Concentration Dependent Improved Spectroscopic Characteristics and Near White Light Emission in Boro Phosphate Glasses Doped with Holmium
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
3.1. XRD Analysis
3.2. Physical Properties with Theoretical Discussion
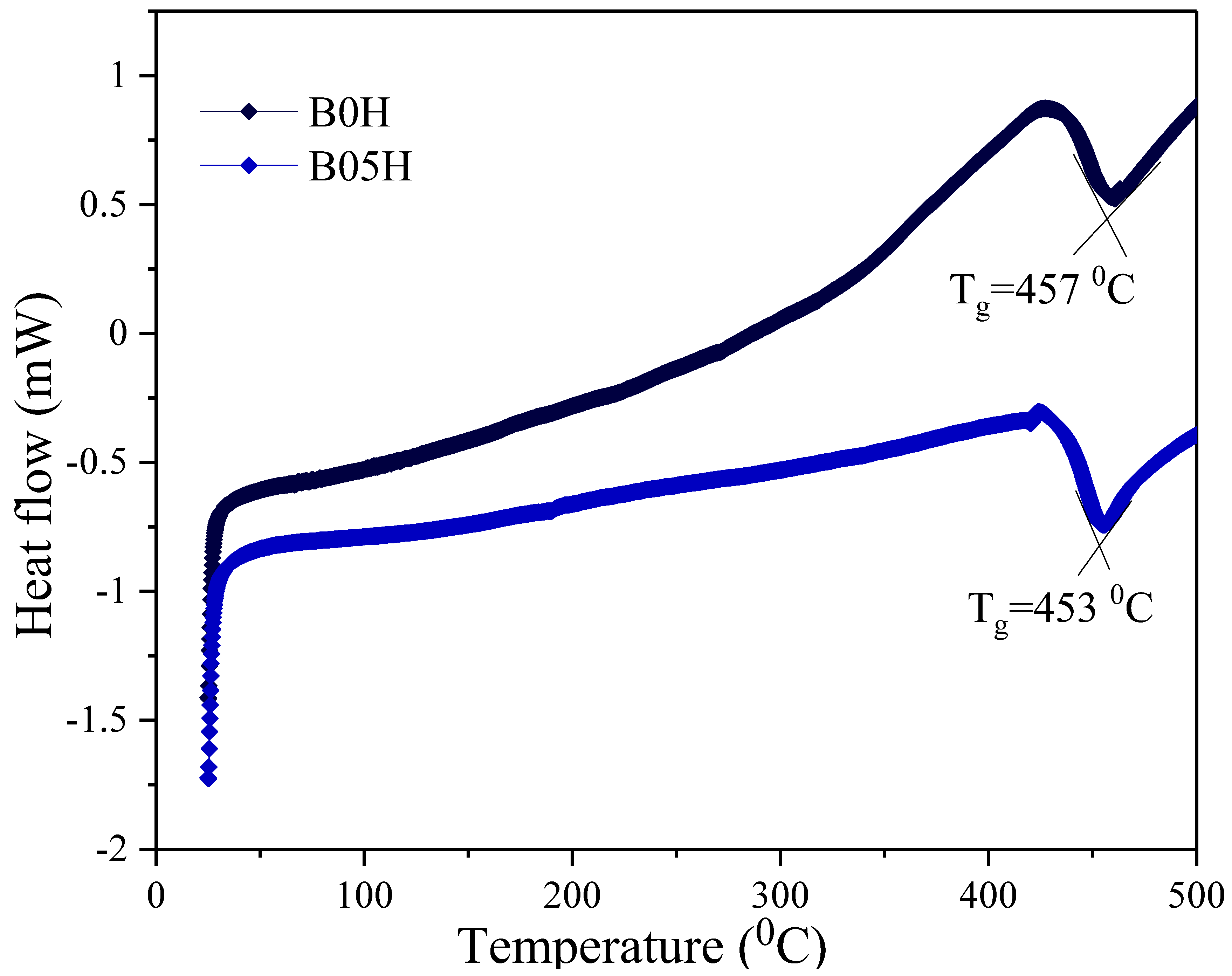
3.3. Glass Transition Temperature
3.4. Structural Analysis from FTIR Spectroscopy
3.5. Optical Properties
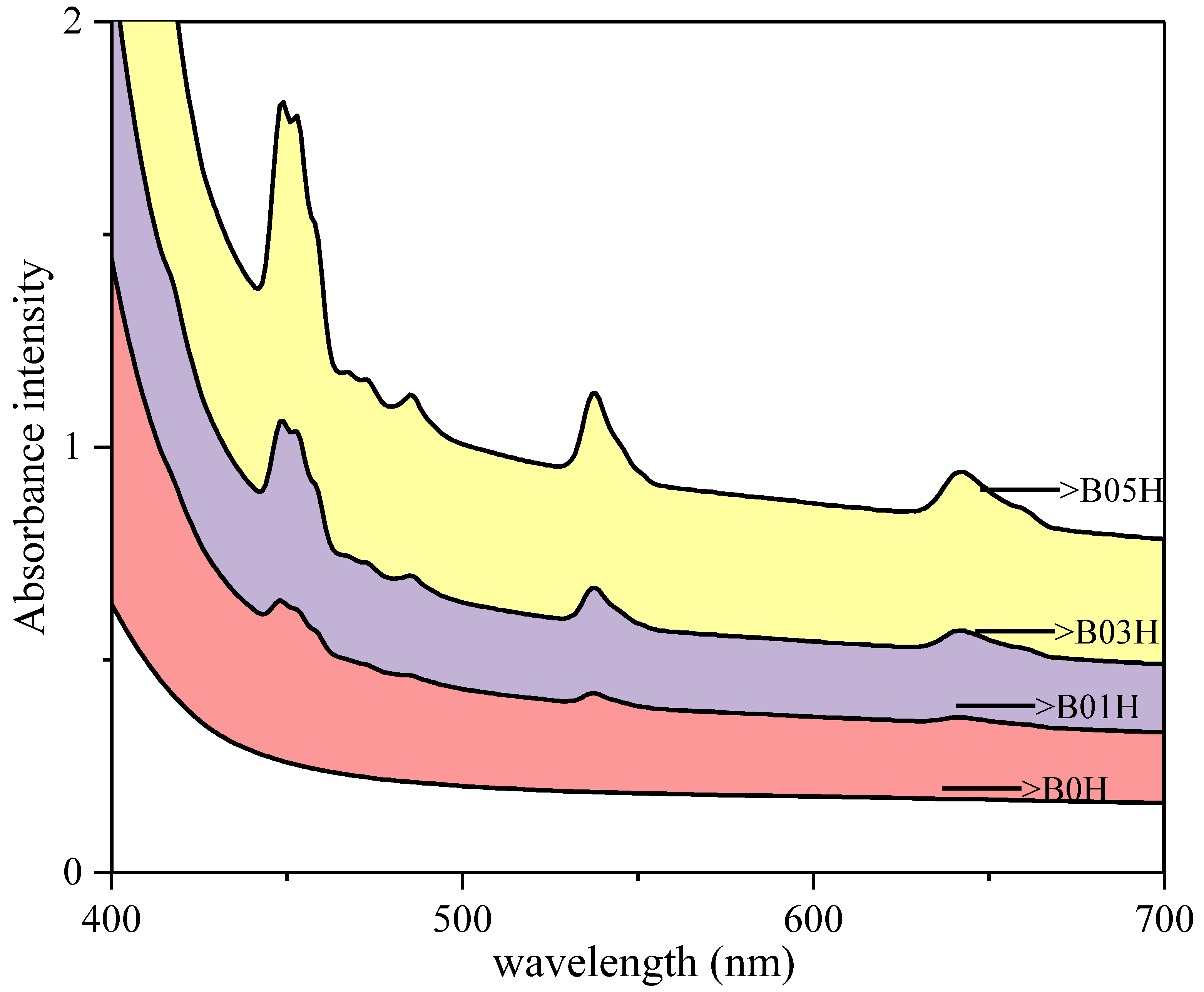
3.5.1. UV-Vis-NIR Analysis with Tauc’s Plots
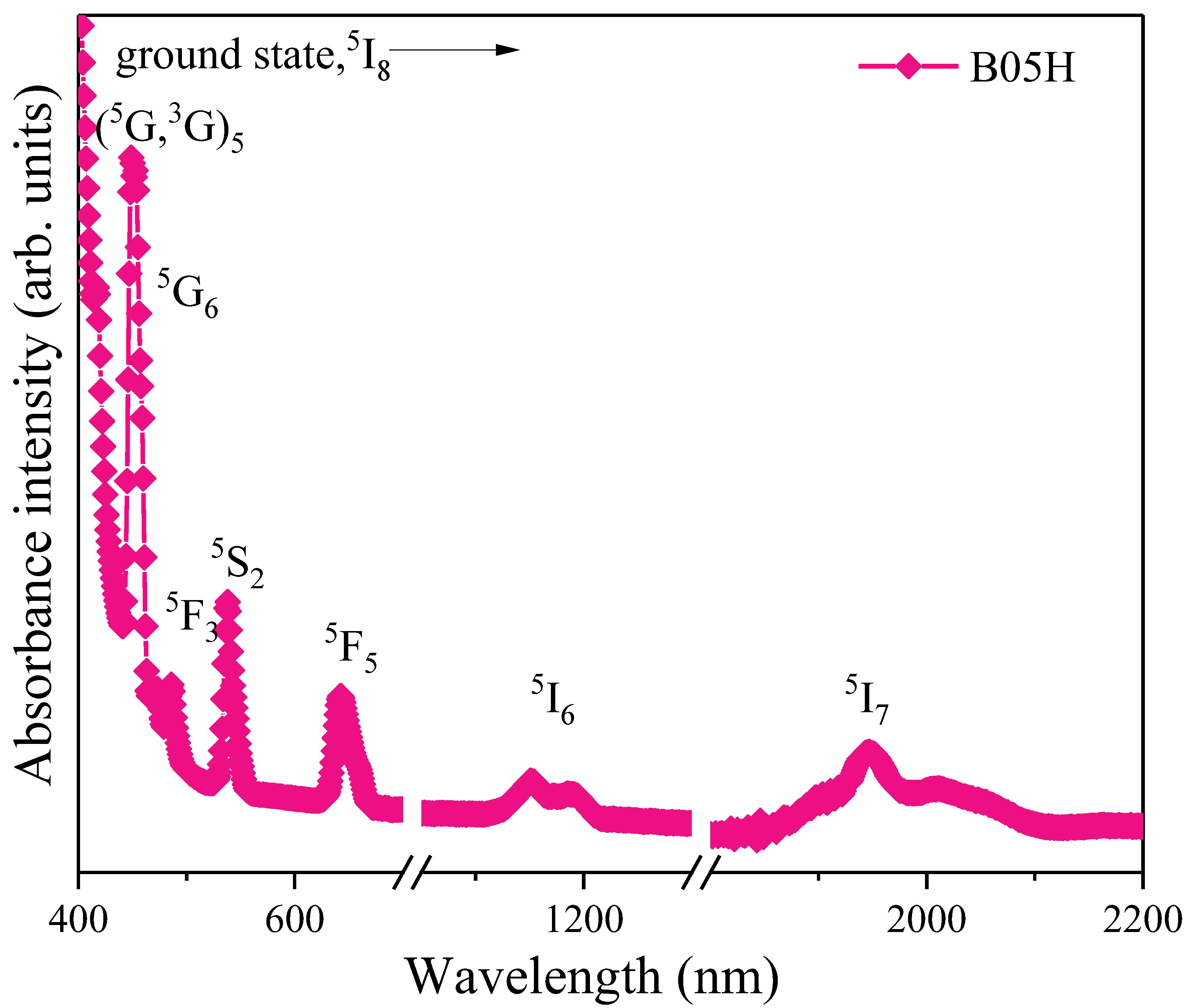
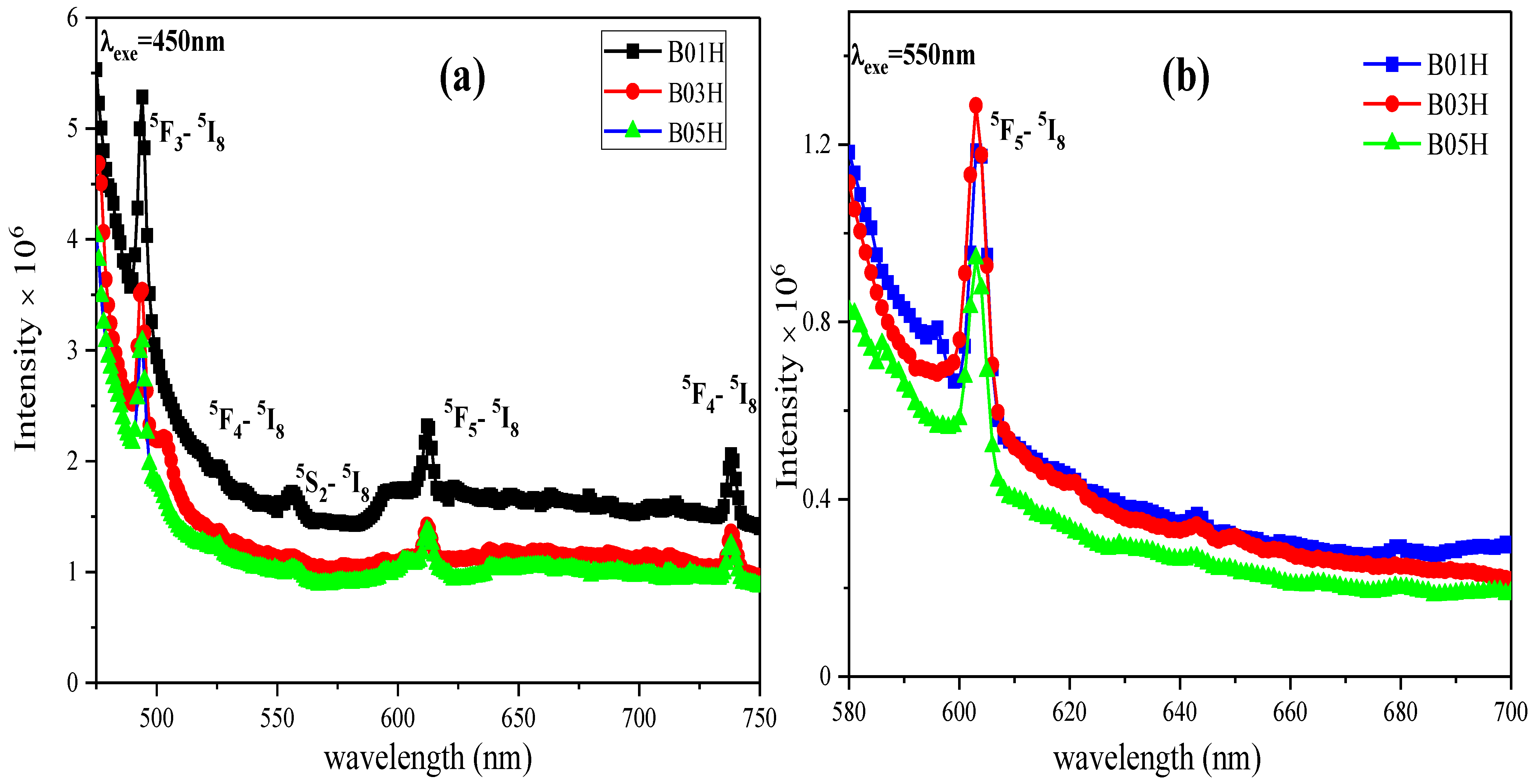
3.5.2. Photoluminescence and CIE Diagram
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakery, A.; Elliott, S.R. Optical properties and applications of chalcogenide glasses: A review. J. Non Cryst. Solids 2003, 330, 1–12. [Google Scholar] [CrossRef]
- Knowles, J.C. Phosphate based glasses for biomedical applications. J. Mater. Chem. 2003, 10, 2395–2401. [Google Scholar] [CrossRef]
- Mariselvam, K.; Liu, J. Green emission and laser properties of Ho3+ doped titano lead borate (TLB) glasses for colour display applications. J. Solid State Chem. 2021, 293, 121793. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hussain, R.; Ghoshal, S.K.; Alamri, S.N.; Yamusa, Y.A.; Jupri, S.A. Intense red and green luminescence from holmium activated zinc-sulfo-boro-phosphate glass: Judd-Ofelt evaluation. J. Alloys Compd. 2019, 808, 151706. [Google Scholar] [CrossRef]
- Lakshminarayan, G.; Baki, S.O.; Lira, A.; Caldino, U.; Rocha, A.N.M.; Kityk, I.V.; Abas, A.F.; Alresheedi, M.T.; Mahdi, M.A. Effect of alkali/mixed alkali metal ions on the thermal and spectral characteristics of Dy3+: B2O3-PbO-Al2O3-ZnO glasses. J. Non Cryst. Solids 2018, 481, 191–201. [Google Scholar] [CrossRef]
- Yu, H.; Xin, R.; Zhang, X.; Liu, H.; Zheng, K.; Zhao, J.; Zhan, L.; Xu, C.; Wan, W.; Zhu, Y.; et al. Crystallization behavior, quantitation of Ce3+/Ce4+ and chemical stability analysis of multiple alkaline earths borosilicate glasses for immobilizing simulated tetravalent actinides. J. Non Cryst. Solids 2021, 558, 120642. [Google Scholar] [CrossRef]
- Reddy, B.N.K.; Raju, B.D.; Thyagarajan, K.; Ramanaiah, R.; Jho, Y.-D.; Reddy, B.S. Optical characterization of Eu3+ ion doped alkali oxide modified borosilicate glasses for red laser and display device applications. Ceram. Int. 2017, 43, 8886–8892. [Google Scholar] [CrossRef]
- Shen, X.; Cheng, G.; Zhang, L.; Wei, W. Fabrication of a hybrid-cladding tellurite glass fiber doped with Tm3+ and Ho3+. J. Lumin. 2020, 227, 117540. [Google Scholar] [CrossRef]
- Bulus, I.; Hussain, R.; Ghoshal, S.K.; Tamuri, A.R.; Danmallam, I.M.; Yamusa, Y.A. Europium-doped boro-telluro-dolomite glasses for red laser applications: Basic insight into spectroscopic traits. J. Non Cryst. Solids 2020, 534, 119949. [Google Scholar] [CrossRef]
- Benouila, O.; Aiadi, K.E.; Rehouma, F.; Poulain, M.; Benhbirech, F. Thermal stability and spectroscopic study of Ho3+/Yb3+ co-doped fluorophosphates glasses. J. King Saud Univ. Sci. 2019, 31, 628–634. [Google Scholar] [CrossRef]
- Jimenez, J.A. Spectroscopic investigation of the influence of Cu+ ions and plasmonic Cu particles on Ho3+ luminescence in phosphate glass. Chem. Phys. 2021, 541, 111045. [Google Scholar] [CrossRef]
- Li, B.; Zhao, X.; Pun, E.Y.B.; Lin, H. Upconversion photon quantification of Ho3+ in highly transparent fluorotellurite glasses. Opt. Laser Technol. 2018, 107, 8–14. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Zmojda, J.; Miluski, P.; Baranowska, A.; Ragin, T.; Dorosz, J.; Kuwik, M.; Pisarski, W.A.; Pisarska, J.; Lesniak, M.; et al. 2 μm emission in gallo-germanate glasses and glass fibers co-doped with Yb3+/Ho3+ and Yb3+/Tm3+/Ho3. J. Lumin. 2019, 211, 341–346. [Google Scholar] [CrossRef]
- Sobczyk, M.; Marek, L. Comparative study of optical properties of Ho3+-doped RE2O3-Na2O-ZnO-TeO2 glasses. J. Lumin. 2019, 206, 308–318. [Google Scholar] [CrossRef]
- Wan, Z.; Li, W.; Mei, B.; Liu, Z.; Yang, Y. Fabrication and spectral properties of Ho-doped calcium fluoride transparent ceramics. J. Lumin. 2020, 223, 117188. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Zmojda, J.; Muluski, P.; Baranowska, A.; Kuwik, M.; Pisarski, W.A.; Pisarska, J.; Dorosz, J.; Dorosz, D. Sensitization of Ho3+-doped fluoroindate glasses for near and mid-infrared emission. Opt. Mater. 2020, 101, 109707. [Google Scholar] [CrossRef]
- Prasad, T.J.; Neelima, G.; Ravi, N.; Kiran, N.; Reddy, N.N.K.; Kummara, V.K.; Suresh, K.; Gadige, P. Optical and spectroscopic properties of Ho3+-doped fluorophosphate glasses for visible lighting applications. Mater. Res. Bull. 2020, 124, 110753. [Google Scholar]
- Rani, P.R.; Venkateshwarlu, M.; Swapna, K.; Mahamuda, S.K.; Prasad, M.V.V.K.S.; Rao, A.S. Spectroscopic and luminescence properties of Ho3+ ions doped Barium Lead AluminoFluoro Borate glasses for green laser applications. Solid. Stat. Sci. 2020, 102, 106175. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Miao, B.; Ma, Q. Thermal, nonlinear, magnetic and faraday rotation properties of sol-gel diamagnetic glass/NaYF4: Fe, Ho3+: Role of magnetic ions. J. Alloys Compd. 2021, 858, 157631. [Google Scholar] [CrossRef]
- Madhu, A.; Eraiah, B.; Manasa, P.; Srinatha, N. Nd3+-doped lanthanum lead boro-tellurite glass for lasing and amplification applications. Opt. Mater. 2018, 75, 357–366. [Google Scholar] [CrossRef]
- Hemalatha, S.; Nagaraja, M.; Madhu, A.; Suresh, K.; Srinatha, N. The role of Sm2O3 on the structural, optical and spectroscopic properties of multi-component ternary borate glasses for orange-red emission applications. J. Non Cryst. Solids 2021, 554, 120602. [Google Scholar] [CrossRef]
- James, J.T.; Jose, J.K.; Manjunatha, M.; Suresh, K.; Madhu, A. Structural, luminescence and NMR studies on Nd3+-doped sodium-calcium-borate glasses for lasing applications. Ceram. Inter. 2020, 46, 27099–27109. [Google Scholar] [CrossRef]
- Algradee, M.A.; Sultan, M.; Samir, O.M.; Alwany, A.E.B. Electronic polarizability, optical basicity and interaction parameter for Nd2O3 doped lithium-zinc-phosphate glasses. J. Appl. Phys. 2017, 6, 163–170. [Google Scholar]
- Nanda, K.; Berwal, N.; Kundu, R.S.; Punia, R.; Kishore, N. Effect of doping of Nd3+ ions in BaO-TeO2-B2O3 glasses: A vibrational and optical study. J. Mol. Struct. 2015, 1088, 147–154. [Google Scholar] [CrossRef]
- Tasheva, T.R.; Dimitrov, V.V. Synthesis, structure and nonlinear optical propertiesof tellurium oxide-bismuth oxide-boron oxide glasses. Bulg. Chem. Commun. 2017, 49, 43–48. [Google Scholar]
- Madhu, A.; Eraiaha, B.; Manasa, P.; Basavapoornima, C. Er3+-ions doped lithium-bismuth-boro-phosphate glass for 1532 nm emission and efficient red emission up conversion for telecommunication and lasing applications. J. Non Cryst. Solids 2018, 495, 35–46. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Goryczka, T.; Wodecka-Dus, B.; Plonska, M.; Pisarska, J. Structure and properties of rare earth-doped lead borate glasses. Mater. Sci. Eng. B 2005, 122, 94–99. [Google Scholar] [CrossRef]
- Madhu, A.; Eraiah, B. The structural studies of vanadium substituted lithium-bismuth-boro-tellurite glass. AIP Conf. Proc. 2018, 1953, 090056. [Google Scholar]
- Pisarska, J.; Kuwik, M.; Górny, A.; Kochanowicz, M.; Zmojda, J.; Dorosz, J.; Dorosz, D.; Sitarz, M.; Pisarski, W.A. Holmium doped barium gallo-germanate glasses for near-infrared luminescence at 2000 nm. J. Lumin. 2019, 215, 116625. [Google Scholar] [CrossRef]
- Jupri, S.A.; Ghoshal, S.K.; Omar, M.F.; Yusof, N.N. Spectroscopic traits of holmium in magnesium zinc sulfophosphate glass host: Judd-Ofelt evaluation. J. Alloys Compd. 2018, 753, 446–456. [Google Scholar] [CrossRef]
- Prasad, V.R.; Damodaraiah, S.; Ratnakaram, Y.C. Optical spectroscopy and luminescence properties of Ho3+ doped zinc fluorophosphate (ZFP) glasses for green luminescent device applications. Opt. Mater. 2018, 78, 63–71. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. A study of the optical bandgap energy and Urbach tail of spray-deposited CdS: In thin films. J. Mater. Res. Technol. 2013, 2, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovice, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Redfield, D. Effect of defect fields on the optical absorption edge. Phys. Rev. 1963, 130, 916. [Google Scholar] [CrossRef]
- Weinstein, I.A.; Zatsepin, A.F.; Kortov, V.S. Effects of structural disorder and Urbach’s rule in binary lead silicate glasses. J. Non Cryst. Solids 2001, 279, 77. [Google Scholar] [CrossRef]
- Fuxi, G. Optical and Spectroscopic Properties of Glass; Springer: Berlin/Heidelberg, Germany, 1962. [Google Scholar]
- Mahr, H. Ultraviolet Absorption of KI Diluted in KCl Crystals. Phys. Rev. 1962, 125, 1510–1516. [Google Scholar] [CrossRef]
- Schubert, F. Light-Emitting Diodes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; Chapter 17; p. 292. [Google Scholar]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Erdem, T.; SNizamoglu Sun, X.W.; Demir, H.V. A photometric investigation of ultra-efficient LEDs with high color rendering index and high luminous efficacy employing nanocrystal quantum dot luminophores. Opt. Express 2010, 18, 340–347. [Google Scholar] [CrossRef] [Green Version]





| Parameters | B0H | B01H | B03H | B05H |
|---|---|---|---|---|
| ρ | 4.041 (1) | 3.802 (1) | 3.873 (1) | 3.934 (1) |
| t | 1.58 (2) | 2.03 (3) | 1.48 (2) | 1.62 (1) |
| M.W | 133.444 (5) | 133.760 (5) | 134.392 (5) | 135.148 (5) |
| N | 1.651 (1) | 1.652 (1) | 1.653 (1) | 1.653 (1) |
| Vm | 33.022 (1) | 35.181 (1) | 34.699 (1) | 34.353 (1) |
| dB-B | 376.121 (1) | 383.905 (1) | 381.685 (1) | 380.000 (1) |
| Vp | 0.631 (1) | 0.592 (1) | 0.601 (1) | 0.607 (1) |
| OPD | 84.790 (1) | 79.586 (1) | 80.691 (1) | 81.504 (1) |
| CNav | 3.850 (6) | 3.853 (6) | 3.859 (6) | 3.871 (6) |
| nb | 0.702 (1) | 0.659 (1) | 0.669 (1) | 0.678 (1) |
| - | 0.351 (1) | 1.067 (1) | 1.800 (1) | |
| ri | - | 3.100 (5) | 2.140 (5) | 1.798 (5) |
| rp | - | 12.492 (3) | 8.628 (3) | 7.250 (3) |
| F | - | 1.922 (2) | 4.029 (2) | 5.707 (2) |
| Rm | 12.059 (1) | 12.863 (1) | 12.702 (1) | 12.576 (1) |
| αm | 4.780 (1) | 5.098 (1) | 5.035 (1) | 4.984 (1) |
| RL (%) | 6.030 (1) | 6.044 (1) | 6.058 (1) | 6.058 (1) |
| ℇ | 2.725 (3) | 2.729 (3) | 2.732 (3) | 2.732 (3) |
| T | 0.886 (1) | 0.886 (1) | 0.885 (1) | 0.885 (1) |
| ℇop | 1.725 (1) | 1.729 (1) | 1.732 (1) | 1.732 (1) |
| M | 0.634 (1) | 0.634 (1) | 0.633 (1) | 0.633 (1) |
| 1.706 (1) | 1.820 (1) | 1.798 (1) | 1.780 (1) | |
| Λ | 0.691 (1) | 0.752 (1) | 0.741 (1) | 0.731 (1) |
| Λth | 0.810 (4) | 0.808 (4) | 0.805 (4) | 0.804 (4) |
| Χ | 1.171 (2) | 1.327 (2) | 1.323 (2) | 1.322 (2) |
| χe | 0.137 (2) | 0.137 (2) | 0.137 (2) | 0.137 (2) |
| χ(3) × 10−14 | 3.555 (2) | 3.582 (2) | 3.610 (2) | 3.610 (2) |
| Samples | Indirect Band Gap (eV) (±0.01) | Urbach Energy (eV) (±0.01) | Steepness Parameter (±0.01) |
|---|---|---|---|
| B0H | 2.50 | 0.34 | 0.07 |
| B01H | 2.89 | 0.23 | 0.11 |
| B03H | 2.88 | 0.22 | 0.11 |
| B05H | 2.78 | 0.26 | 0.09 |
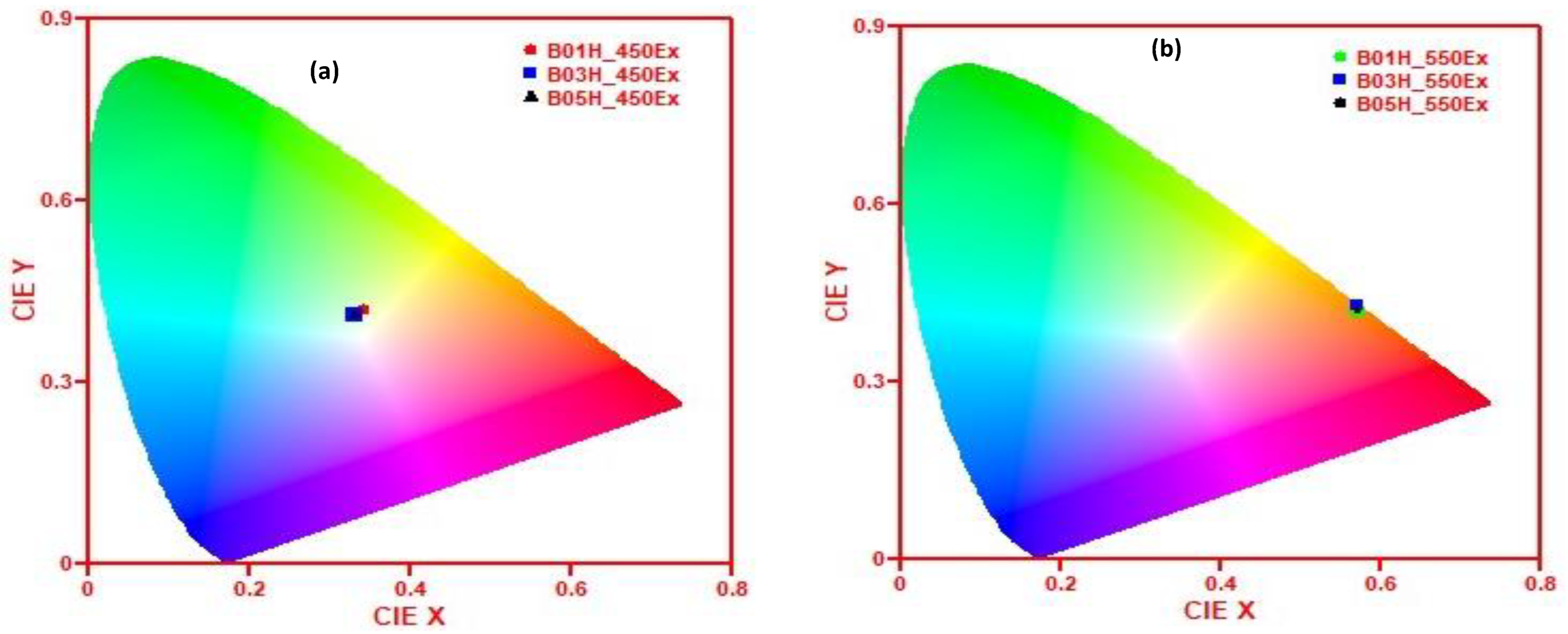
| Sample | Excitation Wavelength (nm) | (x, y) Co-Ordinates | Emission Colour | CCT (K) |
|---|---|---|---|---|
| B01H | 450 | (0.34, 0.42) | Yellowish white | 5287 (cool) |
| B03H | 450 | (0.33, 0.41) | Yellowish white | 5578 (cool) |
| B05H | 450 | (0.33, 0.41) | Yellowish white | 5578 (cool) |
| B01H | 550 | (0.57, 0.42) | Reddish-orange | 1805 |
| B03H | 550 | (0.57, 0.43) | Reddish-orange | 1846 |
| B05H | 550 | (0.58, 0.42) | Reddish-orange | 1771 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, S.A.; Ansari, M.O.; Alshahrie, A.; Shahadat, M.; Parveen, N.; Darwesh, R.; Aboushoushah, S.F. Concentration Dependent Improved Spectroscopic Characteristics and Near White Light Emission in Boro Phosphate Glasses Doped with Holmium. Appl. Sci. 2022, 12, 2632. https://doi.org/10.3390/app12052632
Ansari SA, Ansari MO, Alshahrie A, Shahadat M, Parveen N, Darwesh R, Aboushoushah SF. Concentration Dependent Improved Spectroscopic Characteristics and Near White Light Emission in Boro Phosphate Glasses Doped with Holmium. Applied Sciences. 2022; 12(5):2632. https://doi.org/10.3390/app12052632
Chicago/Turabian StyleAnsari, Sajid Ali, Mohammad Omaish Ansari, Ahmed Alshahrie, Mohammad Shahadat, Nazish Parveen, Reem Darwesh, and Samia Faisal Aboushoushah. 2022. "Concentration Dependent Improved Spectroscopic Characteristics and Near White Light Emission in Boro Phosphate Glasses Doped with Holmium" Applied Sciences 12, no. 5: 2632. https://doi.org/10.3390/app12052632
APA StyleAnsari, S. A., Ansari, M. O., Alshahrie, A., Shahadat, M., Parveen, N., Darwesh, R., & Aboushoushah, S. F. (2022). Concentration Dependent Improved Spectroscopic Characteristics and Near White Light Emission in Boro Phosphate Glasses Doped with Holmium. Applied Sciences, 12(5), 2632. https://doi.org/10.3390/app12052632







