Featured Application
Overview of lympho-epithelial cysts.
Abstract
Oral and cervical lympho-epthelial cysts (LECs) are uncommon lesions with histopathological similarities. The aim of the study is to present clinico-pathological characteristics of oral and cervical LECs with a review of literature in order to create awareness on this uncommon entity. Eighteen new cases of oral and cervical LECs obtained from the archives of the University of Peradeniya and University of Geneva were used for the clinico-pathological analysis. The average age at diagnosis of 7 oral and 11 cervical LECs were 40 and 36 years, respectively. Both showed a female predilection (male:female ratio at 3:4 and 4:7, respectively). The only difference was found in the size of the lesions with oral LECs being significantly smaller than cervical LECs (0.9 cm vs. 4.6 cm). LECs may clinically resemble neoplasms (4/18), including malignancies (1/11 in the present series). None of the 18 LECs recurred following surgical removal. The literature-review-based analysis of 514 oral LECs confirms that the lesions are observed predominantly in adults in 4th–5th decades of life and are relatively small lesions of less than 2 cm in diameter. Oral LECs were found to occur predominantly in the tongue and floor of the mouth, similar to 88% of lesions in literature. In conclusion, oral and cervical LECs are two histologically similar cysts that occur in two distinct sites. The literature review supports the information observed in our study with respect to age of occurrence, site predilections, and size. Cervical LECs, particularly the ones that occur in the parotid region, may require further investigations to exclude BLEL of parotid gland, which occur in HIV infected patients.
1. Introduction
The lympho-epithelial cysts (LECs) may occur within the oral cavity or in the cervical region. Irrespective of the site of occurrence, both show similar histopathology [1,2,3,4]. The cervical LECs were initially referred to as Branchial cysts or Branchial cleft cysts [5,6,7]. However, as the origin of these cysts remain unclear, current terms based on referring to site of occurrence and histopathological presentation seem more appropriate.
The oral LEC is a rare asymptomatic lesion that appears as a whitish or yellowish sub-mucosal nodule that is generally smaller than 1 centimeter and mobile on palpation. It is found mainly on the floor of the mouth and the lateral border of the tongue. In addition, it can also develop in various intraoral locations such as the buccal vestibule and also the soft palate [4]. Two main theories have been proposed concerning the pathogenesis of the LEC. Knapp’s theory [1] suggests that the obstruction of lymphoid crypts induces dilatation and cystic proliferation of the epithelium. Buchner and Hansen’s [2] and Bhaskar’s theories [3] assume that cystic proliferation occurs from epithelial cells that persist in lymphoid tissue during embryogenesis. However, both theories fail to explain how oral LECs occur in sites without lymphoid tissue.
The prevalence of oral LECs is difficult to assess because a large number of cases are probably not histopathologically diagnosed, as they are asymptomatic. According to Yang [4], the age of onset is about 45 years with a female predilection (2:1). The clinical diagnosis of the oral LEC is not obvious. It may be confused with an oral mucocele, a lipoma, and possibly a tumor of the minor salivary glands, but never as a malignancy.
The literature indicates that cervical LECs, on the other hand, appear late in childhood or in young adults, generally as a unilateral asymptomatic mass of slow evolution, localized in the anterior triangle of the neck in the superior third of sternocleidomastoid muscle [5,6,7]. The hypotheses of etiopathogenesis of the cervical LECs indicate that they may arise as a result of an anomaly of fusion of the branchial arches [5], a cystic change of thymic origin [6], or a cystic change in cervical lymph nodes [7], of which the first origin is more widely accepted [5]. In support of the above conclusion, it can be stated that branchial anomalies are frequent and represent about 17% of the cervical masses in pediatric consultations [8]. These abnormalities include cervical lympho-epithelial cysts, but also sinuses and fistulae.
Regarding gender distribution, Agaton-Bonilla et al. [9] and Ford et al. [10] have observed more cases in women than in men, while Gaszynska et al. [11] observed the opposite. McNealy et al. have observed dominance on the right side [6] and Papadogeorgakis et al. on the left side [12]. Literature reveals a wide range of clinical differential diagnoses for cervical LECs, including infections such as tuberculous lymphadenitis, and benign and malignant neoplasms including lipoma, neurofibroma, haemangioma, lymphangioma, carotid body tumours, metastatic neoplasms, lymphomas, teratoma, and parotid neoplasms [13], which may require different management strategies. The cervical LEC is generally asymptomatic. Nevertheless, during super infection, the patient may complain of dysphagia, dyspnea, cough, and dysphonia, depending on the size and location of the cyst.
Accurate histopathological diagnosis is necessary to ensure proper treatment. The treatment of both oral and cervical LEC is surgical excision [4,9,10,11,12,13,14,15]. The aim of the present study is to give a clinico-pathological analysis of 18 new cases of LECs. Our data were compared with those of the literature in order to get a better understanding of the oral and cervical LECs so as to improve the clinical and histopathological diagnoses. The rationale for conducting the study was to highlight the comparison between oral and cervical LEC. None of the clinicopathological studies reviewed contained a comparison between oral and cervical LEC. Authors found it relevant to compare the clinicopathological features of the two entities as these are two lesions that share similar histopathological features but occur at different locations. Comparison was also undertaken to evaluate if there are any striking clinico-pathological differences between the entities other than the location.
2. Materials and Methods
Seven cases of oral LECs were recorded, two at the Unit of Medicine and Oral Pathology at the University Hospitals of Geneva and five at the University of Peradeniya, Sri-Lanka. The 11 cases of cervical LECs come from the University of Peradeniya. Cases were selected on the basis of histological diagnosis. Inclusion criteria were the definitive histological diagnosis of oral or cervical LEC and the adequate clinical information available in respective data bases. The cases were excluded in the event that there was inadequate tissue for new H&E sections.
The preparation of the histological sections was performed with the standard haematoxylin-eosin staining to confirm the diagnoses.
The Ethical Committee of the University Hospitals of Geneva, Geneva, Switzerland, approved the protocol (ID 2017-00015), as well as the ERC of the Faculty of Dental Sciences, Sri-Lanka (ERC/FDC/UOP/I/2016/40).
Clinical and medical history data such as age, sex, location, time since the onset of lesion, size, consistency, and presumptive diagnosis were noted.
The literature review was conducted as follows: an initial search string was carried out to identify articles dealing with LEC with the following keywords: [oral AND lymphoepithelial AND cyst], [intraoral AND lymphoepithelial AND cyst], using several search engines including Google Scholar, Medscape, and Pubmed. Articles published from 1966 to 2021 were included. A total of 154 publications were presented directly with the keywords. Abstracts that were present in English were reviewed. Forty-eight non-relevant articles that dealt with lymphoepithelial carcinoma and lymphoepithelial sialadenitis were excluded initially. Thereafter, lymphoepithelial cysts that were associated with parotid glands and studies that dealt with clinico-pathological presentations of cysts other than LEC were excluded. Finally, a total of 10 articles that analyzed clinico-pathological presentations of at least 05 LEC were identified to present the literature review based presentations of LEC [2,3,4,16,17,18,19,20,21,22]. Four hundred and seventy-nine oral LEC were assessed in these studies. In addition, a further 29 articles describing the clinico-pathological presentations of 35 oral LEC are presented in the Supplementary Materials Table S1 [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
3. Results
Table 1 and Table 2 show the clinical data of oral and cervical LEC. Table 3 shows a summary of clinico-pathological information of both LECs. All oral LECs were asymptomatic lesions, while a few cervical LECs (2/11) presented with features of inflammation such as pain. Of them, 43% and 45% of oral and cervical LECs had occurred by the 3rd decade of life, although the average ages of occurrence for the two lesions were 40 and 36 years, respectively. Both lesions showed a female predilection. Oral LECs were present from 1 to 36 months at the time of consultation, while patients with cervical LECs waited between 8 days and 3.5 years to consult a clinician with lesions of 2 to 8 centimeters in size. The oral LECs were nodular, soft, or firm on palpation and varied in size from 0.2 to 1.5 centimeters with an average of 0.9 centimeters and were mostly on the floor of the mouth 42% (3), but also on other sites: ventral surface of the tongue (1), buccal mucosa (1), tonsillar fossa (1), and soft palate (1) (Figure 1).

Table 1.
Clinical data of the seven oral LEC.

Table 2.
Clinical data of the 11 cervical LEC.

Table 3.
Summary of clinicopathological data of oral and cervical LEC.
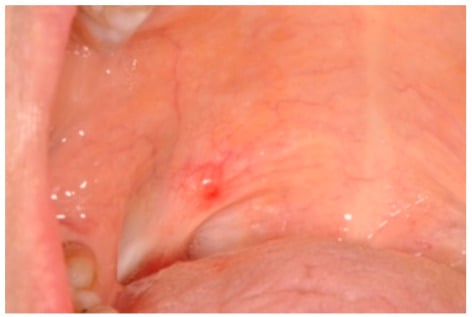
Figure 1.
Clinical view of a palatal lympho-epithelial cyst.
A statistical analysis with Fisher’s exact test considering p < 0.05 as significant revealed that there were no significant differences with respect to age, gender, and the time duration taken to seek a consultation when oral and cervical LEC were compared. However, there were significant differences with respect to the size of the lesion with oral LEC always presenting significantly smaller than cervical LEC (p = 0.000). Invariably, there were statistically significant differences with respect to the site of occurrence with oral LEC showing predilection to the tongue/floor of the mouth and cervical LEC occurring in the region of the parotid or upper part of the neck (p = 0.000).
The different presumptive diagnoses proposed by clinicians for oral LECs included mucocele (2), lipoma (1), fibroma (1), fibro-epithelial lesion (1), and tumor of the accessory salivary glands (1). In contrast, the majority of cervical LECs came with a presumptive diagnosis of branchial cyst (6). However, in two cases the presumptive diagnosis was neoplasms, such as pleomorphic adenoma and lymphoma. Significantly more often, the clinical diagnosis was correct in the case of cervical LEC, but never with respect to oral LEC (p = 0.02). As LECs may clinically and histopathologically mimic neoplasms, awareness among clinicians and pathologists regarding this uncommon entity is important to avoid over-diagnosis and treatment.
The histopathological sections of all oral LECs showed a parakeratinized stratified squamous epithelium, while two cervical LECs were lined by non-keratinized stratified squamous epithelium. In a single oral LEC, the cystic epithelium was seen merging with the surface epithelium. The cystic cavity contained keratin squames and mucinous material, with infected lesions containing neutrophils and macrophages. The pericystic connective tissue contained a dense, chronic inflammatory cell infiltration, predominantly lymphocytic with the presence of one or more germinal centers (Figure 2). None of the cervical LEC with the site of occurrence indicated as the parotid were histopathologically identified to arise within salivary glands and, therefore, the HIV statuses of the patients were not evaluated (Table 2).
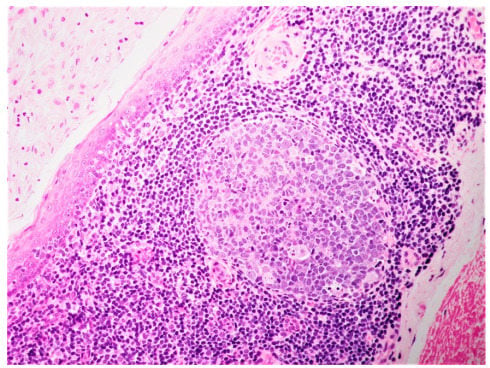
Figure 2.
Histological aspect showing epithelial lining, predominantly lymphocytes, with the presence of one germinal center in the cyst wall (H&S ×20).
The surgical resection using a scalpel was performed for each lesion and no recurrences occurred to the best of our knowledge. For cervical LEC, a series of horizontal incisions, known as a stepladder incision, was made to dissect out the lesion. Any preexisting infection was resolved by antibiotics or incision and drainage before surgical treatment was attempted. In the case of oral LEC, complete enucleation of the cyst under local anesthesia was done. According to follow-up information, oral and cervical LECs were indolent lesions that do not in any way contribute to patient survival.
Table 4 [2,3,4,16,17,18,19,20,21,22] presents a summary of case series dealing with more than five LECs. Case reports dealing with single or two lesions amounting to 35 oral LEC are summarized under the Supplementary Materials Table S1 [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], while the summary is included in Table 4 last row.

Table 4.
Literature-review-based summary of 514 oral LEC.
A literature review based on 514 cases of oral LEC revealed a mean age of 38 years and a female-to-male ratio of 1:1.1. The majority of oral LEC were found to develop in the tongue and floor of the mouth (Table 4). In addition, similar to oral LEC presented, the literature also indicates that they are small lesions that generally do not exceed more than 2 cm [2,3,4,16,17,18,19,20,21,22].
4. Discussion
Of the seven cases of oral LECs studied, we note an average of 40 years (Table 1 and Table 3) compared to 38 years reported according to the literature (Table 4). Cervical LECs were diagnosed at an average age of 36 years, which is slightly above the literature [8,12]. The ratio of female:male for oral LECs is 1.33 (four females to three males), according to our cases (Table 3), and 0.9 (255:258) in the literature (Table 4). Though the majority of studies analyzed reveals female predilection [2,16,17,18,20,21], one large series of cases from China [4] skewed the general trend, resulting in a slight male predilection. The preferred location of the oral LEC is the tongue and floor of the mouth (Table 4), where three (42%) of our cases occurred, followed by single lesion each at the palate, ventral tongue, and tonsillar fossa. A similar trend was seen in the literature, as well, with respect to oral LEC, with 81% of the total lesions occurring in either the tongue or floor of the mouth (Table 4).
The cervical LECs occur in a great majority of cases in the superior anterior triangle of the neck [15] and parotid region [53]. According to Ford et al. [10], they can be found in all the territory arising from the branchial arches. LECs that arise in the parotid region are unique as they have been shown to be associated with HIV infection and are also described as benign lymphoepithelial lesions (BLEL). However, none of the cases described by Wu et al. [53] were found to harbour HIV or EBV infection. Even so, unlike oral and cervical LEC, similar lesions in the parotid would require investigation to exclude HIV, as the management of such lesions is not purely surgical [54].
Presumptive diagnoses of the seven oral LECs were similar to those in the literature [4]. Considering the rarity of the lesion, none of the oral LECs were clinically diagnosed as such. In addition, bearing in mind the inconspicuous nature of the lesion, an inaccurate clinical diagnosis would not hinder the overall management of oral LEC. Presumptive diagnosis of cervical LEC is similar to that found in the literature as well [13]. Unlike oral LECs, clinicians proposed the diagnosis of branchial cyst for majority of patients. However, considering that two lesions were clinically suspected as neoplasms and one carried the clinical diagnosis of lymphoma, the chance of over-treatment is higher with cervical LECs compared to oral LECs. Although we do not have data on special investigations that have been used, it is possible that an MRI, a CT-scan, or an ultrasound would refine the diagnosis of presumption. Ultrasound scanning is the first-line method of imaging choice, as the lesions are situated superficially. It will be useful to determine if there are any fistula connecting different organs, such as throat and ear. On the other hand, it is recommended to perform CT or MRI to determine the exact location of the cyst.
The gold-standard treatment for both lesions is surgical excision. No recurrence is report in the literature for oral LECs [4,17]. The risk of recurrence of cervical LECs is 0–22% according to the studies [8,10,11,12].
The etiopathogenesis of two of the oral LECs described in the present study is compatible with Knapp’s hypothesis [1]. Indeed, one of the histological sections presented a cystic epithelium in continuity with the surface epithelium. An obstruction of the canal and a cystic proliferation of the latter can be envisaged.
Histological analysis of cervical LEC is similar to that of oral LEC. Cervical LECs have a stratified squamous epithelium, keratinized or non-keratinized, but may also be related to a respiratory epithelium [7]. The cystic epithelium is separated from the underlying corium by a basal lamina [7]. The connective tissue is the seat of an important inflammatory infiltrate, predominantly lymphocytic, organized in a diffuse band or arranged in germinal centers [18]. However, unlike in oral LEC, histopathological diagnosis is more difficult in cervical LEC because of mimics such as cystic metastasis of oral cancer, or HPV-induced oropharyngeal cancer, thymic cysts, and salivary lympho-epithelial cysts of HIV-positive individuals [55].
Limitations of the study include the fact that no information could be identified to convincingly support the theory of pathogenesis of all oral LEC, and long-term follow-up was not available for most of the cases to determine the outcome. Strengths include the fact that clinicopathological comparison reliably identified some differences between oral and cervical LEC, such as the size of the lesion. In addition, it was also identified that inflamed cervical LEC may mimic malignant lesions and Clinicians should be careful when deciding on definitive treatment based on clinical diagnoses.
Implications: oral LEC were confirmed to be indolent lesions that are generally not related to the HIV status of the patient. Clinicians should be aware that inflamed cervical LEC has a higher chance of getting misdiagnosed as a malignant lesion.
5. Conclusions
Oral and cervical LECs are two histologically similar cysts that occur in two distinct sites. The main difference between the two lesions was identified as the size of the lesion with oral LEC presenting as significantly smaller lesions compared to cervical LEC. The literature review supports the information observed in our study with respect to age of occurrence, site predilections, and size. Cervical LECs, particularly the ones that occur in the parotid region, may require further investigations to exclude BLEL of parotid.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app12052525/s1, Table S1—Literature review based summary of case reports of LEC.
Author Contributions
L.T.: literature review, initial draft of the manuscript; P.R.J.: draft of the manuscript, critical review; U.W.A.M.L.A.: preparation of images; R.B.R.N.M.; critical review of the manuscript; T.L.: conception, critical review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethical Committee of the University Hospitals of Geneva, Geneva, Switzerland, approved the protocol (ID 2017-00015), as well as the ERC of the Faculty of Dental Sciences, Sri-Lanka (ERC/FDC/UOP/I/2016/40).
Informed Consent Statement
Patient consent was obtained at the time of taking biopsies (incisional/excisional) for diagnostic purposes so that the material could be used for research following deidentification.
Data Availability Statement
The reported cases can be found at the archives of the Department of Oral Pathology, University of Peradeniya and the Unit of Oral Medicine and Oral & Maxillofacial Pathology, University of Geneva.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knapp, M.J. Pathology of oral tonsils. Oral Surg. Oral Med. Oral Pathol. 1970, 29, 295–304. [Google Scholar] [CrossRef]
- Buchner, A.; Hansen, L.S. Lymphoepithelial cysts of the oral cavity: A clinicopathologic study of thirty-eight cases. Oral Surg. Oral Med. Oral Pathol. 1980, 50, 441–449. [Google Scholar] [CrossRef]
- Bhaskar, S.N. Lymphoepithelial cysts of the oral cavity. Report of twenty-four cases. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 120–128. [Google Scholar] [CrossRef]
- Yang, X.; Ow, A.; Zhang, C.P.; Wang, L.Z.; Yang, W.J.; Hu, Y.J.; Zhong, L.P. Clinical analysis of 120 cases of intraoral lymphoepithelial cyst. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Ascherson, G.M. De Fistulis Colli Congenitis Fissuratum Branchialium. In Mammalibus Avibusque Historica Succincta; 1832; pp. 1–21. Available online: https://iiif.wellcomecollection.org/pdf/b22372891 (accessed on 30 December 2021).
- McNealy, R.W. Cystic tumors of the neck: Branchial and thyroglossal cysts. J. Am. Dent. Assoc. 1952, 29, 1808–1818. [Google Scholar] [CrossRef]
- Bhaskar, S.N.; Bernier, J.L. Histogenesis of Branchial Cysts: A Report of 468 cases. Am. J. Pathol. 1959, 35, 407–423. [Google Scholar]
- Choi, S.S.; Zalzal, G.H. Branchial anomalies: A review of 52 cases. Laryngoscope 1995, 105, 909–913. [Google Scholar] [CrossRef]
- Agaton-Bonilla, F.C.; Gay-Escoda, C. Diagnosis and treatment of branchial cleft cysts and fistulae. A retrospective study of 183 patients. Int. J. Oral Maxillofac. Surg. 1996, 25, 449–452. [Google Scholar] [CrossRef]
- Ford, G.; Balakrishnan, A.; Evans, J.; Bailey, C. Branchial cleft and pouch anomalies. J. Laryngol. Otol. 1992, 106, 137–143. [Google Scholar] [CrossRef]
- Gaszynska, E.; Gaszynski, T.; Arkuszewski, P. Diagnosis and Treatment of Cervical Branchial Cleft Cyst based on the Material from the Department of Cranio-Maxillofacial Surgery, Medical University in Lodz and Literature Review. Pol. J. Surg. 2013, 84, 547–550. Available online: https://europepmc.org/article/med/23399617 (accessed on 30 December 2021).
- Papadogeorgakis, N.; Petsinis, V.; Parara, E.; Papaspyrou, K.; Goutzanis, L.; Alexandridis, C. Branchial cleft cysts in adults. Diagnostic procedures and treatment in a series of 18 cases. Oral Maxillofac. Surg. 2009, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Glosser, J.W.; Pires, C.A.S.; Feinberg, S.E. Branchial cleft or cervical lymphoepithelial cysts etiology and management. J. Am. Dent. Assoc. 2003, 134, 81–86. [Google Scholar] [CrossRef]
- Thomaidis, V.; Seretis, K.; Tamiolakis, D.; Papadopoulos, N.; Tsamis, I. Branchial cyst: A report of 4 cases. Acta Dermatovenerol. Alp. Pannonica Adriat. 2006, 15, 85–89. [Google Scholar] [PubMed]
- Panchbhai, A.S.; Choudhary, M.S. Branchial cleft cyst at an unusual location: A rare case with a brief review. Dentomaxillofac. Radiol. 2012, 41, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.L.S.; Roza, A.L.O.C.; Cruz, V.M.S.; Ribeiro, J.L.; Cavalcante, I.L.; Cavalcante, R.B.; Anbinder, A.L.; Abrahão, A.C.; de Andrade, B.A.B.; Romañach, M.J.; et al. Oral Lymphoepithelial Cyst: A Collaborative Clinicopathologic Study of 132 Cases from Brazil. Head Neck Pathol. 2021. [Google Scholar] [CrossRef]
- da Silva, K.D.; Coelho, L.V.; do Couto, A.M.; de Aguiar, M.C.F.; Tarquínio, S.B.C.; Gomes, A.P.N.; Mendonça, E.F.; Batista, A.C.; Nonaka, C.F.W.; de Sena, L.S.B.; et al. Clinicopathological and immunohistochemical features of the oral lymphoepithelial cyst: A multicenter study. J. Oral Pathol. Med. 2020, 49, 219–226. [Google Scholar] [CrossRef]
- Sykara, M.; Ntovas, P.; Kalogirou, E.-M.; Tosios, K.I.; Sklavounou, A. Oral lymphoepithelial cyst: A clinicopathological study of 26 cases and review of the literature. J. Clin. Exp. Dent. 2017, 9, e1035–e1043. [Google Scholar] [CrossRef]
- Chaudhry, A.P.; Yamane, G.M.; Scharlock, E.; Sunderraj, M.; Jain, R. A clinico-pathological study of intraoral lymphoepithelial cysts. J. Oral Med. 1984, 39, 79–84. [Google Scholar]
- Toto, P.D.; Wortel, J.P.; Joseph, G. Lymphoepithelial cysts and associated immunoglobulins. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 59–65. [Google Scholar] [CrossRef]
- Giunta, J.; Cataldo, E. Lymphoepithelial cysts of the oral mucosa. Oral Surg. Oral Med. Oral Pathol. 1973, 35, 77–84. [Google Scholar] [CrossRef]
- Acevedo, A.; Nelson, J.F. Lymphoepithelial cysts of the oral cavity. Report of nine cases. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 632–636. [Google Scholar] [CrossRef]
- Tanaka, S.; Okada, H.; Akimoto, Y.; Yamamoto, H.; Shibutani, J.; Hikiji, A. Lymphoepithelial cyst of the palatine tonsil. Asian J. Oral Maxillofac. Surg. 2004, 16, 123–125. [Google Scholar] [CrossRef]
- Stramandinoli-Zanicotti, R.T.; de Castro Avila, L.F.; Santos de Azevedo Izidoro, A.C.; Alves Izidoro, F.; Schussel, J.L. Lymphoepithelial cysts of oral mucosa: Two cases in different regions. Bull. Tokyo Dent. Coll. 2012, 53, 17–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castro, J.G.L.; Ferreira, G.M.; Mendonça, E.F.; Castro, L.A. A rare occurrence of lymphoepithelial cyst in the palatine tonsil: A case report and discussion of the etiopathogenesis. Int. J. Clin. Exp. Pathol. 2015, 8, 4264–4268. [Google Scholar]
- Sakoda, A.; Kodama, Y.; Shiba, R. Lymphoepithelial cyst of oral cavity: Report of a case and review of the literature. Int. J. Oral Surg. 1983, 12, 127–131. [Google Scholar] [CrossRef]
- Khelemsky, R.; Mandel, L. Lymphoepithelial cyst of the mouth floor. J. Oral Maxillofac. Surg. 2010, 68, 3055–3057. [Google Scholar] [CrossRef]
- Bingöl, F.; Balta, H.; Bingöl, B.O.; Mazlumoglu, R.M.; Kilic, K. Lymphoepithelial cyst in the palatine tonsil. Case Rep. Otolaryngol. 2016, 2016, 6296840. [Google Scholar] [CrossRef]
- Ahamed, A.S.; Kannan, V.S.; Velaven, K.; Sathyanaranayan, G.R.; Roshni, J.; Elavarasi, E. Lymphoepithelial cyst of the submandibular gland. J. Pharm. Bioall. Sci. 2014, 6 (Suppl. S1), 185–187. [Google Scholar] [CrossRef]
- Lopez-Jornet, P. Kyste lympho-épithélial buccal. Ann. Derm. Venereol. 2007, 134, 588. [Google Scholar] [CrossRef]
- Gold, C.; Levittown, N.J. Branchial cleft cyst located in the floor of the mouth. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 1118–1120. [Google Scholar] [CrossRef]
- Vickers, R.A.; Gorlin, R.J.; Smart, E.A. Lymphoepithelial lesions of the oral cavity. Report of four cases. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 1214–1222. [Google Scholar] [CrossRef]
- Calman, H.I. Sublingual branchiogenic cyst. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 333–338. [Google Scholar] [CrossRef]
- Young, W.G.; Claman, S.M. A lymphoepithelial cyst of the oral cavity. Oral Surg. Oral Med. Oral Pathol. 1967, 23, 62–70. [Google Scholar] [CrossRef]
- Merchant, N.E. Lympho-epithelial cyst of the floor of the mouth. A case report. Br. Dent. J. 1972, 132, 271–272. [Google Scholar] [CrossRef]
- Epivatianos, A.; Zaraboukas, T.; Antoniades, D. Coexistence of lymphoepithelial and dermoid cysts on the floor of the mouth: Report of a case. Oral Dis. 2005, 11, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Baba, S.; Hashimoto, K. Lymphoepithelial Cyst in the Sublingual Region: Report of a case and review of litterature. Oral Med. Pathol. 2000, 5, 105–108. [Google Scholar] [CrossRef][Green Version]
- Iwase, T.; Teratani, K.; Saito, A.; Funatsu, K.; Sato, M.; Kiuchi, K.; Umemura, S. Immunohistochemical and Lectin Histochemical Studies on the Lymphoepithelial Cyst of the Oral Cavity and Neck. J. Nihon Univ. Sch. Dent. 1985, 27, 28–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, J.; Kim, K.; Kim, B. Lymphoepithelial cyst of the palatine tonsil. Ear Nose Throat J. 2010, 89, 584–585. [Google Scholar] [PubMed]
- Yamamoto, H.; Fukumoto, M.; Matsumoto, T.; Gunge, M.; Otake, S. An Immunohistochemical study of a lymphoepithelial cyst occurring on the ventral surface of the tongue. J. Nihon Univ. Sch. Dent. 1987, 29, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Won, J.H.; Lee, S.H.; Choi, E.H.; Choi, S.I. Lymphoepithelial cyst associated with epithelial inclusion cyst. Am. J. Dermatopathol. 1996, 18, 424–426. [Google Scholar] [CrossRef]
- Flaitz, C.M. Oral lymphoepithelial cyst in a young child. Pediatric Dent. 2000, 22, 422–423. [Google Scholar]
- Kumara, G.R.; Gillgrass, T.J.; Bridgman, J.B. A lymphoepithelial cyst (branchial cyst) in the floor of the mouth. N. Z. Dent. J. 1995, 91, 14–15. [Google Scholar]
- Chaves, F.N.; Feitosa, S.G.; Pereira, K.M.; Costa, F.W. Multiple lymphoepithelial cysts from oral mucosa of a healthy woman: Case report and literature review. Indian J. Pathol. Microbiol. 2013, 56, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.L.; Weisinger, E.; Manhold, J.H., Jr. Benign lymphoid lesions of oral mucosa. Preliminary classification. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1970, 29, 31–37. [Google Scholar] [CrossRef]
- Flaitz, C.M.; Davis, S.E. Oral and maxillofacial pathology case of the month. Oral lymphoepithelial cyst. Tex. Dent. J. 2004, 121, 624–631. [Google Scholar]
- Pereira, K.M.; Nonaka, C.F.; Santos, P.P.; Medeiros, A.M.; Galvao, H.C. Unusual coexistence of oral lymphoepithelial cyst and benign migratory glossitis. Braz. J. Otorhinolaryngol. 2009, 75, 318. [Google Scholar] [CrossRef]
- Costa, F.W.; Pereira, K.M.; Viana, T.S.; Cavalcante, R.B.; Nogueira, A.S. Simultaneous occurrence of a rare lymphoepithelial cyst and squamous cell carcinoma in the oral cavity. Braz. J. Otorhinolaryngol. 2011, 77, 270. [Google Scholar] [CrossRef]
- Silva, M.M.; Castro, A.L.; Soubhia, A.M.P.; Crivelini, M.M. Lymphoepithelial cyst in jugal mucosa. Int. J. Odontostomat. 2011, 5, 55–58. [Google Scholar]
- de Sousa, L.M.; Albuquerque, A.F.; Silva, P.G.; Bezerra, T.M.; Luna, E.C.; Chaves, F.N.; Carvalho, F.S.; Pereira, K.M.; Alves, A.P.; Ribeiro, T.R.; et al. Unusual occurrence of tongue sensorial disorder after conservative surgical treatment of lymphoepithelial cyst. Case Rep. Dent. 2015, 2015, 352463. [Google Scholar] [CrossRef] [PubMed]
- Marçal, V.E.M.; Palma, V.C.; Semenoff, T.A.D.V.; Aranha, A.M.F.; Filho, F.B.; Borges, A.H. Lymphoepithelial cyst in the palatoglossus arch. Rev. Cuba. Estomatol. 2012, 49, 335–340. [Google Scholar]
- Silva, I.H.; Romanach, M.J.; Carvalho, A.T.; de Almeida, O.P.; Leao, J.C.; Gueiros, L.A. Bilateral lymphoepithelial cyst of the tongue: A case report. Gen. Dent. 2013, 61, 32–34. [Google Scholar] [PubMed]
- Wu, L.; Cheng, J.; Maruyama, S.; Yamazaki, M.; Tsuneki, M.; Lu, Y.; He, Z.; Zheng, Y.; Zhou, Z.; Saku, T. Lymphoepithelial cyst of the parotid gland: Its possible histopathogenesis based on clinicopathologic analysis of 64 cases. Hum. Pathol. 2009, 40, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Mourad, W.F.; Young, R.; Kabarriti, R.; Blakaj, D.M.; Shourbaji, R.A.; Glanzman, J.; Patel, S.; Ohri, N.; Yaparpalvi, R.; Beitler, J.J.; et al. 25-year follow-up of, H.I.V.-positive patients with benign lymphoepithelial cysts of the parotid glands: A retrospective review. Anticancer Res. 2013, 33, 4927–4932. [Google Scholar] [PubMed]
- Odell, E.W.; Morgan, P.R. Chapter 11—Non Odontogenic Cysts. In Biopsy Pathology of Oral Tissues; Chapman and Hall Medical: New York, NY, USA, 1998; pp. 319–321. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).