Quantification of SPECT Concentric Ring Artifacts by Radiomics and Radial Features
Abstract
:1. Introduction
2. Materials and Methods
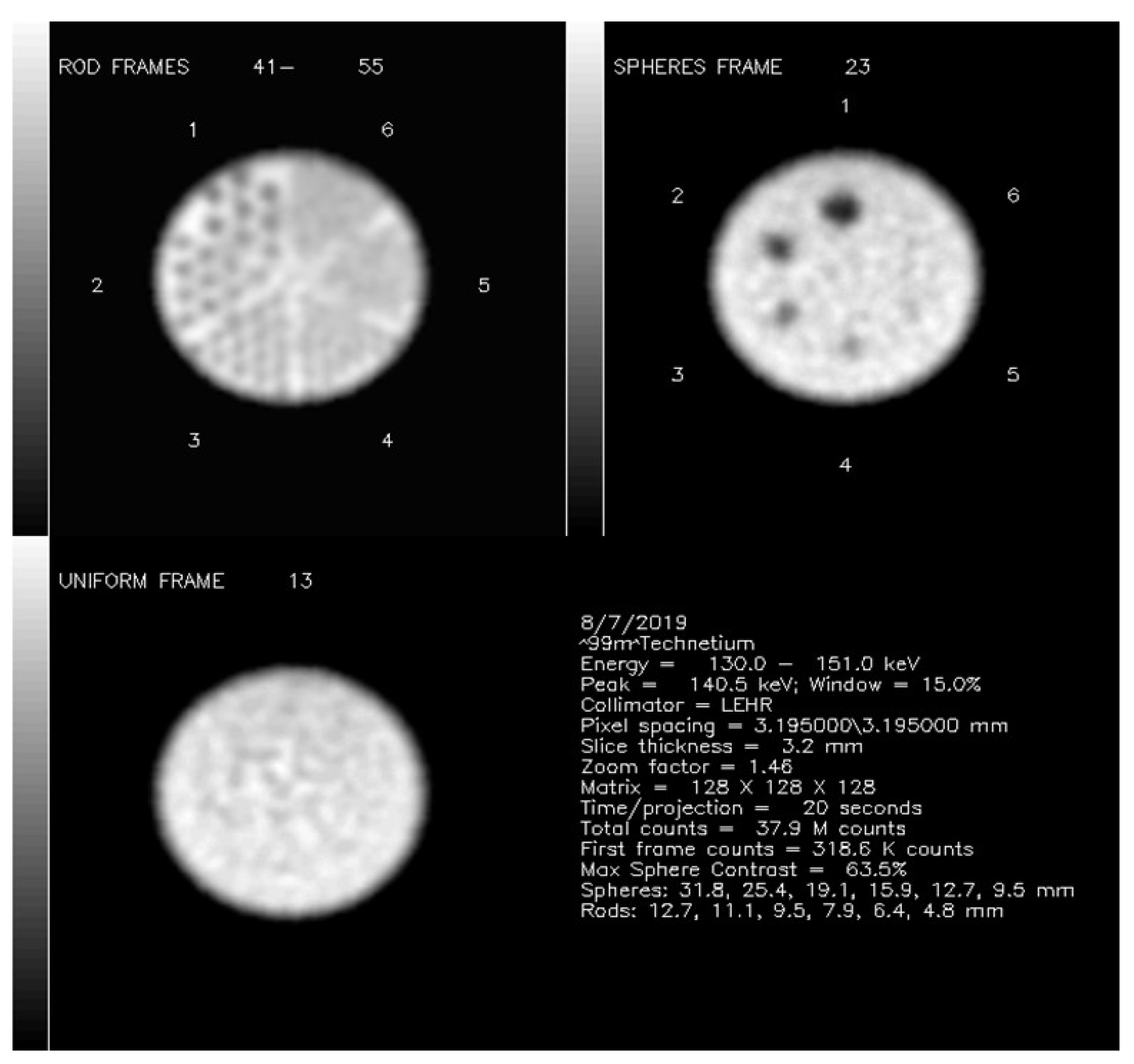
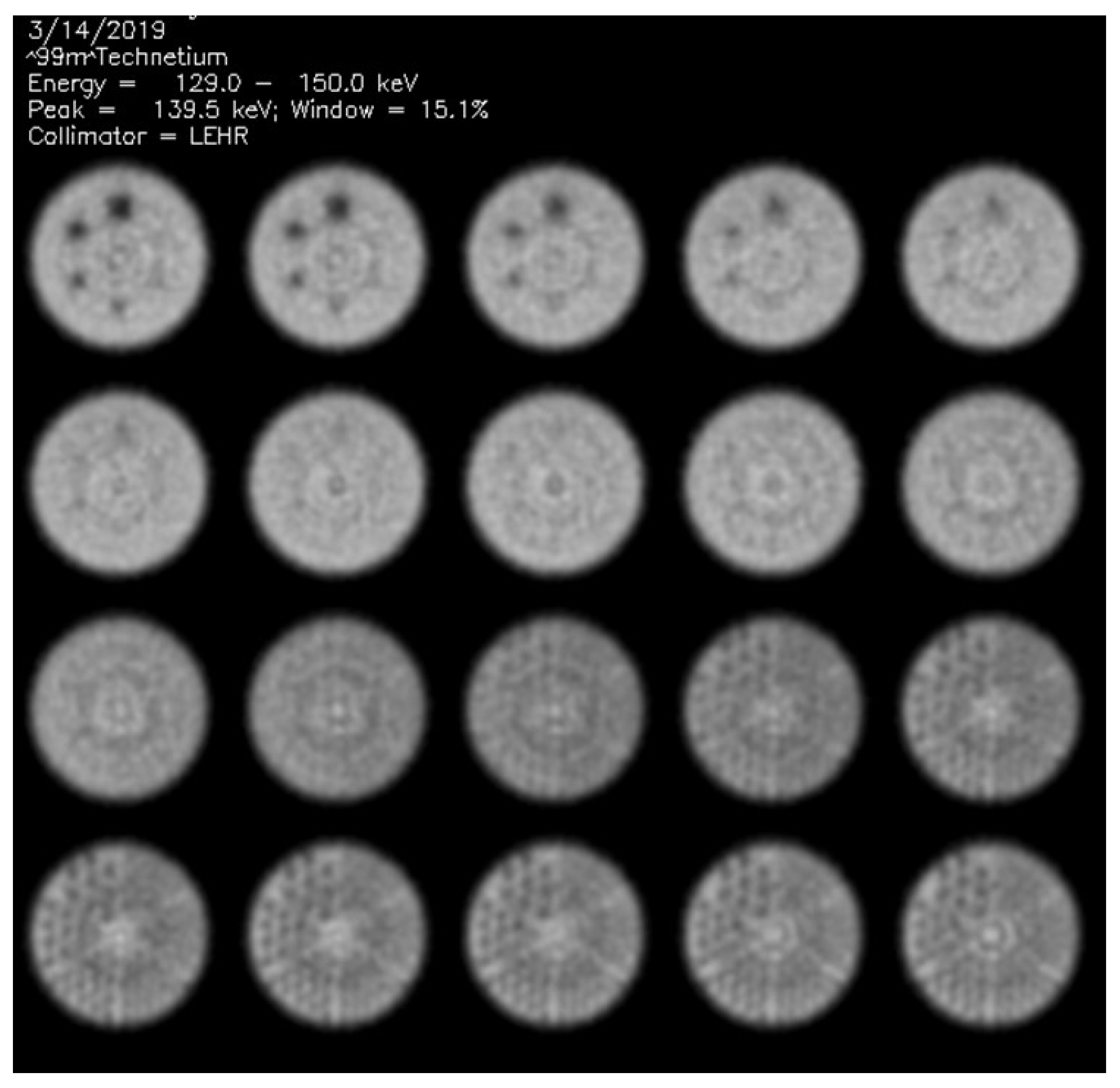
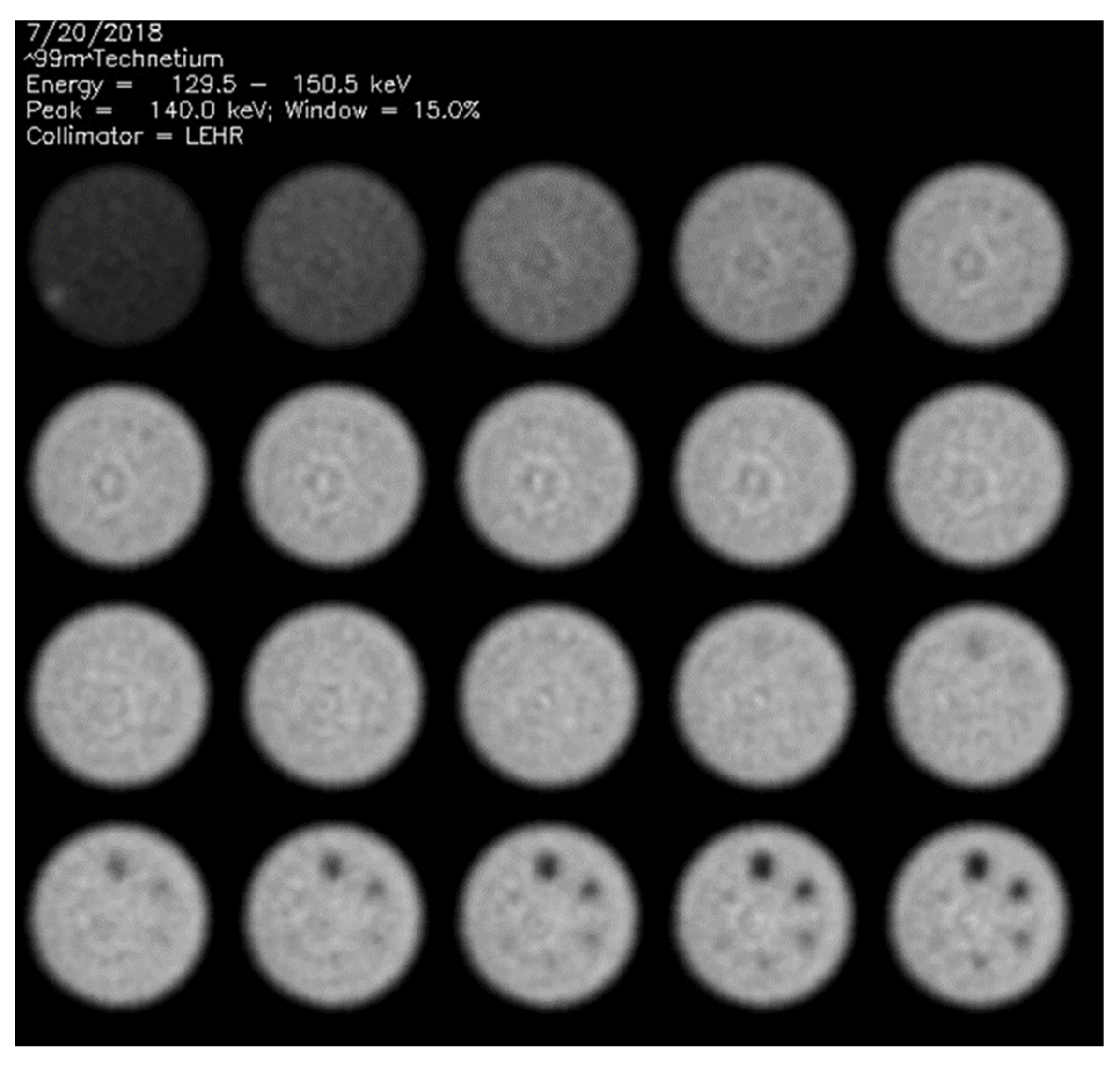
2.1. Phantom Data Acquisitions
2.2. Visual Phantom Readings
2.3. Radiomic Features Extraction
2.4. Radial Features Extraction
2.5. Statistical Analysis
3. Results
3.1. Visual Scores
3.2. Image Analysis Metrics
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, S.; Cullom, J.; Hawkins, W.; Moore, S.C.; Tsui, B.M.W.; Yester, M. Quantitative SPECT imaging: A review and recommendations by the Focus Committee of the Society of Nuclear Medicine Computer and Instrumentation Council. J. Nucl. Med. 1995, 36, 1489–1513. [Google Scholar]
- Celler, A.; Dixon, K.L.; Chang, Z.; Blinder, S.; Powe, J.; Harrop, R. Problems created in attenuation-corrected SPECT images by artifacts in attenuation maps: A simulation study. J. Nucl. Med. 2005, 46, 335–343. [Google Scholar] [PubMed]
- ACR Accreditation. Available online: https://ACR.org/Clinical-Resources/Accreditation (accessed on 15 January 2022).
- Leong, L.K.; Kruger, R.L.; O’connor, M.K. A comparison of the uniformity requirements for SPECT image reconstruction using FBP and OSEM techniques. J. Nucl. Med. Technol. 2001, 29, 79–83. [Google Scholar] [PubMed]
- Gullberg, G.T. An analytic approach to quantify uniformity artifacts for circular and noncircular detector motion in single photon emission computed tomography imaging. Med. Phys. 1987, 14, 105–114. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.K.; Vermeersch, C. Critical examination of the uniformity requirements for single-photon emission computed tomography. Med. Phys. 1991, 18, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.J.; DiFilippo, F.P.; Palestro, C.J. Texture analysis for automated evaluation of Jaszczak phantom SPECT system tests. Med. Phys. 2019, 46, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Filippo, F.P. Assessment of PET and SPECT phantom image quality through automated binary classification of cold rod arrays. Med. Phys. 2019, 46, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, A.; Mezzenga, E.; Vagheggini, A.; Piccinini, F.; Feliciani, G.; Belli, M.L.; Monti, F.; Cremonesi, M.; Cittanti, C.; Martinelli, G.; et al. Texture analysis in 177Lu SPECT phantom images: Statistical assessment of uniformity requirements using texture features. PLoS ONE 2019, 14, e0218814. [Google Scholar] [CrossRef] [Green Version]
- Madsen, M.T. A method for quantifying SPECT uniformity. Med. Phys. 1997, 24, 1696–1700. [Google Scholar] [CrossRef]
- Hirtl, A.; Bergmann, H.; Knausl, B.; Beyer, T.; Figl, M.; Hummel, J. Technical Note: Fully-automated analysis of Jaszczak phantom measurements as part of routine SPECT quality control. Med. Phys. 2017, 44, 1638–1645. [Google Scholar] [CrossRef]
- Jaszczak, R.J. Nuclear Imaging Phantom. U.S. Patent 4,499,375, 12 February 1985. [Google Scholar]
- Data Spectrum Home Page. Available online: http://www.spect.com/pub/Flangeless_Jasczack_Phantoms.pdf (accessed on 15 January 2022).
- MIM Software Home Page. Available online: https://www.mimsoftware.com/ (accessed on 15 January 2022).
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettinelli, A.; Branchini, M.; de Monte, F.; Scaggion, A.; Paiusco, M. An IBEX adaption toward image biomarker standardization. Med. Phys. 2020, 47, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.A. Studies in Optics; University of ChicagoPress: Chicago, IL, USA, 1927. [Google Scholar]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 15 January 2022).
- MedCalc Software Ltd. Medcalc Statistical; version 19.3.1; Software; MedCalc Software Ltd.: Ostend, Belgium, 2020. Available online: https://www.medcalc.org (accessed on 20 January 2022).
- Tibshirani, R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.M.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descendent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Sokole, E.B.; Plachcinska, A.; Britten, A. Acceptance testing for nuclear medicine instrumentation. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 672–681. [Google Scholar] [CrossRef]
- DePuey, E.G. How to detect and avoid myocardial perfusion SPECT artifacts. J. Nucl. Med. 1994, 35, 699–702. [Google Scholar]
- Rose, A. The sensitivity performance of the human eye on an absolute scale. J. Opt. Soc. Am. 1948, 38, 196–208. [Google Scholar] [CrossRef]
- Burgess, A.E. The Rose model, revisited. J. Opt. Soc. Am. A 1999, 16, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shafiq-ul-Hassan, M.; Zhang, G.G.; Latifi, K.; Ullah, G.; Hunt, D.C.; Balagurunathan, Y.; Abdalah, M.A.; Schabath, M.; Goldgof, D.G.; Mackin, D.; et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med. Phys. 2017, 44, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Shafiq-ul-Hassan, M.; Latifi, K.; Zhang, G.; Ullah, G.; Gillies, R.; Moros, E. Voxel size and gray level normalization of CT radiomic features in lung cancer. Sci. Rep. 2018, 8, 10545. [Google Scholar] [CrossRef] [PubMed]


| Manufacturer | Model | Studies |
|---|---|---|
| General Electric | Millenium—dual detector | 17 |
| Philips | Skylight—dual detector | 10 |
| Philips | Adac Argus—single detector | 4 |
| Philips | Adac Argus—single detector | 3 |
| Siemens | Symbia Intevo—dual detector | 2 |
| Siemens | Symbia Intevo—dual detector | 2 |
| Siemens | Symbia Intevo—dual detector | 3 |
| Family Name | Identification |
|---|---|
| Gray-Level Co-Occurrence Matrix (25) | GLCM (3D:mrg) |
| Gray-Level Distance Zone Matrix (16) | GLDZM (3D) |
| Gray-Level Run-Length Matrix (16) | GLRLM (3D:mrg) |
| Gray-Level Size Zone Matrix (16) | GLSZM (3D) |
| Neighborhood Gray-Level Dependence Matrix (17) | NGLDM (3D) |
| Neighborhood Gray-Tone Dependence Matrix (5) | NGTDM (3D) |
| Intensity-Based Statistics (18) | IS |
| Intensity Histogram (23) | IH |
| Intensity Volume Histogram (7) | IVH |
| Morphological (20) | MORPH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzenga, E.; Sarnelli, A.; Bellomo, G.; DiFilippo, F.P.; Palestro, C.J.; Nichols, K.J. Quantification of SPECT Concentric Ring Artifacts by Radiomics and Radial Features. Appl. Sci. 2022, 12, 2726. https://doi.org/10.3390/app12052726
Mezzenga E, Sarnelli A, Bellomo G, DiFilippo FP, Palestro CJ, Nichols KJ. Quantification of SPECT Concentric Ring Artifacts by Radiomics and Radial Features. Applied Sciences. 2022; 12(5):2726. https://doi.org/10.3390/app12052726
Chicago/Turabian StyleMezzenga, Emilio, Anna Sarnelli, Giovanni Bellomo, Frank P. DiFilippo, Christopher J. Palestro, and Kenneth J. Nichols. 2022. "Quantification of SPECT Concentric Ring Artifacts by Radiomics and Radial Features" Applied Sciences 12, no. 5: 2726. https://doi.org/10.3390/app12052726
APA StyleMezzenga, E., Sarnelli, A., Bellomo, G., DiFilippo, F. P., Palestro, C. J., & Nichols, K. J. (2022). Quantification of SPECT Concentric Ring Artifacts by Radiomics and Radial Features. Applied Sciences, 12(5), 2726. https://doi.org/10.3390/app12052726








