudi J. Biol. Sci. 2016, 23, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Maximov, O.B.; Kulesh, N.I.; Stepanenko, L.S.; Dmitrenok, P.S. New prenylated isoflavanones and other constituents of Lespedeza bicolor. Fitoterapia 2004, 75, 96–98. [Google Scholar] [CrossRef]
- Woo, H.S.; Kim, D.W.; Curtis-Long, M.J.; Lee, B.W.; Lee, J.H.; Kim, J.Y.; Kang, J.E.; Park, K.H. Potent inhibition of bacterial neuraminidase activity by pterocarpans isolated from the roots of Lespedeza bicolor. Bioorg. Med. Chem. Lett. 2011, 21, 6100–6103. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, J.H.; Wahedi, H.M.; Pak, C.; Lee, C.H.; Yeo, E.J.; Lim, Y.; Ha, S.K.; Choi, I.; Kim, S.Y. Lespedeza bicolor ameliorates endothelial dysfunction induced by methylglyoxal glucotoxicity. Phytomedicine 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Ko, Y.H.; Shim, K.Y.; Kim, S.K.; Seo, J.Y.; Lee, B.R.; Hur, K.H.; Kim, Y.J.; Kim, S.E.; Do, M.H.; Parveen, A.; et al. Lespedeza bicolor Extract Improves Amyloid Beta25—35-Induced Memory Impairments by Upregulating BDNF and Activating Akt, ERK, and CREB Signaling in Mice. Planta Med. 2019, 85, 1363–1373. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, H.; Kim, S.Y.; Lim, Y. Lespedeza bicolor Extract Ameliorated Renal Inflammation by Regulation of NLRP3 Inflammasome-Associated Hyperinflammation in Type 2 Diabetic Mice. Antioxidants 2020, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Tan, J.; Zou, B.; Liu, X.; Yu, Y. Exploring the potential effect and mechanisms of protocatechuic acid on human hair follicle melanocytes. Acta Pharm. 2020, 70, 539–549. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Alvarez-Fernandez, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.A.; Garcia-Parrilla, M.A.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-beta and alpha-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.B. Growth of the hair. Physiol. Rev. 1954, 34, 113–126. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Attia, S.; Saleh, F.; Bassyouni, M.I.; El-Fakahany, H.; Abdel-Wahab, H. Proliferation, DNA repair and apoptosis in androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Boyera, N.; Galey, I.; Bernard, B.A. Biphasic effects of minoxidil on the proliferation and differentiation of normal human keratinocytes. Skin Pharmacol. 1997, 10, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Arch. Pharm. Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, S.; Handjiski, B.; Peters, E.M.; Paus, R. A guide to assessing damage response pathways of the hair follicle: Lessons from cyclophosphamide-induced alopecia in mice. J. Investig. Dermatol. 2005, 125, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Shin, W.S.; Ho, J. Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice. J. Ethnopharmacol. 2011, 138, 340–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).