Damage Characteristics of Thermally Deteriorated Carbonate Rocks: A Review
Abstract
1. Introduction
2. Thermal Dilatancy and Alteration in Rock Fabric
2.1. Thermal Decomposition of Calcite and Dolomite
2.2. Reasons behind the Thermal Expansion
2.3. Thermal Cracking and Microstructural Variations
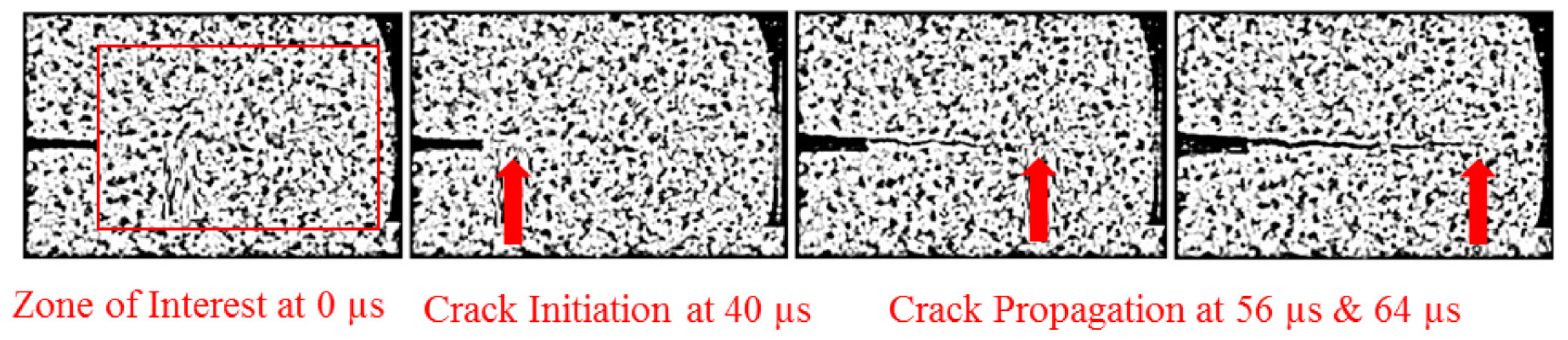
3. Crack Initiation and Propagation
Brittle–Ductile Transition
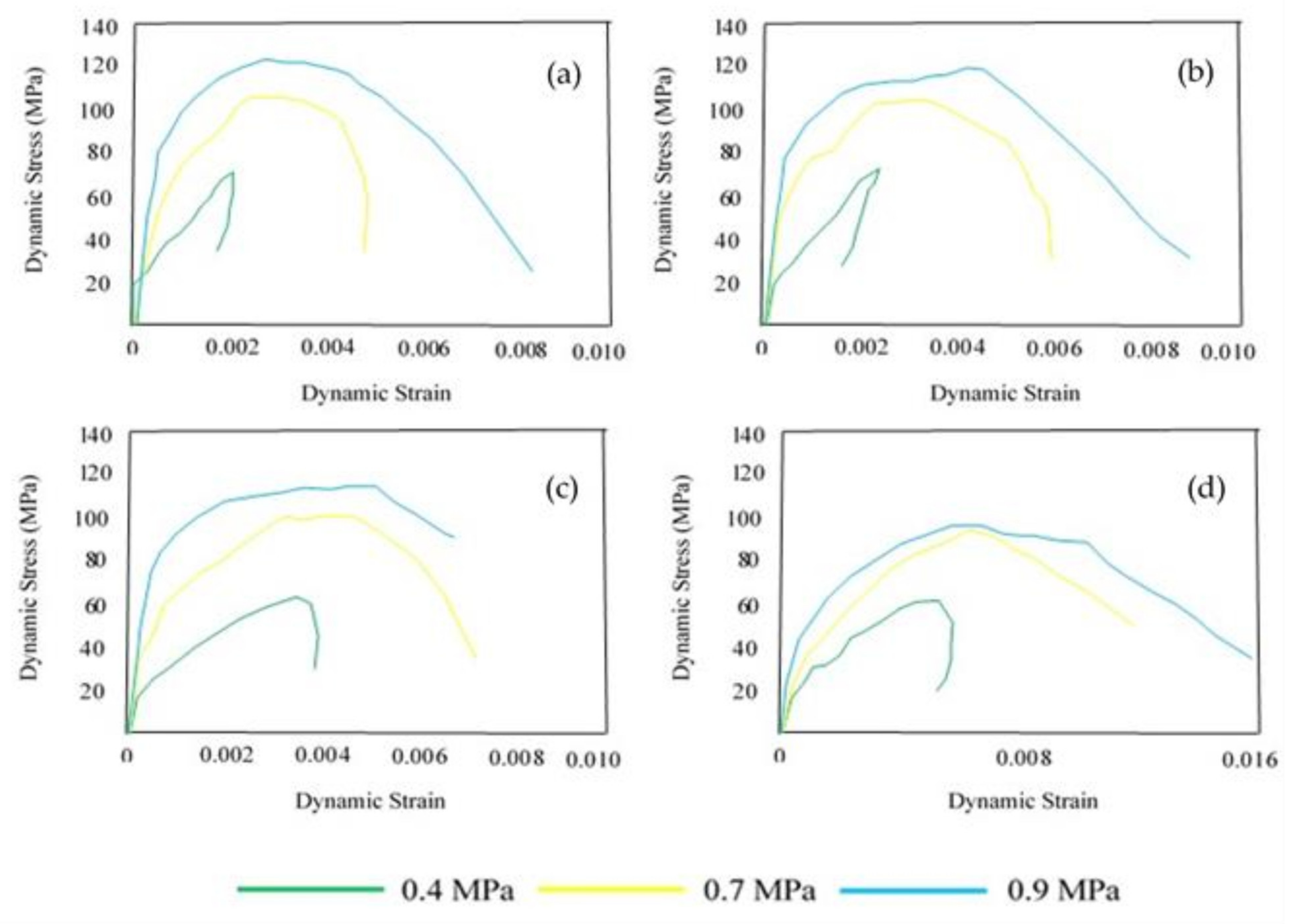
4. Dynamic Fracture Toughness and Failure Modes
5. Thermal Deterioration of Porous Network
Heat Transport Properties
6. Conclusions
7. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Behnia, D.; Ahangari, K.; Moeinossadat, S.R. Modeling of shear wave velocity in limestone by soft computing methods. Int. J. Min. Sci. Technol. 2017, 27, 423–430. [Google Scholar] [CrossRef]
- Lai, H.P.; Wang, S.Y.; Xie, Y.L. Experimental research on temperature field and structure performance under different lining water contents in a road tunnel fire. Tunn. Undergr. Space Technol. 2014, 43, 327–335. [Google Scholar] [CrossRef]
- Nasseri, M.H.B.; Schubnel, A.; Young, R.P. Coupled evolutions of fracture toughness and elastic wave velocities at high crack density in thermally treated Westerly granite. Int. J. Rock Mech. Min. Sci. 2007, 44, 601–616. [Google Scholar] [CrossRef]
- Heuze, F.E. High-temperature mechanical, physical, and thermal properties of granitic rocks—A review. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1983, 20, 3–10. [Google Scholar] [CrossRef]
- Smith, B.M.; Reynolds, S.J.; Day, H.W.; Bodnar, R.J. Deep-seated fluid involvement in ductile-brittle deformation and mineralization, South Mountains metamorphic core complex, Arizona. Geol. Soc. Am. Bull. 1991, 103, 559–569. [Google Scholar] [CrossRef]
- Waqas, U.; Ahmed, M.F. Prediction Modeling for the Estimation of Dynamic Elastic Young’s Modulus of Thermally Treated Sedimentary Rocks Using Linear–Nonlinear Regression Analysis, Regularization, and ANFIS. Rock Mech. Rock Eng. 2020, 53, 5411–5428. [Google Scholar] [CrossRef]
- Idris, M.A. Effects of elevated temperature on physical and mechanical properties of carbonate rocks in South-Southern Nigeria. Min. Miner. Depos. 2018, 12, 20–27. [Google Scholar] [CrossRef]
- Waqas, U.; Ahmed, M.F.; Arshad, M. Classification of the intact carbonate and silicate rocks based on their degree of thermal cracking using discriminant analysis. Bull. Eng. Geol. Environ. 2020, 79, 2607–2619. [Google Scholar] [CrossRef]
- Yang, J.; Fu, L.Y.; Zhang, W.; Wang, Z. Mechanical property and thermal damage factor of limestone at high temperature. Int. J. Rock Mech. Min. Sci. 2019, 117, 11–19. [Google Scholar] [CrossRef]
- Merriman, J.D.; Hofmeister, A.M.; Roy, D.J.; Whittington, A.G. Temperature-dependent thermal transport properties of carbonate minerals and rocks. Geosphere 2018, 14, 1961–1987. [Google Scholar] [CrossRef]
- Nicolas, A.; Fortin, J.; Regnet, J.B.; Dimanov, A.; Guéguen, Y. Brittle and semi-brittle behaviors of a carbonate rock: Influence of water and temperature. Geophys. J. Int. 2016, 206, 438–456. [Google Scholar] [CrossRef]
- Sengun, N. Influence of Thermal Damage on the Physical and Mechanical Properties of Carbonate Rocks. Arab. J. Geosci. 2014, 7, 5543–5551. [Google Scholar] [CrossRef]
- Yavuz, H.; Demirdag, S.; Caran, S. Thermal effect on the physical properties of carbonate rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 94–103. [Google Scholar] [CrossRef]
- Hajpál, M. Fire Damaged Stone Structures in Historical Monuments. Laboratory Analyses of Changes in Natural Stones by Heat Effect; CIB Publication: Salford, UK, 2010; pp. 164–173. [Google Scholar]
- Sury, M.; White, M.; Kirton, J.; Carr, P.; Woodbridge, R.; Mostade, M.; Chappell, R.; Hartwell, D.; Hunt, D.; Rendell, N. Review of Environmental Issues of Underground Coal Gasification–Best Practice Guide; Report No. COAl R273 DTi/Pub URN; U.S. Department of Energy: Oak Ridge, VA, USA, 2004; Volume 4, p. 1881.
- Behn, M.D.; Kelemen, P.B.; Hirth, G.; Hacker, B.R.; Massonne, H.J. Diapirs as the source of the sediment signature in arc lavas. Nat. Geosci. 2011, 4, 641–646. [Google Scholar] [CrossRef]
- Philpotts, A.R.; Ague, J.J. Principles of Igneous and Metamorphic Petrology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Deegan, F.M.; Troll, V.R.; Freda, C.; Misiti, V.; Chadwick, J.P.; McLeod, C.L.; Davidson, J.P. Magma–carbonate interaction processes and associated CO2 release at Merapi Volcano, Indonesia: Insights from experimental petrology. J. Petrol. 2010, 51, 1027–1051. [Google Scholar] [CrossRef]
- Kılıc, Ö. The influence of high temperatures on limestone P-wave velocity and Schmidt hammer strength. Int. J. Rock Mech. Min. Sci. 2006, 43, 980–986. [Google Scholar] [CrossRef]
- González-Gómez, W.S.; Quintana, P.; May-Pat, A.; Avilés, F.; May-Crespo, J.; Alvarado-Gil, J.J. Thermal effects on the physical properties of limestones from the Yucatan Peninsula. Int. J. Rock Mech. Min. Sci. 2015, 75, 182–189. [Google Scholar] [CrossRef]
- Lippmann, F. Sedimentary Carbonate Minerals; Springer Science & Business Media: Berlin, Germany, 1973. [Google Scholar]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Asaki, Z.; Fukunaka, Y.; Nagase, T.; Kondo, Y. Thermal decomposition of limestone in a fluidized bed. Metall. Trans. 1974, 5, 381–390. [Google Scholar] [CrossRef]
- Sanders, J.P.; Gallagher, P.K. Kinetic analyses using simultaneous TG/DSC measurements: Part II: Decomposition of calcium carbonate having different particle sizes. J. Therm. Anal. Calorim. 2005, 82, 659–664. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Karunadasa, K.S.; Manoratne, C.H.; Pitawala, H.M.; Rajapakse, R.M. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Hartman, M.; Trnka, O.; Vesely, V.; Svoboda, K. Predicting the rate of thermal decomposition of dolomite. Chem. Eng. Sci. 1996, 51, 5229–5232. [Google Scholar] [CrossRef]
- Barcina, L.M.; Espina, A.; Suárez, M.; Garcia, J.R.; Rodriguez, J. Characterization of monumental carbonate stones by thermal analysis (TG, DTG, and DSC). Ther. Acta 1997, 290, 181–189. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar] [CrossRef]
- Kök, M.U.; Smykatz-Kloss, W. Characterization, correlation, and kinetics of dolomite samples as outlined by thermal methods. J. Therm. Anal. Calorim. 2008, 91, 565–568. [Google Scholar] [CrossRef]
- Hossain, F.M.; Długogorski, B.Z.; Kennedy, E.M.; Belova, I.V.; Murch, G.E. First-principles study of the electronic, optical, and bonding properties in dolomite. Comput Mater Sci. 2011, 50, 1037–1042. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, J.; Zhang, G.; Gong, X.; Nie, L.; Liu, J. Polymorph and morphology control of CaCO3 via temperature and PEG during the decomposition of Ca(HCO3)2. J. Am. Ceram. Soc. 2012, 95, 3735–3738. [Google Scholar] [CrossRef]
- Samtani, M.; Dollimore, D.; Wiburn, F.W.; Alexander, K. Isolation and identification of the intermediate and final products in the thermal decomposition of dolomite in an atmosphere of carbon dioxide. Ther. Acta 2001, 367, 285–295. [Google Scholar] [CrossRef]
- Gallagher, P.; Johnson, D.W., Jr. The effects of sample size and heating rate on the kinetics of the thermal decomposition of CaCO3. Thermochim. Acta 1973, 6, 67–83. [Google Scholar] [CrossRef]
- Caldwell, K.M.; Gallagher, P.K.; Johnson, D.W., Jr. Effect of thermal transport mechanisms on the thermal decomposition of CaCO3. Thermochim. Acta 1977, 18, 15–19. [Google Scholar] [CrossRef]
- Maciejewski, M.; Oswald, H.R. Morphological observations on the thermal decomposition of calcium carbonate. Thermochim. Acta 1985, 85, 39–42. [Google Scholar] [CrossRef]
- Engler, P.; Santana, M.W.; Mittleman, M.L.; Balazs, D. Non-isothermal, in situ XRD analysis of dolomite decomposition. Thermochim. Acta 1989, 140, 67–76. [Google Scholar] [CrossRef]
- Rao, T.R. Kinetic parameters for the decomposition of calcium carbonate. Can. J. Chem. Eng. 1993, 71, 481–484. [Google Scholar]
- Wang, Y.; Thomson, W.J. The effects of steam and carbon dioxide on calcite decomposition using dynamic X-ray diffraction. Chem. Eng. Sci. 1995, 50, 1373–1382. [Google Scholar] [CrossRef]
- Rao, T.R. Kinetics of calcium carbonate decomposition. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 1996, 19, 373–377. [Google Scholar] [CrossRef]
- Dollimore, D.; Tong, P.; Alexander, K.S. The kinetic interpretation of the decomposition of calcium carbonate by use of relationships other than the Arrhenius equation. Thermochim. Acta 1996, 282, 13–27. [Google Scholar] [CrossRef]
- Kristóf-Makó, É.; Juhász, A.Z. The effect of mechanical treatment on the crystal structure and thermal decomposition of dolomite. Thermochim. Acta 1999, 342, 105–114. [Google Scholar] [CrossRef]
- Dash, S.; Kamruddin, M.; Ajikumar, P.K.; Tyagi, A.K.; Raj, B. Nanocrystalline and metastable phase formation in vacuum thermal decomposition of calcium carbonate. Thermochim. Acta 2000, 363, 129–135. [Google Scholar] [CrossRef]
- Zucchini, A.; Comodi, P.; Nazzareni, S.; Hanfland, M. The effect of cation ordering and temperature on the high-pressure behaviour of dolomite. Phys. Chem. Miner. 2014, 41, 783–793. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Kuang, C. A modified random pore model for carbonation reaction of CaO-based limestone with CO2 in different calcination-carbonation cycles. Energy Procedia 2017, 105, 1924–1931. [Google Scholar] [CrossRef]
- Subagjo; Wulandari, W.; Adinata, P.M.; Fajrin, A. Thermal decomposition of dolomite under CO2-air atmosphere. AIP Conf. Proc. 2017, 1805, 040006. [Google Scholar]
- Momenzadeh, L.; Moghtaderi, B.; Buzzi, O.; Liu, X.; Sloan, S.W.; Murch, G.E. The thermal conductivity decomposition of calcite calculated by molecular dynamics simulation. Comput. Mater. Sci. 2018, 141, 170–179. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, J.; Tao, L.; Li, Z.; Wang, Q. A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate. Crystals 2020, 10, 849. [Google Scholar] [CrossRef]
- Reeder, R.J. Crystal chemistry of the rhombohedral carbonates. Rev. Mineralogy. 1983, 11, 1–47. [Google Scholar]
- Bater, G. Thermal expansion anisotropy of dolomite-type borates Me2 + Me4 + B2O6. Z. Krist. Cryst. Mater. 1971, 133, 85–90. [Google Scholar] [CrossRef]
- Barber, D.J.; Heard, H.C.; Wenk, H.R. Deformation of dolomite single crystals from 20–800 °C. Phys. Chem. Miner. 1981, 7, 271–286. [Google Scholar] [CrossRef]
- Reeder, R.J.; Markgraf, S.A. High-temperature crystal chemistry of dolomite. Am. Mineral. 1986, 71, 795–804. [Google Scholar]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Markgraf, S.A.; Reeder, R.J. High-temperature structure refinements of calcite and magnesite. Am. Mineral. 1985, 70, 590–600. [Google Scholar]
- Hazen, R.M.; Prewitt, C.T. Effects of temperature and pressure on interatomic distances in oxygen-based minerals. Am. Mineral. 1977, 62, 309–315. [Google Scholar]
- Hazen, R.M.; Finger, L.W. Comparative Crystal Chemistry; John Wiley: New York, NY, USA, 1982. [Google Scholar]
- Sygała, A.; Bukowska, M.; Janoszek, T. High temperature versus geomechanical parameters of selected rocks–the present state of research. J. Sustain. Min. 2013, 12, 45–51. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Swift, G.; Chen, J. Laboratory investigation of the effects of temperature on the mechanical properties of sandstone. Geotech. Geol. Eng. 2013, 31, 809–816. [Google Scholar] [CrossRef]
- Coppola, M.; Correale, A.; Barberio, M.D.; Billi, A.; Cavallo, A.; Fondriest, M.; Nazzari, M.; Paonita, A.; Romano, C.; Stagno, V.; et al. Meso-to nano-scale evidence of fluid-assisted co-seismic slip along the normal Mt. Morrone Fault, Italy: Implications for earthquake hydrogeochemical precursors. Earth Planet. Sci. Lett. 2021, 568, 117010. [Google Scholar] [CrossRef]
- Zhi-jun, W.; Yang-Sheng, Z.; Yuan, Z.; Chong, W. Research status quo and prospection of mechanical characteristics of rock under high temperature and high pressure. Procedia Earth Planet. Sci. 2009, 1, 565–570. [Google Scholar]
- Małkowski, P.; Skrzypkowski, K.; Bożęcki, P. Zmiany zachowania się skał pod wpływem wysokich temperatur w rejonie georeaktora. Pr. Nauk. GIG Górnictwo Środowisko 2011, 4, 259–272. [Google Scholar]
- Małkowski, P.; Kamiński, P.; Skrzypkowski, K. Impact of Heating of carboniferous rocks on their mechanical parameters. AGH J. Min. Geoengin. 2012, 36, 231–242. [Google Scholar]
- Ranjith, P.G.; Viete, D.R.; Chen, B.J.; Perera, M.S.A. Transformation plasticity and the effect of temperature on the mechanical behavior of Hawkesbury sandstone at atmospheric pressure. Eng. Geol. 2012, 151, 120–127. [Google Scholar]
- Chen, L.J.; Jun, H.E.; Chao, J.Q.; Qin, B.D. Swelling and breaking characteristics of limestone under high temperatures. Min. Sci. Technol. 2009, 19, 503–507. [Google Scholar] [CrossRef]
- Sivrikaya, O. A study on the physicochemical and thermal characterization of dolomite and limestone samples for use in ironmaking and steelmaking. Ironmak. Steelmak. 2018, 45, 764–772. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q. Identification of primary mineral elements and macroscopic parameters in thermal damage process of limestone with canonical correlation analysis. Rock Mech. Rock Eng. 2018, 51, 1287–1292. [Google Scholar] [CrossRef]
- Meng, Q.B.; Wang, C.K.; Liu, J.F.; Zhang, M.W.; Lu, M.M.; Wu, Y. Physical and microstructural characteristics of limestone after high-temperature exposure. Bull. Eng. Geol. Environ. 2020, 79, 1259–1274. [Google Scholar] [CrossRef]
- Paterson, M.S.; Wong, T.F. Experimental Rock Deformation—The Brittle Field; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Hoek, E.; Martin, C.D. Fracture initiation and propagation in intact rock—A review. J. Rock Mech. Geotech. Eng. 2014, 6, 287–300. [Google Scholar] [CrossRef]
- Fredrich, J.T.; Evans, B.; Wong, T.F. Effect of grain size on brittle and semi brittle strength: Implications for micromechanical modeling of failure in compression. J. Geophys. Res. Solid Earth 1990, 95, 10907–10920. [Google Scholar] [CrossRef]
- Hoagland, R.G.; Hahn, G.T.; Rosenfield, A.R. Influence of microstructure on fracture propagation in rock. Rock Mech. 1973, 5, 77–106. [Google Scholar] [CrossRef]
- Holm, P.E. Effects of temperature and strain rate on the experimental deformation of an early-cemented Jurassic Limestone. J. Geol. 1983, 91, 714–719. [Google Scholar] [CrossRef]
- Vajdova, V.; Zhu, W.; Chen, T.M.N.; Wong, T.F. Micromechanics of brittle faulting and cataclastic flow in Tavel limestone. J. Struct. Geol. 2010, 32, 1158–1169. [Google Scholar] [CrossRef]
- De Bresser, J.H.P.; Spiers, C.J. Strength characteristics of the r, f, and c slip systems in calcite. Tectonophysics 1997, 272, 1–23. [Google Scholar] [CrossRef]
- Schmid, S.M.; Boland, J.N.; Paterson, M.S. Superplastic flow in fine-grained limestone. Tectonophysics 1977, 43, 257–291. [Google Scholar] [CrossRef]
- Li, W.; An, X.; Li, H. Limestone mechanical deformation behavior and failure mechanisms: A review. Acta Geochim. 2018, 37, 153–170. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Jin, Z.M. Superplastic deformation of roks and its dynamic implication. Geol. Sci. Technol. Inf. 2003, 22, 17–23. [Google Scholar]
- Myer, L.R.; Kemeny, J.M.; Zheng, Z.; Suarez, R.; Ewy, R.T.; Cook, N.G.W. Extensile cracking in the porous rock under differential compressive stress. Appl. Mech. Rev. 1992, 45, 263–280. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, P. Rock Mechanics; Central South University Press: Chang Sha, China, 2013; pp. 15–17. [Google Scholar]
- Mao, X.B.; Zhang, L.Y.; Li, T.Z.; Liu, H.S. Properties of failure mode and thermal damage for limestone at high temperature. Min. Sci. Technol. 2009, 19, 290–294. [Google Scholar] [CrossRef]
- Castagna, A.; Ougier-Simonin, A.; Benson, P.M.; Browning, J.; Walker, R.J.; Fazio, M.; Vinciguerra, S. Thermal damage and pore pressure effects of the Brittle-Ductile transition in comiso limestone. J. Geophys. Res. Solid Earth 2018, 123, 7644–7660. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, X.; Lu, A. Experimental study on the mechanical properties of rocks at high temperature. Sci. China Ser. E Technol. Sci. 2009, 52, 641–646. [Google Scholar] [CrossRef]
- Brotons, V.; Tomás, R.; Ivorra, S.; Alarcón, J.C. Temperature influence on the physical and mechanical properties of a porous rock: San Julian’s calcarenite. Eng. Geol. 2013, 167, 117–127. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Hao, S.; Wang, B. Experimental study on the thermal damage characteristics of limestone and the underlying mechanism. Rock Mech. Rock Eng. 2016, 49, 2999–3008. [Google Scholar] [CrossRef]
- Kramer, S.L. Geotechnical Earthquake Engineering; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Zhang, Q. Mechanical Behavior of Rock Materials under Dynamic Loading (No. THESIS); EPFL: Lausanne, Switzerland, 2014. [Google Scholar]
- Waqas, U.; Ahmed, M.F.; Rogers, J.D. Effect of loading frequencies on the dynamic properties of thermally treated rock samples. In 52nd US Rock Mechanics/Geomechanics Symposium; American Rock Mechanics Association: Alexandria, VA, USA, 2018. [Google Scholar]
- Johansson, E.; Rautakorpi, J. Rock Mechanics Stability at Olkiluoto, Hästholmen, Kivetty and Romuvaara (No. POSIVA--00-02); Posiva Oy: Eurajoki, Finland, 2000. [Google Scholar]
- Ping, Q.; Zhang, C.; Su, H.; Zhang, H. Experimental Study on Dynamic Mechanical Properties and Energy Evolution Characteristics of Limestone Specimens Subjected to High Temperature. Adv. Civ. Eng. 2020, 2020, 8875568. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Waqas, U.; Arshad, M.; Rogers, J.D. Effect of heat treatment on dynamic properties of selected rock types taken from the Salt Range in Pakistan. Arab. J. Geosci. 2018, 11, 728. [Google Scholar] [CrossRef]
- Yu, L.; Su, H.; Liu, R.; Jing, H.; Li, G.; Li, M. Effect of Thermal Treatment on the Dynamic Behaviors at a Fixed Loading Rate of Limestone in Quasi-vacuum and Air-filled Environments. Lat. Am. J. Solids Struct. 2018, 15, 15. [Google Scholar] [CrossRef]
- Crosby, Z.K. Effects of Thermally Induced Microcracking on the Quasi-Static and Dynamic Response of Salem Limestone (No. ERDC/GSL TR-17-15); US Army Engineer Research and Development Center, Geotechnical and Structures Laboratory Vicksburg United States: Vicksburg, MS, USA, 2017. [Google Scholar]
- Zou, F.; Fang, Z.F.; Xia, M.Y. Study on Dynamic Mechanical Properties of Limestone under Uniaxial Impact Compressive Loads. Math. Probl. Eng. 2016, 2016, 5207457. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xia, K.W.; Li, X.B.; Li, H.B.; Ma, G.W.; Zhao, J.; Zhou, Z.L.; Dai, F. Suggested methods for determining the dynamic strength parameters and mode-I fracture toughness of rock materials. In The ISRM Suggested Methods for Rock Characterization, Testing, and Monitoring: 2007–2014; Springer: Cham, Switzerland, 2011; pp. 35–44. [Google Scholar]
- Yuan, F.; Prakash, V. Use of a modified torsional Kolsky bar to study frictional slip resistance in rock-analog materials at coseismic slip rates. Int. J. Solids Struct. 2008, 45, 4247–4263. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.Z.; Li, W.; Song, X.L. A method for testing dynamic tensile strength and elastic modulus of rock materials using SHPB. Pure Appl. Geophys. 2006, 163, 1091–1100. [Google Scholar] [CrossRef]
- Zhao, H.; Gary, G. On the use of SHPB techniques to determine the dynamic behavior of materials in the range of small strains. Int. J. Solids Struct. 1996, 33, 3363–3375. [Google Scholar] [CrossRef]
- Lindholm, U.S.; Yeakley, L.M.; Nagy, A. The dynamic strength and fracture properties of dresser basalt. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1974, 11, 181–191. [Google Scholar] [CrossRef]
- Kumar, A. The effect of stress rate and temperature on the strength of basalt and granite. Geophysics 1968, 33, 501–510. [Google Scholar] [CrossRef]
- Kolesnikov, Y.I. Dispersion effect of velocities on the evaluation of material elasticity. J. Min. Sci. 2009, 45, 347. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhao, J. A review of dynamic experimental techniques and mechanical behavior of rock materials. Rock Mech. Rock Eng. 2014, 47, 1411–1478. [Google Scholar] [CrossRef]
- Fjær, E. Relations between static and dynamic moduli of sedimentary rocks. Geophys. Prospect. 2019, 67, 128–139. [Google Scholar] [CrossRef]
- Regnet, J.B.; David, C.; Robion, P.; Menéndez, B. Microstructures and physical properties in carbonate rocks: A comprehensive review. Mar. Pet. Geol. 2019, 103, 366–376. [Google Scholar] [CrossRef]
- Lucia, F.J. Carbonate Reservoir Characterization: An Integrated Approach; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bisai, R.; Palaniappan, S.K.; Pal, S.K. Effects of high-temperature heating and cryogenic quenching on the physicomechanical properties of limestone. SN Appl. Sci. 2020, 2, 158. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Tian, W.; Wu, X. Investigating and predicting permeability variation in thermally cracked dry rocks. Int. J. Rock Mech. Min. Sci. 2018, 103, 77–88. [Google Scholar] [CrossRef]
- Trippetta, F.; Carpenter, B.M.; Mollo, S.; Scuderi, M.M.; Scarlato, P.; Collettini, C. Physical and transport property variations within carbonate-bearing fault zones: Insights from the Monte Maggio Fault (central Italy). Geochem. Geophys. Geosyst. 2017, 18, 4027–4042. [Google Scholar] [CrossRef]
- Berryman, J.G.; Wang, H.F. Elastic wave propagation and attenuation in a double-porosity dual-permeability medium. Int. J. Rock Mech. Min. Sci. 2000, 37, 63–78. [Google Scholar] [CrossRef]
- Dvorkin, J.; Nur, A.; Yin, H. Effective properties of cemented granular materials. Mech. Mater. 1994, 18, 351–366. [Google Scholar] [CrossRef]
- Walton, K. The effective elastic moduli of a random packing of spheres. J. Mech. Phys. Solids 1987, 35, 213–226. [Google Scholar] [CrossRef]
- Berryman, J.G. Long-wavelength propagation in composite elastic media II. Ellipsoidal inclusions. J. Acoust. Soc. Am. 1980, 68, 1820–1831. [Google Scholar] [CrossRef]
- Bagrintseva, K.I. Conditions of Generation and Properties of Carbonate Reservoirs of Oil and Gas; RGGU: Moscow, Russia, 1999. (In Russian) [Google Scholar]
- Zinszner, B.; Pellerin, F.M. A Geoscientist’s Guide to Petrophysics; Editions Technip: Paris, France, 2007; p. 384. [Google Scholar]
- Mukerji, T.; Dvorkin, J. The Rock Physics Handbook, 2nd ed.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Homand-Etienne, F.; Troalen, J.P. Behaviour of granites and limestones subjected to slow and homogeneous temperature changes. Eng. Geol. 1984, 20, 219–233. [Google Scholar] [CrossRef]
- Lion, M.; Skoczylas, F.; Ledésert, B. Effects of heating on the hydraulic and poroelastic properties of Bourgogne limestone. Int. J. Rock Mech. Min. Sci. 2005, 42, 508–520. [Google Scholar] [CrossRef]
- Heap, M.J.; Mollo, S.; Vinciguerra, S.; Lavallée, Y.; Hess, K.U.; Dingwell, D.B.; Baud, P.; Iezzi, G. Thermal weakening of the carbonate basement under Mt. Etna volcano (Italy): Implications for volcano instability. J. Volcanol. Geotherm. Res. 2013, 250, 42–60. [Google Scholar] [CrossRef]
- Li, F.B.; Sheng, J.C.; Zhan, M.L.; Xu, L.M.; Qiang Wu Jia, C.L. Evolution of limestone fracture permeability under coupled thermal, hydrological, mechanical, and chemical conditions. J. Hydrodyn. Ser. B 2014, 26, 234–241. [Google Scholar] [CrossRef]
- Min, S.H.; Jianfeng, S.H.; Anjiang, S.H.; Liyin, P.A.; Anping, H.U.; Yuanyuan, H.U. Experimental simulation of dissolution law and porosity evolution of carbonate rock. Pet. Explor. Dev. 2016, 43, 616–625. [Google Scholar]
- Timoshenko, S.P.; Goodier, J.N. Two-dimensional problems in polar coordinates. In Theory of Elasticity, 3rd ed.; McGraw-Hill: New York, NY, USA, 1970; pp. 65–149. [Google Scholar]
- Çanakci, H.; Demirboğa, R.; Karakoç, M.B.; Şirin, O. Thermal conductivity of limestone from Gaziantep (Turkey). Build. Environ. 2007, 42, 1777–1782. [Google Scholar] [CrossRef]
- Demirboǧa, R. Influence of mineral admixtures on thermal conductivity and compressive strength of mortar. Energy Build. 2003, 35, 189–192. [Google Scholar] [CrossRef]
- Robertson, E.C. Thermal Properties of Rocks. 1988. Available online: https://pubs.usgs.gov/of/1988/0441/report.pdf (accessed on 1 March 2022).
- Lo, K.Y.; Wai, R.S.C. Thermal expansion, diffusivity, and cracking of rock cores from Darlington, Ontario. Can. Geotech. J. 1982, 19, 154–166. [Google Scholar] [CrossRef]
- Hanley, E.J.; Dewitt, D.P.; Roy, R.F. The thermal diffusivity of eight well-characterized rocks for the temperature range 300–1000 K. Eng. Geol. 1978, 12, 31–47. [Google Scholar] [CrossRef]
- Freeman, D.C.; Sawdye, J.A.; Mumpton, F.A. The mechanism of thermal spalling in rocks. Colo. Sch. Mines Q. 1963, 58, 225–252. [Google Scholar]
- Clark, S.P. (Ed.) Handbook of Physical Constants; Memoir-Geological Society of America: New York, NY, USA, 1966; Volume 97. [Google Scholar]
- Wong, T.F.; Brace, W.F. Thermal expansion of rocks: Some measurements at high pressure. Tectonophysics 1979, 57, 95–117. [Google Scholar] [CrossRef]
- Pertermann, M.; Whittington, A.G.; Hofmeister, A.M.; Spera, F.J.; Zayak, J. Transport properties of low-sanidine single-crystals, glasses and melts at high temperature. Contrib. Mineral. Petrol. 2008, 155, 689–702. [Google Scholar] [CrossRef]
- Hofmeister, A.M. Thermal diffusivity of garnets at high temperature. Phys. Chem. Miner. 2006, 33, 45–62. [Google Scholar] [CrossRef]
- Popov, Y.A.; Pribnow, D.F.; Sass, J.H.; Williams, C.F.; Burkhardt, H. Characterization of rock thermal conductivity by high-resolution optical scanning. Geothermics 1999, 28, 253–276. [Google Scholar] [CrossRef]
- Degiovanni, A.; Andre, S.; Maillet, D. Phonic conductivity measurement of a semi-transparent material. Therm. Conduct. 1993, 22, 623. [Google Scholar]
- Fei, Y. Thermal Expansion, Mineral Physics and Crystallography: A Handbook of Physical Constants; AUG: Washington, DC, USA, 1995; Volume 2, pp. 29–44. [Google Scholar]
- Shen, Y.; Yang, Y.; Yang, G.; Hou, X.; Ye, W.; You, Z.; Xi, J. Damage characteristics and thermo-physical properties changes of limestone and sandstone during thermal treatment from −30° C to 1000° C. Heat Mass Transf. 2018, 54, 3389–3407. [Google Scholar] [CrossRef]
- Miao, S.; Zhou, Y. Temperature dependence of thermal diffusivity and conductivity for sandstone and carbonate rocks. J. Therm. Anal. Calorim. 2018, 131, 1647–1652. [Google Scholar] [CrossRef]
- Khan, L.A.; Maqsood, A. Prediction of effective thermal conductivity of porous consolidated media as a function of temperature: A test example of limestones. J. Phys. D Appl. Phys. 2007, 40, 4953. [Google Scholar]
- Vosteen, H.D.; Schellschmidt, R. Influence of temperature on thermal conductivity, thermal capacity, and thermal diffusivity for different types of rock. Phys. Chem. Earth Parts A/B/C 2003, 28, 499–509. [Google Scholar] [CrossRef]
- Mirkovich, V.V. Experimental study relating thermal conductivity to the thermal piercing of rocks. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1968, 5, 205–218. [Google Scholar] [CrossRef]
- Birch, A.F.; Clark, H. The thermal conductivity of rocks and its dependence upon temperature and composition. Am. J. Sci. 1940, 238, 529–558. [Google Scholar] [CrossRef]
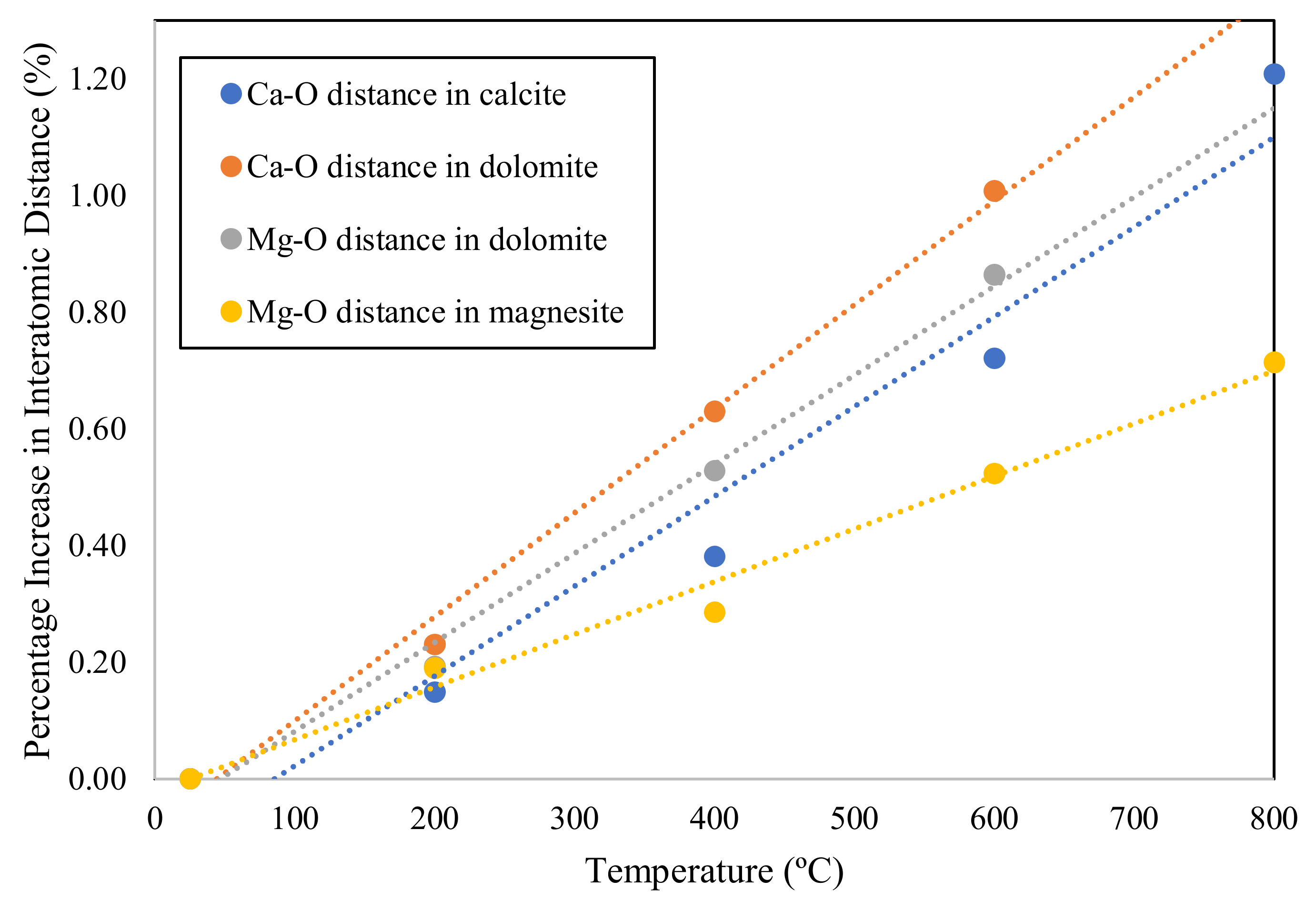
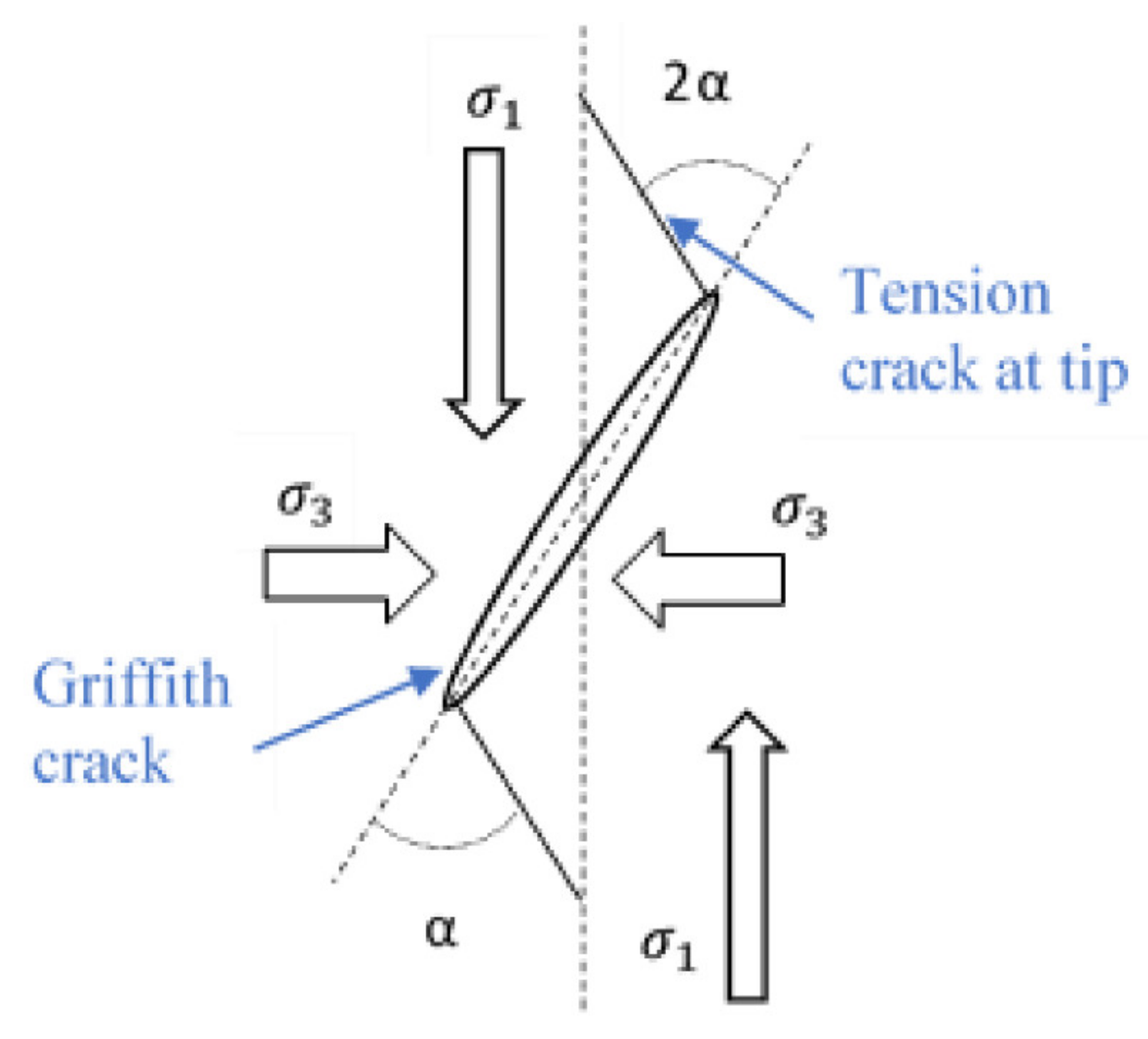

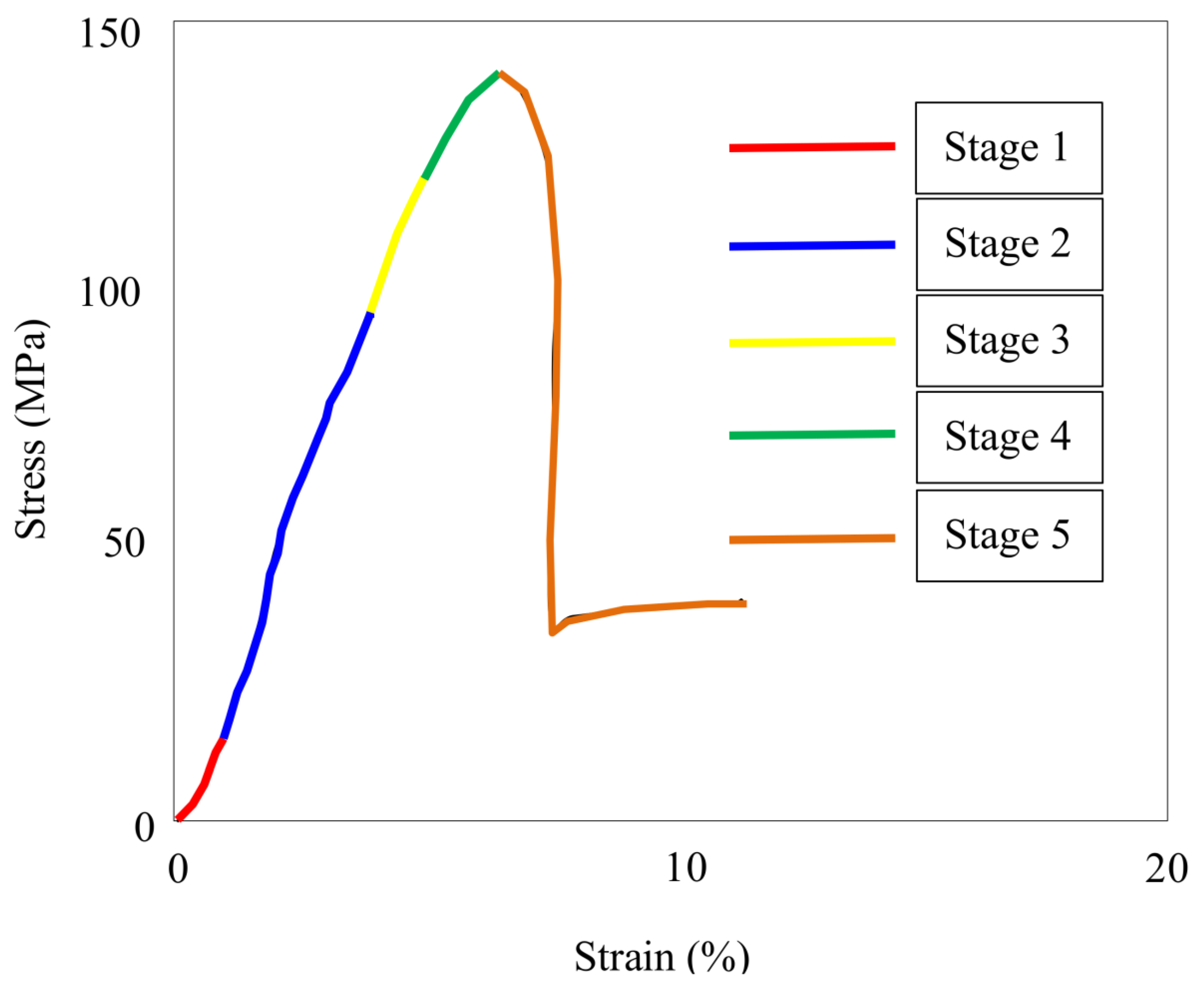

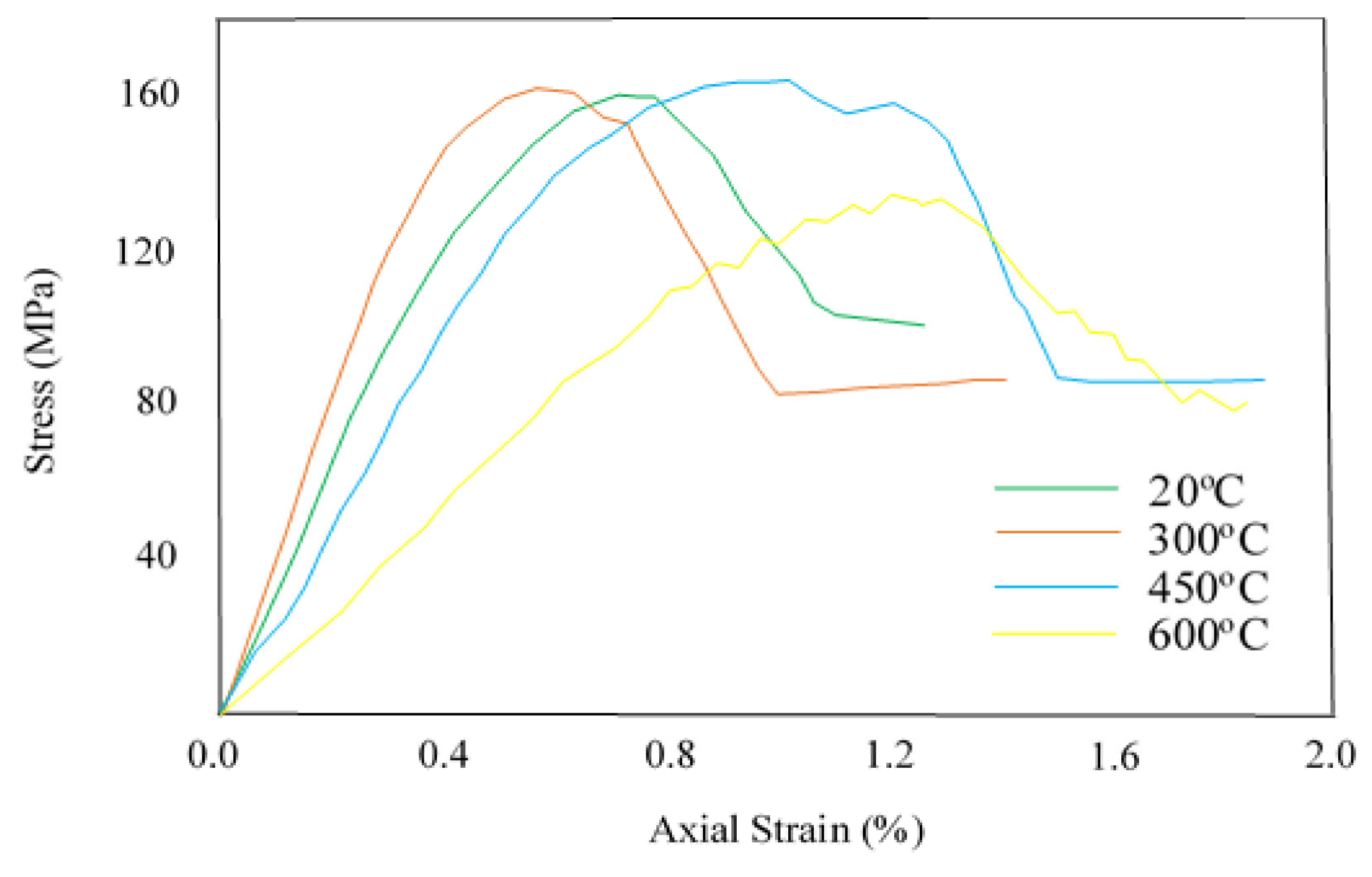

| Mineral Type | Major Developments | Reference |
|---|---|---|
| Calcite | The rate of mass loss was studied using isothermal and dynamic methods. | [34] |
| Calcite | A comparative study was conducted using isothermal–dynamic techniques and thermogravimetric analysis. | [35] |
| Calcite | Morphological variations were studied in polycrystalline CaCO3 under temperature and pressure. | [36] |
| Dolomite | Thermal decomposition and weight loss analysis was carried out under non-isothermal conditions using in situ X-ray diffraction and thermogravimetry. | [37] |
| Calcite | Reaction rate constants were determined based on the grain model using thin slab-type pellets. | [38] |
| Calcite | Thermal decomposition was analyzed using dynamic X-ray diffraction under the effect of steam and CO2. | [39] |
| Dolomite | Prediction of rate of reaction using stoichiometric analysis and thermogravimetric analysis. | [27] |
| Calcite | Thermal decomposition was investigated using thermogravimetric analysis subjected to non-isothermal conditions. | [40] |
| Calcite | The kinetic parameters were obtained from a new method that avoids the Arrhenius equation. | [41] |
| Dolomite | Thermo-mechanical damage was examined under intensive grinding using X-ray diffraction and thermal analysis. | [42] |
| Calcite | The solid-state transformation was evaluated using thermogravimetric analysis, evolved gas analysis-mass spectrometry, and high-temperature XRD. | [43] |
| Dolomite | The thermal decomposition mechanism was explained in detail using thermogravimetry and X-ray powder diffraction. | [33] |
| Dolomite | Thermal expansion and decomposition behavior were investigated using thermogravimetric analysis, differential thermal analysis, XRD, and scanning electron microscopy. | [29] |
| Dolomite | Stoichometric ordered and disordered single crytal dolomite was studied using X-ray diffraction under high pressure and temperature conditions. | [44] |
| Dolomite | Investigation of kinetics of isothermal and non-isothermal decompositions. | [25] |
| Calcite | A simulated model was presented that effectively predicted the conversion time curve and described the calcination–carbonation cycle after performing thermogravimetric analysis. | [45] |
| Dolomite | Differential scanning calorimetry and thermogravimetric analysis based on non-isothermal calcination carried out under varying CO2–air environments. | [46] |
| Calcite | Thermo-physical decomposition was studied under equilibrium dynamic simulation. | [47] |
| Calcite | Parameters including unit cell volume alteration, thermal expansion, variations along lattice axis, and thermal strains were studied using high-temperature X-ray powder diffraction. | [26] |
| Calcite | Thermal decomposition analysis was performed to validate improved reaction kinetic equation based on the pore structure model. | [48] |
| Temperature Range | Major Findings | Reference |
|---|---|---|
| 200–800 °C | They analyzed the thermally treated limestones using scanning electron microscopy. They showed orientation of thermal tension and shear cracks developed in limestone. The cracks were straight, curved, parallel, vertical, oblique, and crossed layers. | [64] |
| 100–500 °C | They studied the monomineralic carbonate rocks subjected to various temperature ranges. They observed that, in these kinds of carbonate rocks, thermal damage was the function of anisotropic dilation of calcite and shrinkage of clay minerals. The mineral expansion was observed at a temperature range of 100–200 °C, whereas intergranular and intragranular cracking was noted at 300–500 °C. | [13] |
| 25–600 °C | They demonstrated the thermal deterioration of the limestone in terms of spectral reflectance. They found that, at an initial level of temperature, mineral expansion under elastic constraints increased the spectral reflectance, and, at a temperature above 500 °C, the thermal degradation of minerals decreased their spectral reflectance. | [20] |
| 20–1000 °C | He investigated the effect of mineral crystal structures on the thermal behavior of carbonate rocks. In the case of dolostone, he observed that larger crystals of dolomite minerals decomposed more than the dolostone containing the smaller size dolomite crystals. | [65] |
| 25–800 °C. | XRF technique was used to investigate the microstructural changes in limestones. They noticed an appreciable alteration in the percentage of the mineral content at a temperature window of 400–700 °C. | [66] |
| 20–800 °C | They studied microstructural variations in carbonate rocks and found no noticeable changes in the chemical composition at a temperature below 400 °C. Furthermore, they observed that calcite and dolostone were decomposed at 400–500 °C and clay minerals started to decay at a temperature above 500 °C. In the case of trace minerals and impurities, their concentration was decreased gradually up to 400 °C and then increased sharply above 600 °C. | [67] |
| Material Properties | Major Findings | Reference |
|---|---|---|
| Compressive Strength | At a temperature of 100–500 °C, linear changes were observed because ultimate rock strength was higher than the induced thermal strains. Above 500 °C, about 70% reduction was recorded in the peak strength, which was a result of plastic deformation. | [82] |
| Compressive Strength & Elastic Modulus | They tested the San Julian’s calcarenite at a temperature of 100–600 °C to investigate the behavior of porous carbonate rocks. At 600 °C, they found a reduction in peak strength and elastic moduli by 35% and 75%, respectively. | [83] |
| Compressive Strength & Elastic Modulus | They studied the mechanical behavior of thermally deteriorated carbonate rocks at a temperature window of 25–900 °C. They observed that, beyond the brittle–ductile transformation phase, the slippage–twining effect in crystal lattice governed the plastic deformation and significantly reduced the peak strength and elastic modulus | [84] |
| Compressive Strength & Elastic Modulus | They found a significant reduction in elastic modulus at a temperature of 600 °C. Beyond this critical temperature (i.e., 600 °C), rock strength dropped to 81%. The decomposition of carbonate at elevated temperature was considered the main reason for depreciation in mechanical characteristics. | [9] |
| Compressive & Tensile Strength | They investigated the thermal cycling effect on carbonate rocks at a temperature of 200 °C. They noticed a considerable decrement in compressive strength and tensile strength of dolostone by 27% and 25%, respectively. | [6] |
| Material Properties | Major Findings | Reference |
|---|---|---|
| Dynamic Compressive Strength | They studied the thermal effect on the dynamic behavior of carbonate rock in two different scenarios. Firstly, they tested the heated rock specimens at a temperature above 300 °C. Secondly, they tested the air-cooled thermally treated rock samples. They found very similar results in both cases and concluded that, at this temperature range, thermal deterioration did not affect the dynamic strength of rocks to a great extent. | [82] |
| Dynamic Elastic Modulus | They noticed a linear change in the dynamic elastic modulus of porous carbonate rocks with rising temperatures. They recorded decrements in limestones by 10%, 60%, and 75% at 200 °C, 400 °C, and 600 °C, respectively. | [83] |
| Dynamic Compressive Strength | They performed a uniaxial impact compressive load test on limestone samples to discern their dynamic–mechanical behavior. The test results showed that the dynamic compressive strength of limestone had an exponential rise with the strain rate under an increasing impact pressure. | [93] |
| Dynamic Compressive Strength & Dynamic Elastic Modulus | He investigated the effect of temperature on the dynamic properties of carbonate rocks. He tested limestone at undamaged, moderate damaged (heated at 450 °C), and high damaged (heated at 800 °C) conditions under an increasing strain rate. He noted that, on account of anisotropic effects, the dynamic strength of damaged samples was found greater than the strength of undamaged samples. Furthermore, he found that the elastic modulus calculated by using ultrasonic wave velocities was dropped to 92% at 800 °C. | [92] |
| Dynamic Elastic Modulus | They heated the limestone from its ambient temperature to 900 °C and found a 70% reduction in dynamic elastic modulus at 600 °C. This temperature was considered as a critical temperature after which limestone started to change its behavior from brittle to ductile. | [91] |
| Damping Ratio, Damping Capacity, & Loss Factor | They studied the cyclic effect of temperature on the dynamic properties of selected carbonate rocks. In the case of dolostone, they noticed a significant appreciation in its damping ratio, specific damping capacity, and loss factor by 15%, 13%, and 12%, respectively. | [90] |
| Dynamic Elastic Modulus | They observed that the thermal cycling effect significantly reduced the dynamic elastic modulus of dolostone by 38% at 200 °C. | [8] |
| Material Properties | Major Findings | Reference |
|---|---|---|
| Permeability | They noticed that, at a low temperature, crystalline limestone showed slight variations in its permeability. Moreover, the thermal cracking of limestone at a temperature above 400 °C produced an appreciable increment in its permeability. | [115] |
| Porosity & Permeability | They studied the temperature effect on the hydraulic and poroelastic properties of limestone. They found that the permeability of limestone was increased by 3% and 8% at the temperatures of 150 °C and 250 °C, respectively. | [116] |
| P-wave Velocity | They investigated the thermo-physical behavior of limestones at varying temperatures. They noticed slight variations in P-wave velocities at a temperature of 150 °C and a linearly decreasing trend by 55% at 500 °C. | [13] |
| P-wave Velocity | He studied thermal effects on the physical properties of carbonate rocks. At the initial temperature (i.e., 200 °C), only 4% depreciation was recorded in the P-wave velocity. However, at a temperature of 600 °C, due to the brittle–ductile transformation, its value reduced to 36%. | [12] |
| Porosity | They investigated the thermal damage effect on the porosity of air-cooled and water-cooled limestone specimens. They found that, at 200 °C, both limestone samples showed a very similar increase in porosity (i.e., 1–1.5%). At the temperature of 300 °C, air-cooled and water-cooled limestone samples showed a 3% and 6% rise in porosity, respectively. On further increase in temperature of 600 °C, water-cooled limestone samples demonstrated slight variations in their porosity, whereas air-cooled limestone samples followed a linear increasing trend in porosity by 11%. | [83] |
| Porosity | They observed no significant variations in the total porosity of carbonate rocks at ambient temperature. However, at 800 °C, ductile behavior considerably changed the porosity by 13%. | [117] |
| Permeability | They found a reduction in the permeability of pre-heated fractured carbonate rocks with a slight rise in temperature (i.e., 60 °C). The obvious reason for this behavior is that, at a low temperature, rock dilates to enhance stiffness, which ultimately reduces permeability. On the other hand, at higher temperatures, fluid transport properties improve on account of thermal cracking and brittle–ductile transformation. | [118] |
| Porosity | They evaluated the porosity of carbonate rocks under different conditions. For porous dolostone, they found that the temperature effect was larger than the pressure effect and the rate of dissolution was inversely proportional to temperature. | [119] |
| Permeability | They observed no changes in the permeability of limestone at low temperature and then recorded an increase in permeability by 2%, 10%, and 126% at the temperatures of 600 °C, 700 °C, and 800 °C, respectively. Beyond the temperature of 700 °C, limestone lost its integrity, which is why a sharp increase was noted in its permeability. | [106] |
| P-wave Velocity | They observed that thermal exploitation reduces the stiffness of porous rocks, turns them into a more compressible state, and hinders wave propagation by limiting their quality. | [90] |
| P-wave Velocity | They studied the coupling effect of high temperature and liquid nitrogen quenching on the physical characteristics of limestones. They found an appreciable reduction in P-wave velocities by 47% and 88% at a temperature of 400 °C and 600 °C, respectively. | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas, U.; Rashid, H.M.A.; Ahmed, M.F.; Rasool, A.M.; Al-Atroush, M.E. Damage Characteristics of Thermally Deteriorated Carbonate Rocks: A Review. Appl. Sci. 2022, 12, 2752. https://doi.org/10.3390/app12052752
Waqas U, Rashid HMA, Ahmed MF, Rasool AM, Al-Atroush ME. Damage Characteristics of Thermally Deteriorated Carbonate Rocks: A Review. Applied Sciences. 2022; 12(5):2752. https://doi.org/10.3390/app12052752
Chicago/Turabian StyleWaqas, Umer, Hafiz Muhammad Awais Rashid, Muhammad Farooq Ahmed, Ali Murtaza Rasool, and Mohamed Ezzat Al-Atroush. 2022. "Damage Characteristics of Thermally Deteriorated Carbonate Rocks: A Review" Applied Sciences 12, no. 5: 2752. https://doi.org/10.3390/app12052752
APA StyleWaqas, U., Rashid, H. M. A., Ahmed, M. F., Rasool, A. M., & Al-Atroush, M. E. (2022). Damage Characteristics of Thermally Deteriorated Carbonate Rocks: A Review. Applied Sciences, 12(5), 2752. https://doi.org/10.3390/app12052752






