The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery
Abstract
:1. Introduction
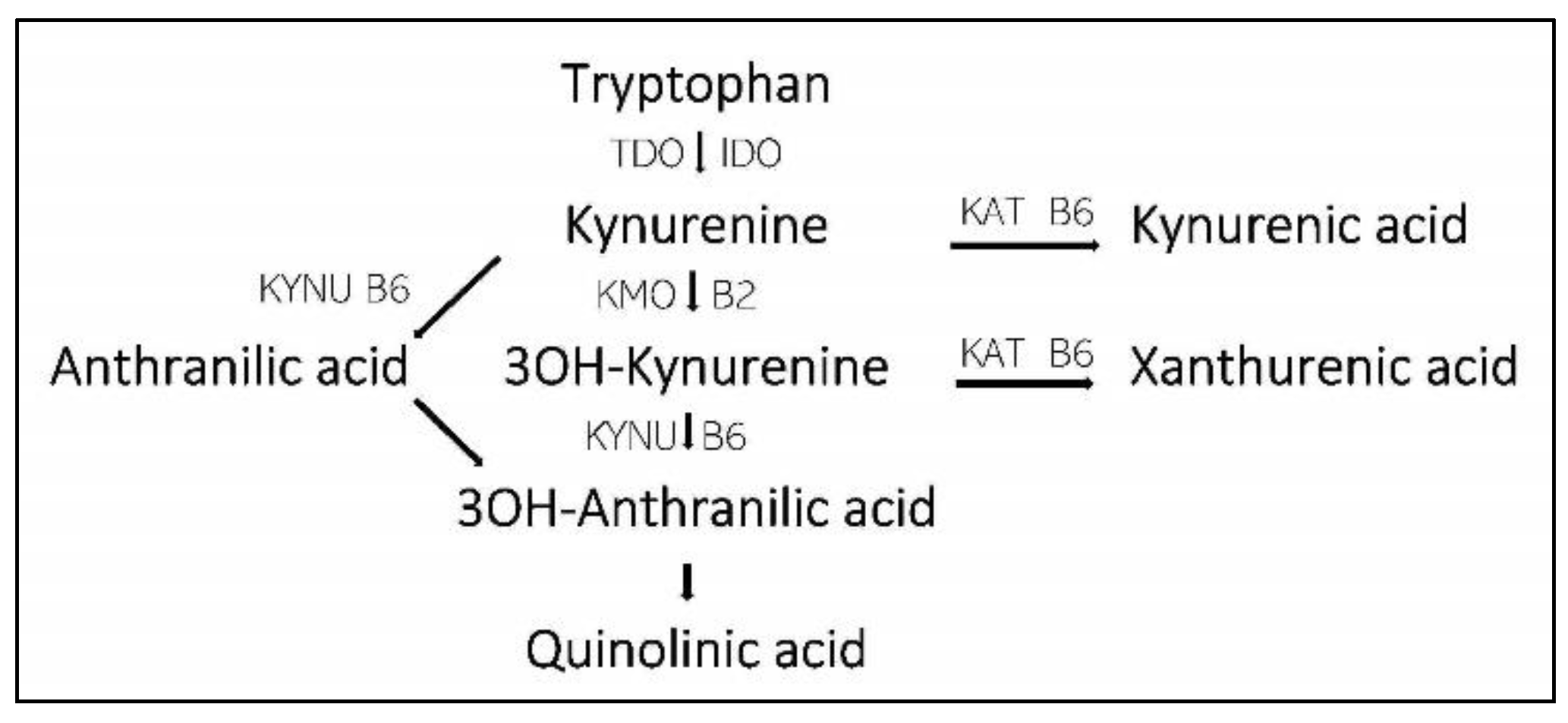
- Tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3 dioxygenase (IDO) regulate the Trypt to Kyn step.
- Kynurenine aminotransferase (KAT) regulate the steps from Kyn to Kynurenine acid (KA) and 3OH-Kyn to Xanthurenic acid (XA).
- Kynureninase (KYNU) and kynurenine 3-monooxygenase (KMO) are involved in the steps from Kyn to Quinolinic acid (QA).
2. Materials and Methods
2.1. Data Materials and Study Design
2.2. Participants and Inclusion Criteria
2.3. Interventions
2.4. Variables
- Demographic and anthropometric data: age (years), gender (male/female), height (m), body weight (kg), body mass index (BMI: kg/m2), and ethnicity.
- Social factors: cohabitation (yes/no), employed (yes/no), and smoking (daily/not daily).
- The physical activity score was the sum of two scores: easy activity (not sweaty/breathless): none; <1 h/week, 1–2 h/week, and >3 h/week (scores 0–3); and strenuous activity (sweaty/breathless): none, <1 h/week, 1–2 h/week, and >3 h/week (scores 0, 3, 4, and 5). The sum score for physical activity was 0–8.
- General health: Bad (0), Not quite good (1), Good (2), and Very good (3), score 0–3.
- Comorbidities: 12 current or previous disorders were noted (yes/no), score 0–12.
- Musculoskeletal pain from six parts of the body: none (0), mild (1), and severe (2), score 0–12.
- The World Health Organization—Five Well-Being Index (WHO-5) (scores 0–100, scores ≤ 50 indicate low mood, and scores ≤ 28 indicate likely depression) [24].
- Hopkins Symptom Checklist 10 measures psychological distress (scores 1–4, scores ≥ 1.85 indicate mental distress) [25].
- Fatigue Severity Scale: a validated Norwegian translation of the Fatigue Severity Scale was used (scores 1–7; scores ≥ 4 indicate further evaluation) [26].
- Epworth Sleepiness Scale: a validated Norwegian translation was used (scores 0–24; normal 0–10, mild 11–14, moderate 15–18, and severe 19–25) [29].
- Sense of Humour Questionnaire: a Norwegian version of the short form SHQ-6: “Attitudes towards humour” was used (scores 6–24) [30].
2.5. The Kynurenine Pathway
2.6. Statistics
3. Results
3.1. Participants
3.2. The Kynurenine Pathway
3.3. The Psychosomatic Disorders
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J. Clin. Med. 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Verbanck, M.; Pigeyre, M.; Caiazzo, R.; Raverdy, V.; Verkindt, H.; Leloire, A.; Guillemin, G.J.; Yengo, L.; et al. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS ONE 2016, 11, e0158051. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Movassat, J.; Portha, B. Emerging role for kynurenines in metabolic pathologies. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 82–90. [Google Scholar] [CrossRef]
- Christensen, M.H.E.; Fadnes, D.J.; Rost, T.H.; Pedersen, E.R.; Andersen, J.R.; Vage, V.; Ulvik, A.; Midttun, O.; Ueland, P.M.; Nygard, O.K.; et al. Inflammatory markers, the tryptophan-kynurenine pathway, and vitamin B status after bariatric surgery. PLoS ONE 2018, 13, e0192169. [Google Scholar] [CrossRef] [Green Version]
- Groven, N.; Reitan, S.K.; Fors, E.A.; Guzey, I.C. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology 2021, 131, 105287. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vecsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitron, R.; Ugalde Muniz, P.; Pineda, B.; Pedraza-Chaverri, J.; Rios, C.; Perez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The weight of nutrients: Kynurenine metabolites in obesity and exercise. J. Intern. Med. 2018, 284, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleb, O.; Maammar, M.; Klein, C.; Maitre, M.; Mensah-Nyagan, A.G. A Role for Xanthurenic Acid in the Control of Brain Dopaminergic Activity. Int. J. Mol. Sci. 2021, 22, 6974. [Google Scholar] [CrossRef]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Blankfield, A. A Brief Historic Overview of Clinical Disorders Associated with Tryptophan: The Relevance to Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). Int. J. Tryptophan Res. 2012, 5, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Farup, P.G. Are the Results of a Combined Behavioural and Surgical Treatment of Morbid Obesity Satisfactory and Predictable? Nutrients 2020, 12, 1997. [Google Scholar] [CrossRef]
- Farup, P.G.; Rootwelt, H.; Hestad, K. APOE—A genetic marker of comorbidity in subjects with morbid obesity. BMC Med. Genet. 2020, 21, 146. [Google Scholar] [CrossRef]
- Aasbrenn, M.; Lydersen, S.; Farup, P.G. A Conservative Weight Loss Intervention Relieves Bowel Symptoms in Morbidly Obese Subjects with Irritable Bowel Syndrome: A Prospective Cohort Study. J. Obes. 2018, 2018, 3732753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, P.R.; Ikramuddin, S.; Hamad, G.; Eid, G.M.; Mattar, S.; Cottam, D.; Ramanathan, R.; Gourash, W. Laparoscopic gastric bypass surgery: Current technique. J. Laparoendosc. Adv. Surg. Tech. A 2003, 13, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Roa, P.E.; Kaidar-Person, O.; Pinto, D.; Cho, M.; Szomstein, S.; Rosenthal, R.J. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: Technique and short-term outcome. Obes. Surg. 2006, 16, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Topp, C.W.; Ostergaard, S.D.; Sondergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Søgaard, A.J.; Bjelland, I.; Tell, G.S.; Røysamb, E. A comparison of the CONOR Mental Health Index to the HSCL-10 and HADS. Norsk Epidemiol. 2003, 13, 279–284. [Google Scholar]
- Lerdal, A.; Wahl, A.; Rustoen, T.; Hanestad, B.R.; Moum, T. Fatigue in the general population: A translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand. J. Public Health 2005, 33, 123–130. [Google Scholar] [CrossRef]
- Alessandri, G.; Vecchione, M.; Eisenberg, N.; Laguna, M. On the factor structure of the Rosenberg (1965) General Self-Esteem Scale. Psychol. Assess. 2015, 27, 621–635. [Google Scholar] [CrossRef] [Green Version]
- von Soest, T. Rosenbergs selfølelsesskala: Validering av en norsk oversettelse. J. Norwegian. Psychol. Assoc. 2005, 42, 226–228. [Google Scholar]
- Beiske, K.K.; Kjelsberg, F.N.; Ruud, E.A.; Stavem, K. Reliability and validity of a Norwegian version of the Epworth sleepiness scale. Sleep Breath. 2009, 13, 65–72. [Google Scholar] [CrossRef]
- Svebak, S. The development of the Sense of Humor Questionnaire: From SHQ to SHQ-6. Humor-Int. J. Humor Res. 1996, 9, 341–362. [Google Scholar] [CrossRef]
- Mume, C.O. Excessive daytime sleepiness among depressed patients. Libyan J. Med. 2010, 5, 4626. [Google Scholar] [CrossRef] [PubMed]
- Schwieler, L.; Samuelsson, M.; Frye, M.A.; Bhat, M.; Schuppe-Koistinen, I.; Jungholm, O.; Johansson, A.G.; Landen, M.; Sellgren, C.M.; Erhardt, S. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J. Neuroinflammation 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, H.; Toldi, J.; Vecsei, L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Hagman, D.K.; Larson, I.; Kuzma, J.N.; Cromer, G.; Makar, K.; Rubinow, K.B.; Foster-Schubert, K.E.; van Yserloo, B.; Billing, P.S.; Landerholm, R.W.; et al. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism 2017, 70, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. 1), 9–14. [Google Scholar] [CrossRef] [Green Version]
- Theofylaktopoulou, D.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygard, O.; Eussen, S.J. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Kolen, A.M.; Romeijn, M.M.; Holthuijsen, D.D.B.; Janssen, L.; Greve, J.W.M.; Leclercq, W.K.G.; van Dielen, F.M.H. Current preoperative strategies applied in the Dutch bariatric centers: A national survey. Clin. Obes. 2021, 11, e12461. [Google Scholar] [CrossRef]
- Kim, J.J.; Rogers, A.M.; Ballem, N.; Schirmer, B.; American Society for Metabolic; Bariatric Surgery Clinical Issues Committee. ASMBS updated position statement on insurance mandated preoperative weight loss requirements. Surg. Obes. Relat Dis 2016, 12, 955–959. [Google Scholar] [CrossRef]
| Patient Characteristics | At Inclusion Mean (SD) (No 141) | 6 Months after Surgery Mean (SD) (No 95) | Difference Compared with Inclusion (Paired Data) | Statistics p-Values |
|---|---|---|---|---|
| Gender (female) | 116 (82%) | 77 (81%) | ||
| Age (years) | 43.0 (8.7) | |||
| BMI (kg/m2) | 42.1 (3.8) | 30.2 (3.6) | −11.7 (2.7) | <0.001 |
| Cohabitation (yes) | 115 (82%) | |||
| Employed (yes) | 107 (76%) | |||
| Daily smoker (yes) | 25 (18%) | 7/89 (5%) | ||
| Physical activity (score 0–8) | 4.47 (2.2) | 5.4 (2.2) | 0.8 (2.5) | 0.007 |
| General health (score 0–3) | 1.30 (0.7) | 2.03 (0.70) | 0.72 (0.75) | <0.001 |
| Somatic disorders (score 0–12) | 1.8 (1.4) | 1.7 (1.5) | −0.2 (0.9) | 0.091 |
| Muscle-skeletal pain (score 0–12) | 4.25 (2.94) | 3.04 (2.57) | −1.81 (2.50) | <0.001 |
| WHO-5 Well-Being (score 0–100) | 59.5 (17.7) | 71.9 (16.1) | 14.6 (17.8) | <0.001 |
| Hopkins Symptoms Checklist (score 1–4) | 1.58 (0.54) | 1.32 (0.39) | −0.26 (0.48) | <0.001 |
| Fatigue Severity Score (score 1–7) | 3.95 (1.63) | 3.14 (1.70) | −0.90 (1.76) | <0.001 |
| Rosenberg Self-Esteem Scale (range 0–30) | 18.4 (5.3) | 22.2 (5.4) | 3.9 (5.5) | <0.001 |
| Epworth Sleepiness Scale (score 0–24) | 8.0 (4.7) | 5.8 (4.1) | −2.4 (3.2) | <0.001 |
| Sense of humour (score 6–24) | 19.2 (2.7) | 19.7 (2.0) | 0.3 (1.9) | 0.144 |
| CRP (mg/L) | 7.1 (6.3) | 1.87 (2.51) | −4.92 (4.6) | <0.001 |
| Diabetes mellitus (yes) | 26/137 (18%) | 16/90 (18%) | ||
| Glucose (mmol/L) | 6.6 (3.1) | 5.4 (1.2) | −1.1 (2.6) | <0.001 |
| HbA1c (%) | 5.9 (1.4) | 5.1 (0.8) | −0.7 (0.9) | <0.001 |
| Vitamin B6 (15–160 nmol/l) | 31 (27) | 44 (30) | 10 (32) | 0.003 |
| Tryptophan (Trypt) (ng/mL) | 12,630 (2777) | 11,339 (1912) | −788 (2649) | 0.005 |
| Kynurenine (Kyn) (ng/mL) | 488 (143) | 378 (91) | −85 (111) | <0.001 |
| Kynurenic acid (KA) (ng/mL) | 14.2 (7.1) | 8.5 (3.9) | −4.8 (4.7) | <0.001 |
| Quinolinic acid (QA) (ng/mL) | 111 (45) | 84 (30) | −22 (30) | <0.001 |
| Xanthurenic acid (QA) (ng/mL) | 5.3 (1.2) | 4.6 (0.9) | −0.4 (1.1) | 0.001 |
| Kyn/Trypt ratio × 1000 | 39 (11) | 34 (9) | −5 (7) | <0.001 |
| KA/Kyn ratio × 1000 | 30 (12) | 22 (8) | −7 (11) | <0.001 |
| KA/QA ratio × 1000 | 138 (70) | 107 (52) | 27 (60) | <0.001 |
| KA/XA ratio | 2.77 (1.27) | 1.84 (0.81) | −0.88 (0.11) | <0.001 |
| QA/XA ratio | 21.9 (8.8) | 18.4 (6.3) | −3.4 (8.0) | <0.001 |
| Dependent Variables | Independent Variables (B-Values with 95% CI, All p-Values and q-Values * < 0.10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Typt-Kyn Metabolites | Gender | Age | BMI | Smoking | Physical Activity | Diabetes Mellitus | CRP | Vitamin B6 | Time |
| Tryptophan | 620 (−457; 1798) p = 0.256 | −18 (−72; 35) p = 0.498 | −10 (−128; 109) p = 0.875 | −230 (−1317; 856) p = 0.676 | 90 (−91; 271) p = 0.328 | 163 (−955; 1280) p = 0.773 | −67 (−141; 6) p = 0.073 | 0 (−15; 15) p = 0.997 | −1784 (−3430; −139) p= 0.034 |
| Kynurenine | 4 (−54; 61) p = 0.902 | 4 (0.6; 6.4) p= 0.017 | 3 (−3; 9) p = 0.379 | −14 (−67; 38) p = 0.589 | 7 (−1; 16) p = 0.103 | −60 (−119; −0.7) p= 0.047 | 2 (−2; 5) p = 0.305 | 0 (−0.7; 0.7) p = 0.984 | −70 (−151; 10) p = 0.087 |
| Kynurenic acid (KA) | −0.18 (−3.18; 2.8) p = 0.906 | 0.19 (0.03; 0.34) p= 0.015 | 0.23 (−0.06; 0.52) p = 0.115 | 0.65 (−1.79; 3.08) p = 0.599 | 0.21 (−0.19; 0.61) p = 0.297 | −3.17 (−6.18; −0.15) p= 0.040 | −0.13 (−0.30; 0.04) p = 0.132 | 0.02 (−0.01; 0.06) p = 0.141 | −3.7 (−7.6; 0.09) p = 0.055 |
| Quinolinic acid (QA) | 9.8 (−8.4; 28.0) p = 0.288 | 0.9 (−0.03; 1.8) p = 0.058 | 1.3 (−0.5; 3.1) p = 0.156 | −15.5(−30.8; 0.26) p= 0.046 | −1.2 (−3.7; 1.3) p = 0.355 | −21.9 (−40.3; −3.5) p= 0.020 | 1.0 (−0.1; 2.0) p = 0.076 | −0.0 (−0.23; 0.17) p = 0.757 | −3.7 (−27.7; 20.3) p = 0.759 |
| Xanthurenic acid (XA) | 0.3 (−0.1; 0.8) p = 0.137 | −0.01 (−0.03; 0.02) p = 0.607 | 0.0 (−0.05; 0.05) p = 0.938 | −0.04 (−0.50; 0.41) p = 0.851 | 0.03 (−0.04; 0.11) p = 0.368 | 0.11 (−0.36; 0.59) p = 0.633 | −0.03 (−0.06; 0.00) p = 0.066 | −0.00 (−0.00; 0.00) p = 0.939 | −0.77 (−1.47; −0.08) p= 0.028 |
| Kyn/Trypt ratio × 1000 | −1.2 (−5.6; 3.1) p = 0.577 | 0.3 (0.095; 0.53 p= 0.005 | 0.1 (−0.3; 0.5) p = 0.549 | −0.8 (−4.1; 2.4) p = 0.604 | 0.6 (0.05; 1.1) p= 0.032 | −5.9 (−10.2; −1.5) p= 0.008 | 0.5 (0.3; 0.7) p= 0.000061 q= 0.005 | 0.005 (−0.00; 0.047) p = 0.819 | −2.7 (−8.0; 2.5) p = 0.315 |
| KA/Kyn ratio × 1000 | −0.4 (−5.1; 4.3) p = 0.857 | 0.1 (−0.2; 0.3) p = 0.546 | 0.4 (−0.1; 0.9) p = 0.150 | 0.3 (−4.3; 4.9) p = 0.902 | −0.2 (−0.9; 0.6) p = 0.698 | −1.9 (−6.7; 3.0) p = 0.447 | −0.3 (−0.7; −0.03) p= 0.032 | 0.02 (−0.05; 0.08) p = 0.650 | −4.7 (−11.7; 2.3) p = 0.186 |
| KA/QA ratio × 1000 | −9 (−38; 20) p = 0.532 | 0.4 (−1.1; 1.8) p = 0.628 | 0.6 (−2.3; 3.8) p = 0.627 | 34 (7; 61) p= 0.013 | 0.7 (−3.7; 5.1) p = 0.746 | −7 (−36; 23) p = 0.665 | −1.7 (−3.5; 0.1) p = 0.069 | 0.3 (−0.06; 0.7) p = 0.100 | −36 (−77; 5) p = 0.089 |
| KA/XA ratio | −0.2 (−0.7; 0.3) p = 0.452 | 0.03 (0.01; 0.06) p= 0.016 | 0.04 (−0.01; 0.10) p = 0.117 | −0.04 (−0.52; 0.44) p = 0.872 | 0.01 (−0.07; 0.09) p = 0.743 | −0.62 (−1.14; −0.10) p= 0.021 | −0.01 (−0.04; 0.03) p = 0.672 | 0.00 (−0.005; 0.01) p = 0.581 | −0.5 (−1.2; 0.2) p = 0.167 |
| QA/XA ratio | 0.1 (−3.3; 3.5) p = 0.954 | 0.2 (0.02; 0.35) p= 0.030 | 0.3 (−0.1; 0.6) p = 0.142 | −3.0 (−6.2; 1.3) p = 0.060 | −0.3 (−0.8; 0.2) p = 0.252 | −5.3 (−8.8; −1.9) p= 0.003 q= 0.090 | 0.37 (0.16; 0.59) p= 0.0008 q= 0.036 | −0.01 (−0.05; 0.03) p = 0.691 | 1.6 (−3.3; 6.4) p = 0.519 |
| Dependent Variable | Independent Variables, One at a Time (B-Values with 95% Confidence Interval and p-Values *) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan × 105 | Kynurenine × 104 | Kyn ac × 103 | Quin ac × 103 | Xanth ac × 102 | Kyn/Trypt Ratio | KA/Kyn Ratio | KA/QA Ratio | KA/XT Ratio × 102 | QA/XT Ratio × 103 | |
| General health | 1.7 (−2.1; 5.5) p = 0.384 | −1.1 (−9.0; 6.8) p = 0.790 | 0.4 (−16; 15) p = 0.962 | −1.4 (−4.0; 1.1) p = 0.269 | 4 (−5; 13) p = 0.379 | −6 (−17; 5) p = 0.278 | −0.1 (−9; 9) p = 0.979 | 0.6 (−1.0; 2.1) p = 0.466 | 4 (−13; 4) p = 0.340 | −9 (−22; 4) p = 0.168 |
| Muscle-skeletal pain | −1.1 (−15; 14) p = 0.887 | 9 (−22; 40) p = 0.560 | 32 (−31; 96) p = 0.316 | 1.7 (−8.5; 12) p = 0.740 | −6 (−42; 29) p = 0.722) | 15 (−30; 59) p = 0.512 | −3 (−39; 32) p = 0.848 | 0.6 (−5.6; 6.7) p = 0.858 | 17 (−18; 51) p = 0.337 | 6 (−45; 58) p = 0.807 |
| WHO−5 Well-Being | 17 (75; 108) p = 0.719) | 61 (−132; 253) p = 0.533 | 75 (−317; 468) p = 0.705 | 40 (−22; 103) p = 0.208 | 85 (−134; 303) p = 0.446 | 13 (−258; 284) p = 0.923 | −2 (−225; 221) p = 0.986 | −14 (−53; 26) p = 0.473 | −44 (−260; 172) p = 0.689 | 142 (−177; 462) p = 0.380 |
| Hopkins Symptom Checklist−5 | 0.2 (−2.5; 2.9) p = 0.890 | 0.2 (−5.4; 5.9) p = 0.937 | 1 (−10; 12) p = 0.867 | −0.7 (−2.6; 1.1) p = 0.453 | −0.3 (−6.7; 6.0) p = 0.909 | 1 (−7; 9) p = 0.779 | −0.3 (−7; 6) p = 0.918 | 0.4 (−0.7; 1.5) p = 0.428 | 2.0 (−4.2; 8.2) p = 0.529 | −5 (−14; 5) p = 0.338) |
| Fatigue Severity Score | 1.6 (−7.1; 10.3) p = 0.713 | 14 (−4; 32) p = 0.134 | 11 (−26; 47) p = 0.560 | 2.1 (−3.7; 8.1) p = 0.473 | −0.7 (−22; 20) p = 0.943 | 17 (−8; 43) p = 0.186 | −14 (−35; 6) p = 0.160 | −0.5 (−4.0; 3.1) p = 0.802 | 5.3 (−14.7; 25.4) p = 0.598 | 2 (−27; 32) p = 0.886 |
| Rosenberg Self- Esteem Scale | 4 (−25; 34) p = 0.755 | 9 (−70; 53) p = 0.785 | 77 (−47; 200) p = 0.222 | 2.3 (−17; 22) p = 0.821 | 21 (−50; 92) p = 0.561 | −32 (−119; 53) p = 0.453 | 72 (1.9; 142) p= 0.044 | 5 (−7; 17) p = 0.413 | 30 (−38; 98) p = 0.381 | 20 (82; 122) p = 0.697 |
| Epworth Sleepiness Scale | 6 (−15; 28) p = 0.547 | 24 (−25; 72) p = 0.337 | −52 (−151; 48) p = 0.305 | 11 (−5; 27) p = 0.191 | 9 (−43; 61) p = 0.739 | 23 (−49; 95) p = 0.525 | −45 (−97;7) p = 0.089 | −10 (−19; −0.7) p= 0.035 | −23 (−75; 28) p = 0.376 | 23 (−54; 101) p = 0.558 |
| Sense of humour | −2 (−14; 10) p = 0.729 | −5 (−32; 23) p = 0.741 | −32 (−87; 22) p = 0.246 | −3 (−12; 5) p = 0.445 | −1.6 (−31; 28) p = 0.914 | 9 (−30; 48) p = 0.652 | 0.2 (−29; 29) p = 0.988 | −0.7 (−6; 4.5) p = 0.800 | −8 (−37; 20) p = 0.567 | −3 (−45; 41) p = 0.913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farup, P.G.; Hestad, K. The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery. Appl. Sci. 2022, 12, 2648. https://doi.org/10.3390/app12052648
Farup PG, Hestad K. The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery. Applied Sciences. 2022; 12(5):2648. https://doi.org/10.3390/app12052648
Chicago/Turabian StyleFarup, Per G., and Knut Hestad. 2022. "The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery" Applied Sciences 12, no. 5: 2648. https://doi.org/10.3390/app12052648
APA StyleFarup, P. G., & Hestad, K. (2022). The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery. Applied Sciences, 12(5), 2648. https://doi.org/10.3390/app12052648






