Long Term Trends of Base Cation Budgets of Forests in the UK to Inform Sustainable Harvesting Practices
Abstract
1. Introduction
2. Methodology
2.1. Site Details: ICP Forest Level II Intensive Monitoring Sites
2.2. Base Cation Balance Calculations
2.3. Inputs: Base Cation Deposition
2.4. Input: Base Cation Weathering
2.5. Input: Pre-Existing Vegetation
2.6. Input: Fertiliser
2.7. Outputs: Base Cation Leaching
2.8. Outputs: Base Cation Uptake by Trees
2.9. Harvesting Scenarios
3. Results
3.1. Site-Specific Base Cation Budgets
3.2. Temporal Changes in Base Cation Budgets
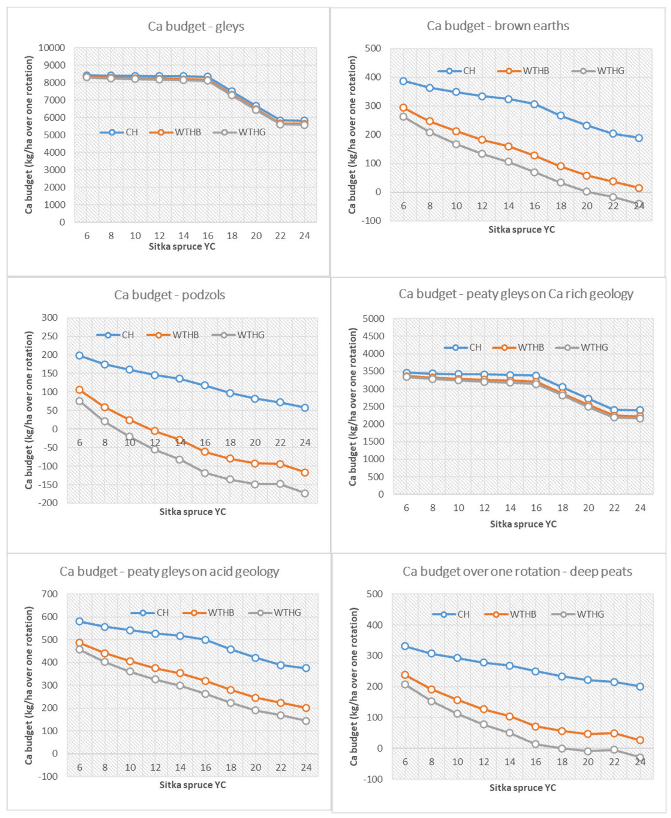
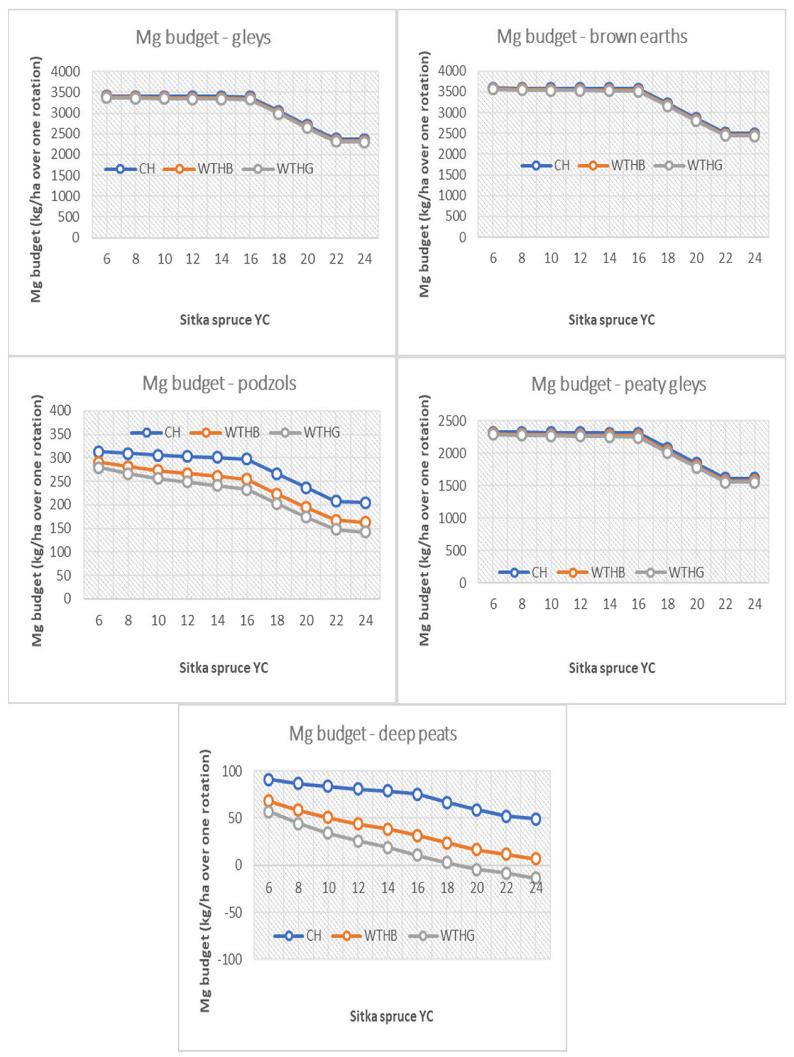
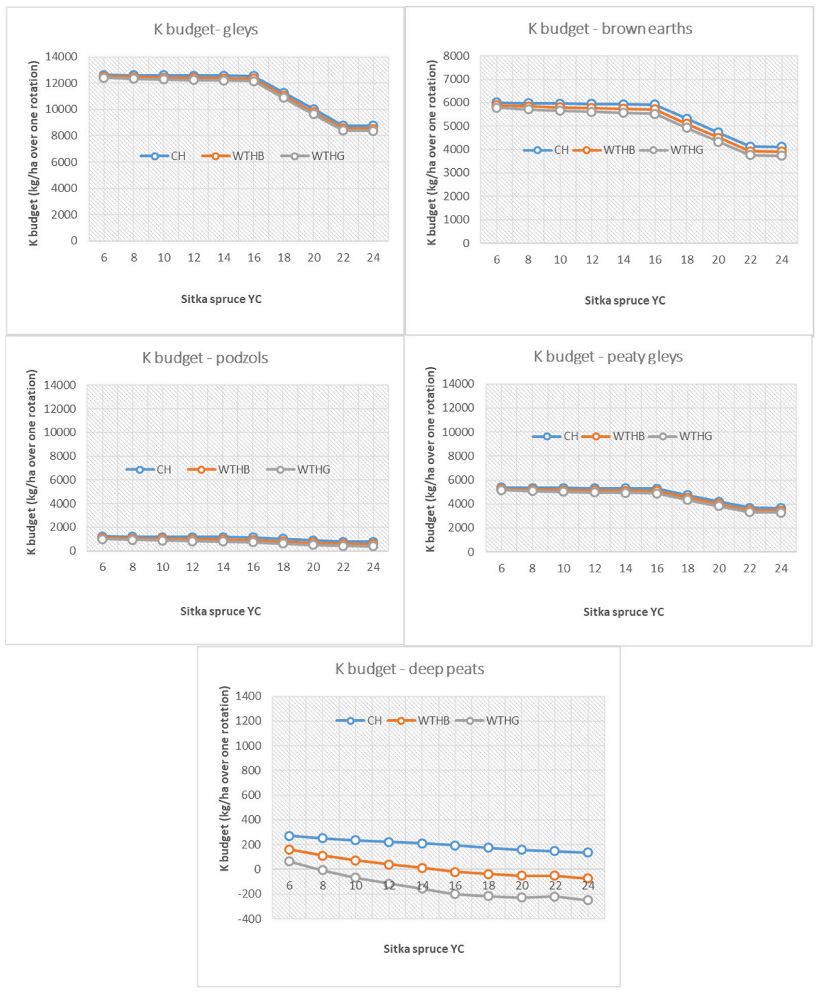
3.3. Base Cation Harvesting Scenarios
4. Discussion
4.1. Base Cation Inputs and Outputs in Forest Ecosystems
4.2. Forest Base Cation Budget
4.3. Long Term Changes in Base Cation Budgets
4.4. Biomass Removal Decision Support Matrix for Nutrient Sustainability
- (1)
- The base cation budgets are limited to first rotation Sitka spruce and will change for subsequent rotations, most notably in terms of the loss of the base cation input from the pre-forest vegetation. This will make the budgets less positive or more negative, although the deficit could be at least partly offset by greater soil weathering and/or organic matter mineralisation due to restock cultivation, provided these rates are sustainable.
- (2)
- The calculated base cation budgets are imprecise and subject to significant uncertainty, especially when applied to individual sites. Consequently, the decision matrix should be used with care and it is recommended that its application is monitored and reviewed to inform future assessments and refinement of the practice. One issue is the estimation of soil mineral weathering, which tends to dominate base cation inputs. Although several methods were used to estimate this key component, they all have high uncertainties [35]. Others found variability in weathering estimates to be too high to set thresholds for harvesting practice since the increase in base cation export due to residue harvesting was considerably smaller than the uncertainties associated with mineral weathering estimates [66]. A similar conclusion was reached by [65] in Ireland, where the high uncertainties prevented the definition of clear boundaries for separating the impact of harvest residue removal from that of CH on the base cation balance.
- (3)
- Another issue concerns the calculated water fluxes used to estimate base cation losses by leaching, which was based on modelled potential rather than measured actual evapotranspiration. It has been shown [67] that actual evapotranspiration by forests can be 20% lower than potential rates estimated using the Penman–Monteith equation. This suggests that our base cation losses through leaching may have been underestimated and the budget overestimated, especially on the more drought-prone sites. The error is unlikely to be large enough to turn the long-term base cation balance for any site from positive to negative (except for Mg in the case of Rogate; Table 3 and Table 4) but will make the values more negative for the most sensitive sites. This is likely to reduce the Yield Class threshold for sustainable harvesting of brown or green brash by one or two classes for the highlighted soil types. Application of a full water balance model to calculate actual evapotranspiration losses is recommended to improve the quantification of base cation leaching and resulting budgets for individual sites.
- (4)
- The decision matrix only considers the impact of brash removal on base cation sustainability and it is important to note and manage the risks posed to other essential nutrients such as nitrogen and phosphorus, as well as related impacts of practice on other aspects of the forest environment, including ground damage, soil acidity and soil carbon loss.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Royo, J.; Sebastián, F.; García-Galindo, D.; Gómez, M.; Díaz, M. Large-scale Analysis of GHG (Greenhouse Gas) Reduction by Means of Biomass Co-Firing at Country-Scale: Application to the Spanish Case. Energy 2012, 48, 255–267. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody Biomass Energy Potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- Kazagic, A.; Music, M.; Smajevic, I.; Ademovic, A.; Redzic, E. Possibilities and Sustainability of “Biomass for Power” Solutions in the Case of a Coal-Based Power Utility. Clean. Technol. Environ. Pol. 2016, 18, 1675–1683. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Hazlett, P.W.; Gunn, J.M. Logging Impacts on the Biogeochemistry of Boreal Forest Soils and Nutrient Export to Aquatic Systems: A Review. Environ. Rev. 2008, 16, 157–179. [Google Scholar] [CrossRef]
- Thiffault, E.; Hannam, K.D.; Paré, D.; Titus, B.D.; Hazlett, P.W.; Maynard, D.G.; Brais, S. Effects of Forest Biomass Harvesting on Soil Productivity in Boreal and Temperate Forests—A Review. Environ. Rev. 2011, 19, 278–309. [Google Scholar] [CrossRef]
- Achat, D.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying Consequences of Removing Harvesting Residues on Forest Soils and Tree Growth—A Meta-Analysis. Ecol. Manag. 2015, 348, 124–241. [Google Scholar] [CrossRef]
- De Oliveira, G.W.; Amann, T.; Hartmann, J. Increasing Biomass Demand Enlarges Negative Forest Nutrient Budget Areas in Wood Export Regions. Sci. Rep. 2018, 8, 5280. [Google Scholar] [CrossRef]
- Akselsson, C.; Westling, O.; Sverdrup, H.; Holmqvist, J.; Thelin, G.; Uggla, E.; Malm, G. Impact of Harvest Intensity on Long-Term Base Cation Budgets in Swedish Forest Soils. Water Air Soil Pollut. Focus 2007, 7, 201–210. [Google Scholar] [CrossRef]
- Aherne, J.; Posch, M.; Forsius, M.; Lehtonen, A.; Härkönen, K. Impacts of Forest Biomass Removal on Soil Nutrient Status under Climate Change: A Catchment-Based Modelling Study for Finland. Biogeochemistry 2012, 107, 471–488. [Google Scholar] [CrossRef]
- Vangansbeke, P.; De Schrijver, A.; De Frenne, P.; Verstraeten, A.; Gorissen, L.; Verheyen, K. Strong Negative Impacts of Whole Tree Harvesting in Pine Stands on Poor, Sandy Soils: A Long-Term Nutrient Budget Modelling Approach. Ecol. Manag. 2015, 356, 101–111. [Google Scholar] [CrossRef]
- Knust, C.; Schua, K.; Feger, K.-H. Estimation of Nutrient Exports Resulting from Thinning and Intensive Biomass Extraction in Medium-Aged Spruce and Pine Stands in Saxony, Northeast Germany. Forests 2016, 7, 302. [Google Scholar] [CrossRef]
- Clarke, N.; Kiær, L.P.; Kjønaas, O.J.; Bárcena, T.G.; Vesterdal, L.; Stupak, I.; Finér, L.; Jacobson, S.; Armolaitis, K.; Lazdina, D.; et al. Effects of Intensive Biomass Harvesting on Forest Soils in the Nordic Countries and the UK: A Meta-Analysis. Ecol. Manag. 2020, 482, 118877. [Google Scholar] [CrossRef]
- Iwald, J.; Löfgren, S.; Stendahl, J.; Karltun, E. Acidifying Effect of Removal of Tree Stumps and Logging Residues as Compared to Atmospheric Deposition. Ecol. Manag. 2013, 290, 49–58. [Google Scholar] [CrossRef]
- McGivney, E.; Gustafsson, J.P.; Belyazid, S.; Zetterberg, T.; Löfgren, S. Assessing the Impact of Acid Rain and Forest Harvest Intensity with the HD-MINTEQ Model–Soil Chemistry of Three Swedish Conifer Sites from 1880 to 2080. Soil 2019, 5, 63–77. [Google Scholar] [CrossRef]
- Johnson, J.; Pannatier, E.G.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The Response of Soil Solution Chemistry in European Forests to Decreasing Acid Deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Duval, B.D.; Blankinship Djikstra, P.; Hungate, B.A. CO2 Effects on Plant Nutrient Concentration Depend on Plant Functional Group and Available Nitrogen: A Meta-Analysis. Plant Ecol. 2012, 213, 505–521. [Google Scholar] [CrossRef]
- Terrer, C.; Vicca, S.; Hungate, B.A.; Phillips, R.P.; Prentice, I.C. Mycorrhizal Association as a Primary Control of the CO2 Fertilization Effect. Science 2016, 353, 72–74. [Google Scholar] [CrossRef]
- Kreutzer, K. Ecological Questions about the Full Tree Harvest. Forstw. Cbl. 1979, 98, 298–308. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.E. Nutrient Cycling. Science 1967, 155, 424–429. [Google Scholar] [CrossRef]
- Kolka, R.K.; Grigal, D.F.; Nater, E.A. Forest Soil Mineral Weathering Rates: Use of Multiple Approaches. Geoderma 1996, 73, 1–21. [Google Scholar] [CrossRef]
- Ouimet, R.; Duchesne, L. Base Cation Mineral Weathering and Total Release from Soils in Three Calibrated Forest Watersheds on the Canadian Boreal Shield. Can. J. Soil Sci. 2005, 85, 245–260. [Google Scholar] [CrossRef]
- Futter, M.; Klaminder, J.; Lucas, R.W.; Laudon, H.; Köhler, S.J. Uncertainty in Silicate Mineral Weathering Rate Estimates: Source Partitioning and Policy Implications. Environ. Res. Lett. 2012, 7, 024025. [Google Scholar] [CrossRef]
- Vadeboncoeur, M.A.; Hamburg, S.P.; Yanai, R.D.; Blum, J.D. Rates of Sustainable Forest Harvest Depend on Rotation Length and Wathering of Soil Minerals. Ecol. Manag. 2014, 318, 194–205. [Google Scholar] [CrossRef]
- Lucas, R.W.; Holmström, H.; Lämås, T. Intensive Forest Harvesting and Pools of Base Cations in Forest Ecosystems: A Modeling Study Using the Heureka Decision Support System. Ecol. Manag. 2014, 325, 26–36. [Google Scholar] [CrossRef]
- Van Breemen, N.; Mulder, J.; Driscoll, C.T. Acidification and Alkalinization of Soils. Plant Soil 1983, 75, 283–308. [Google Scholar] [CrossRef]
- Akselsson, C.; Belyazid, S. Critical Biomass Harvesting—Applying a New Concept for Swedish Forest Soils. Ecol. Manag. 2018, 409, 67–73. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Barsoum, N.; Benham, S.; Broadmeadow, M.; Moffat, A.; Nisbet, T.; Pitman, R. Ten Years of Intensive Environmental Monitoring of British Forests. For. Comm. Inf. Note 2007, 88, 11. [Google Scholar]
- Vanguelova, E.I.; Benham, S.; Pitman, R.; Moffat, A.; Broadmeadow, M.; Nisbet, T.; Durrant, D.; Barsoum, N.; Wilkinson, M.; Bochereau, F.; et al. Chemical Fluxes in Time through Forest Ecosystems in the UK–Soil Response to Pollution Recovery. Environ. Pollut. 2010, 158, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Sverdrup, H.; Warfvinge, P. Calculating Field Weathering Rates Using a Mechanistic Geochemical Model PROFILE. Appl. Geochem. 1993, 8, 273–283. [Google Scholar] [CrossRef]
- Clarke, N.; Žlindra, D.; Ulrich, E.; Mosello, R.; Derome, J.; Derome, K.; König, N.; Lövblad, G.; Draaijers, G.P.J.; Hansen, K.; et al. Part XIV: Sampling and Analysis of Deposition. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 32. Available online: http://www.icpforests.org/Manual.htm (accessed on 2 February 2022)ISBN 978–3-86576–162–0.
- Ulrich, B. Interaction at forest canopies with atmospheric constituents: SO2, Alkali and Earth Alkali Cations and Chloride. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Ulrich, B., Pankrath, J., Eds.; Reidel: Dordrecht, The Netherlands, 1983; pp. 33–45. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Naderi, M. Chapter Fourteen-Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Steve, T., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 585–608. ISBN 9780123847461. [Google Scholar]
- Kennedy, F.; Rowell, D.; Singh, B.; Moffat, A. The Use of Soil Mineral Weathering Rates in the Calculation of Critical Loads of Sulphur Deposition Using the Simple Mass Balance equation. In Proceedings of the ISSS 16th World Soils Congress, Montpellier, France, 20–26 August 1998. [Google Scholar]
- Kennedy, F.; Rowell, D.; Moffat, A.J.M.; Singh, B. An Analysis of the Structure of the Simple Mass Balance Equation-Implications for Testing National Critical Loads Maps. Water Air Soil Pollut. Focus 2001, 1, 281–298. [Google Scholar] [CrossRef]
- Taylor, C.M.A. Forest Fertilisation in Britain; Forestry Commission Bulletin 95: London, UK, 1991. [Google Scholar]
- Nieminen, T.M.; De Vos, B.; Cools, N.; König, N.; Fischer, R.; Iost, S.; Meesenburg, H.; Nicolas, M.; O’Dea, P.; Cecchini, G.; et al. Part XI: Soil Solution Collection and Analysis. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 20. Available online: http://www.icp-forests.org/manual.htm (accessed on 2 February 2022)ISBN 978–3-86576–162–0.
- Monteith, J.; Unsworth, M. Principles of Environmental Physics, 3rd ed.; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Hough, M.N.; Jones, R.J.A. The United Kingdom Meteorological Office Bulk Precipitation and Evaporation Calculation System: MORECS Version 2.0-an Overview. Hydrol. Earth Syst. Sci. 1997, 1, 227–239. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Kennedy, F. Base Cation Removal in Harvesting and Biological Limit Terms for Use in the Simple Mass Balance Equation to Calculate Critical Loads for Forest Soils. Water Air Soil Pollut. 2003, 145, 409–427. [Google Scholar] [CrossRef]
- Matthews, R.W.; Duckworth, R.R. BSORT: A Model of Tree and Stand Biomass Development and Production in Great Britain. In Proceedings of the World Renewable Energy Congress (WREC 2005), Aberdeen, UK, 22–27 May 2005; Imbabi, M.S., Mitchell, C.P., Eds.; Elsevier: Oxford, UK, 2005; pp. 404–409. Available online: https://www.forestresearch.gov.uk/documents/2785/BSORT.pdf (accessed on 2 February 2022).
- Jenkins, T.A.R.; Mackie, E.D.; Matthews, R.W.; Miller, G.; Randle, T.J.; White, M.E. FC Woodland Carbon Code: Carbon Assessment Protocol; Forestry Commission: Edinburgh, UK, 2014. Available online: www.forestry.gov.uk/carboncode (accessed on 2 February 2022).
- Forest Research. Forestry Statistics 231; Corstorphine Road: Edinburgh, UK, 2021. Available online: https://www.forestresearch.gov.uk/tools-and-resources/statistics/forestry-statistics/ (accessed on 2 February 2022).
- Reynolds, B.; Stevens, P.A.; Adamson, J.K.; Hughes, S.; Roberts, J.D. Effects of Clearfelling on Stream and Soil Water Aluminium Chemistry in Three UK Forests Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 1992; Volume 77, pp. 157–165. [Google Scholar]
- Mannerkoski, H.; Finér, L.; Piirainen, S.; Starr, M. Effects of Clear-Cutting and Site Preparation on the Level and Quality of Groundwater in Some Headwater Catchments in Eastern Finland. For. Ecol. Manag. 2005, 220, 107–117. [Google Scholar] [CrossRef]
- Aherne, J.; Farrell, E.P. Deposition of Sulphur, Nitrogen and Acidity in Precipitation Over Ireland: Chemistry, Spatial Distribution and Long-Term Trends. Atmos. Environ. 2002, 36, 1379–1389. [Google Scholar] [CrossRef]
- Guerrieri, R.; Vanguelova, E.I.; Michalski, G.; Heaton, T.H.E.; Mencuccini, M. Isotopic Evidence for the Occurrence of Biological Nitrification and Nitrogen Deposition Processing in Forest Canopies. Glob. Chang. Biol. 2015, 21, 4613–4626. [Google Scholar] [CrossRef]
- Pitman, R.; Vanguelova, E.I.; Benham, S. Effects of Phytophagous Insects on the Nutrient Concentrations and Fluxes through Forest Stands in the UK Level II Network. Sci. Total Environ. 2010, 409, 169–181. [Google Scholar] [CrossRef]
- Sawicka, D.E.; Monteith, D.T.; Vanguelova, E.I.; Wade, A.J.; Clark, J.M. Fine-Scale Temporal Characterization of Trends in Soil Water Dissolved Organic Carbon and Potential Drivers. Ecol. Indic. 2016, 68, 35–51. [Google Scholar] [CrossRef]
- Sawicka, K.; Rowe, E.; Evans, C.; Monteith, D.; Vanguelova, E.I.; Wade, A.; Clark, J.M. Modelling Impacts of Atmospheric Deposition and Temperature on Long-Term DOC Trends. Sci. Total Environ. 2016, 578, 323–336. [Google Scholar] [CrossRef]
- RoTAP. Review of Transboundary Air Pollution: Acidification, Eutrophication, Ground Level Ozone and Heavy Metals in the UK Contract Report to Defra; Centre for Ecology and Hydrology (CEH): Penicuik, UK, 2012. [Google Scholar]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment; Cambridge University Press: Cambridge, UK, 2011; Available online: http://go.nature.com/5n9 (accessed on 2 February 2022).
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a Good Thing? Nature 2015, 472, 159–161. [Google Scholar] [CrossRef]
- Langan, S.; Fransson, L.; Vanguelova, E. Dynamic Modelling of UK Forest Soils to Changes in Acid Deposition Using the SAFE Model. Sci. Total Environ. 2009, 407, 5605–5619. [Google Scholar] [CrossRef] [PubMed]
- Vanguelova, E.; Pitman, R.; Luiro, J.; Helmisaari, H.S. Long Term Effects of Whole Tree Harvesting on Soil Carbon and Nutrient Sustainability in the UK. Biogeochemistry 2010, 101, 43–59. [Google Scholar] [CrossRef]
- Reynolds, B.; Wood, M.J.; Truscott, A.M.; Williams, D.L. Cycling of Nutrient Base Cations in a Twelve Year Old Sitka Spruce Plantation in Upland Mid-Wales. Hydrol. Earth Syst. Sci. 2000, 4, 311–321. [Google Scholar] [CrossRef][Green Version]
- Reynolds, B.; Stevens, P.A. Assessing Soil Calcium Depletion Following Growth and Harvesting of Sitka Spruce Plantation Forestry in the Acid Sensitive Welsh Uplands. Hydrol. Earth Syst. Sci. Discuss. 1998, 2, 345–352. [Google Scholar] [CrossRef]
- Roberts, J.; Rosier, P. The Impact of Broadleaved Woodland on Water Resources in Lowland UK: I. Soil Water Changes below Beech Woodland and Grass on Chalk Sites in Hampshire. Hydrol. Earth Syst. Sci. Discuss. 2005, 9, 596–606. [Google Scholar] [CrossRef]
- Walmsley, J.; Jones, D.; Reynolds, B.; Price, M.; Healey, J. Whole Tree Harvesting Can Reduce Second Rotation Forest Productivity. For. Ecol. Manag. 2009, 257, 1104–1111. [Google Scholar] [CrossRef]
- Olsson, B.A.; Bengtsson, J.; Lundkvist, H. Effects of Different Forest Harvest Intensities on the Pools of Exchangeable Cations in Coniferous Forest Soils. For. Ecol. Manag. 1996, 84, 135–147. [Google Scholar] [CrossRef]
- Johnson, D.W.; Todd, D.E. Harvesting Effects on Long-Term Changes in Nutrient Pools of Mixed Oak Forest. Soil Sci. Soc. Am. J. 1998, 62, 1725–1735. [Google Scholar] [CrossRef]
- Bélanger, N.; Paré, D.; Yamasaki, S.H. The Soil Acid Base Status of Boreal Black Spruce Stands after Whole-Tree and Stem-only Harvesting. Can. J. For. Res. 2003, 33, 1874–1879. [Google Scholar] [CrossRef]
- Belleau, A.; Brais, S.; Paré, D. Soil Nutrient Dynamics after Harvesting and Slash Treatments in Boreal Aspen Stands. Soil Sci. Soc. Am. J. 2006, 70, 1189–1199. [Google Scholar] [CrossRef]
- Thiffault, E.; Paré, D.; Bélanger, N.; Munson, A.; Marquis, F. Harvesting Intensity at Clear-Felling in the Boreal Forest. Soil Sci. Soc. Am. J. 2006, 70, 691–701. [Google Scholar] [CrossRef]
- Johnson, J.; Aherne, J.; Cummins, T. Base Cation budgets under Residue Removal in Temperate Maritime Plantation Forests. For. Ecol. Manag. 2015, 343, 144–156. [Google Scholar] [CrossRef]
- Klaminder, J.; Lucas, R.; Futter, M.; Bishop, K.; Köhler, S.; Egnell, G.; Laudon, H. Silicate Mineral Weathering Rate Estimates: Are they Precise enough to Be Useful when Predicting the Recovery of Nutrient Pools after Harvesting? For. Ecol. Manag. 2011, 261, 1–9. [Google Scholar] [CrossRef]
- Hammel, K.; Kennel, M. Charakterisierung und Analyse der Wasserverfügbarkeit und des Wasserhaushalts von Waldstandorten in Bayern mit dem Simulationsmodell Brook90; Forstliche Forschungsberichte München 185: Munich, Germany, 2001. [Google Scholar]
- Titus, B.D.; Brown, K.; Helmisaari, H.-S.; Vanguelova, E.; Stupak, I.; Evans, A.; Clarke, N.; Guidi, C.; Bruckman, V.J.; Varnagiryte-Kabasinskiene, I.; et al. Sustainable Forest Biomass: A Review of Current Residue Harvesting Guidelines. Energy Sustain. Soc. 2021, 11, 10. [Google Scholar] [CrossRef]
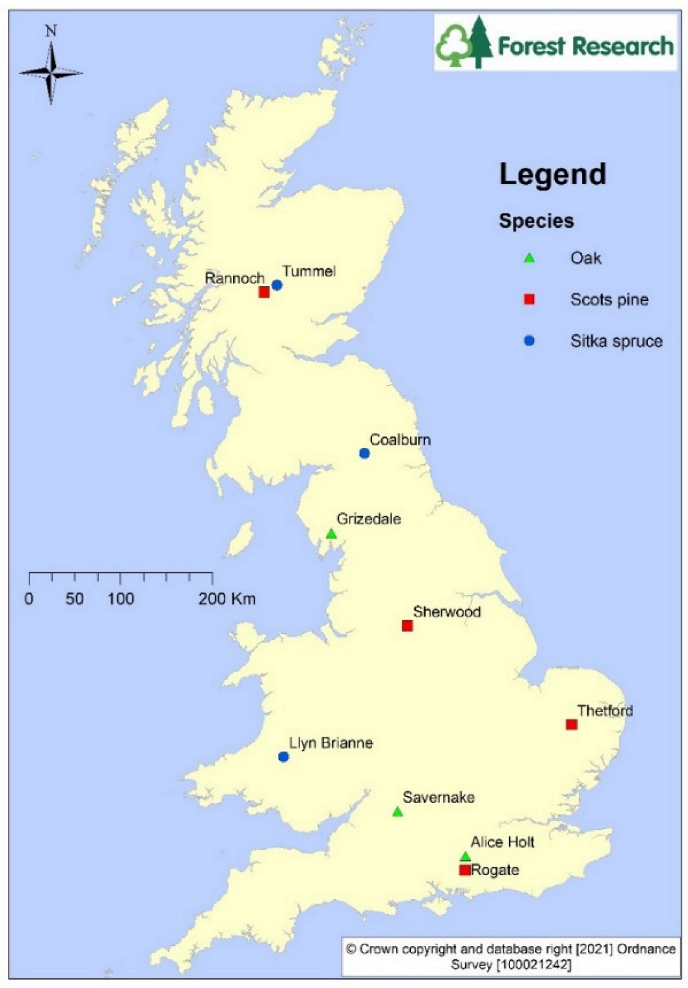
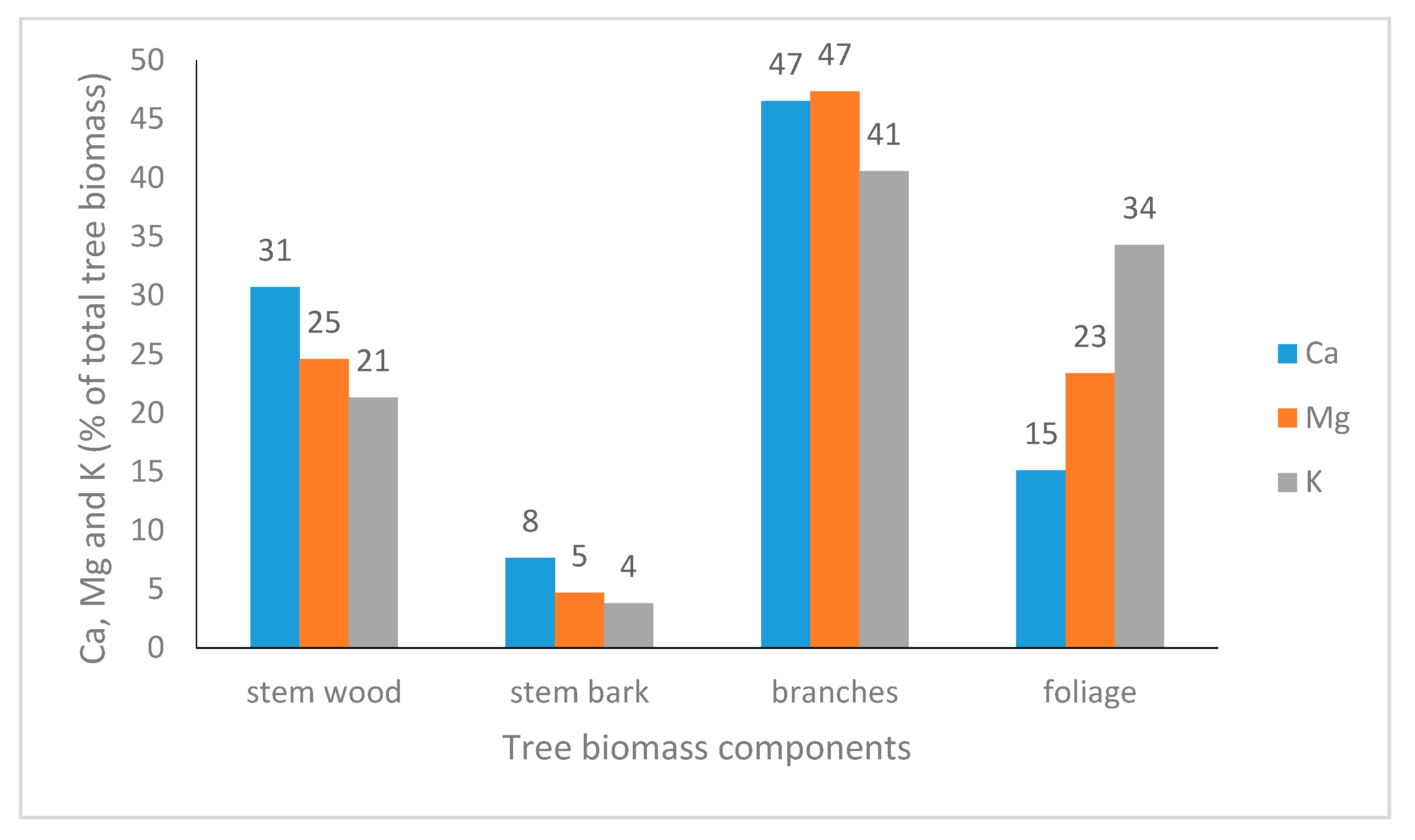
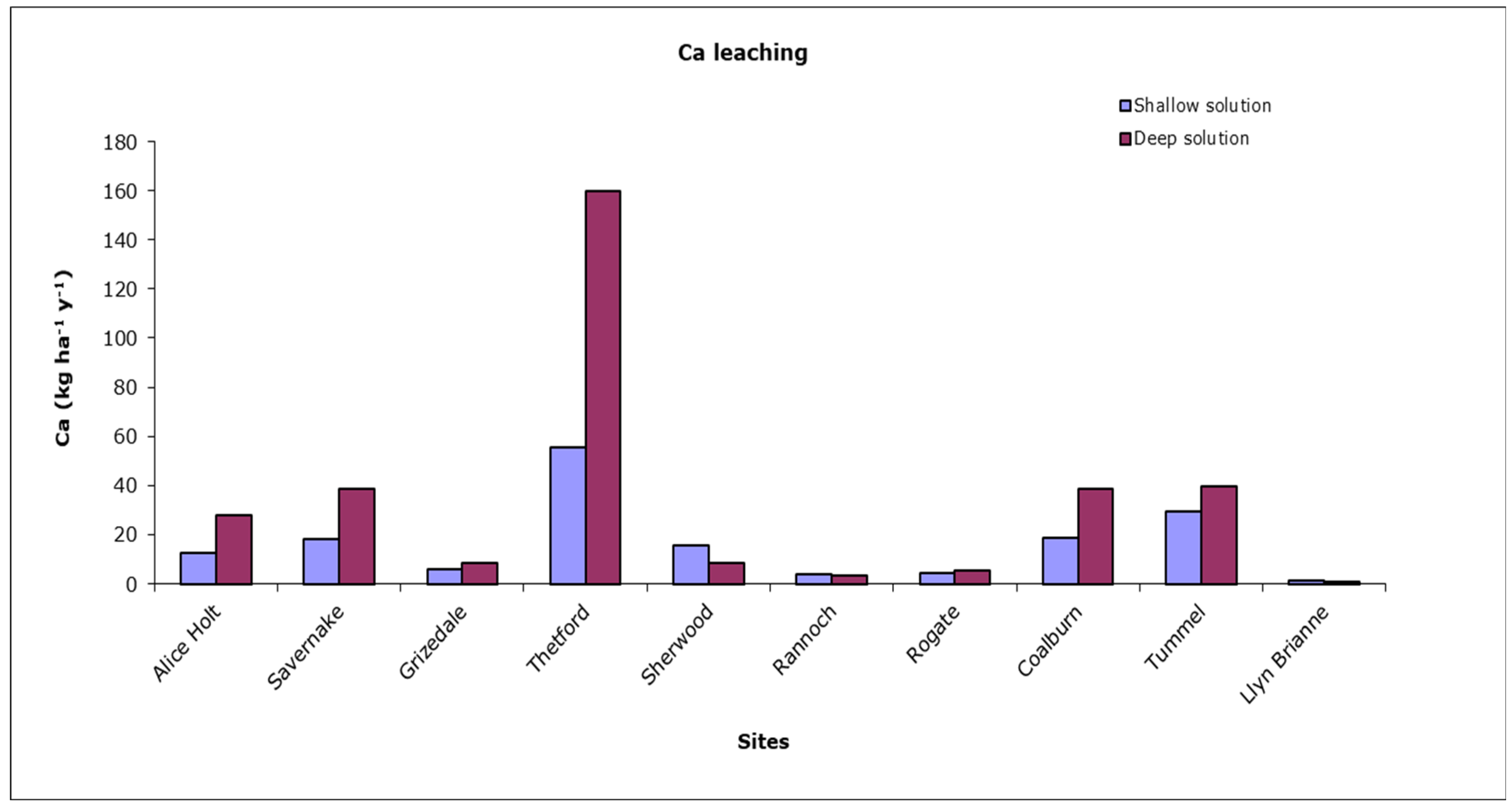
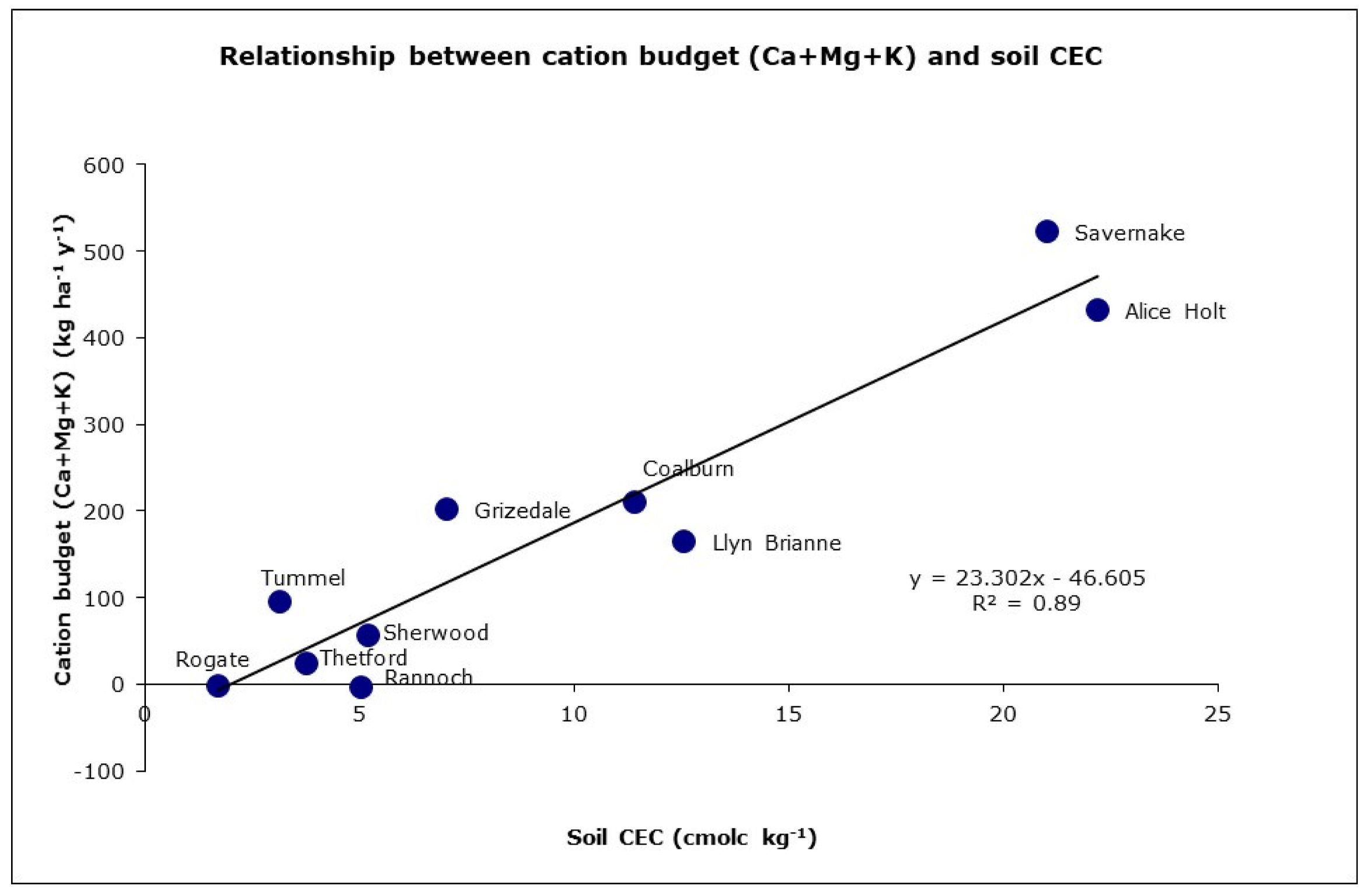
| Site Name | Tree Species | Monitoring Period | Previous Vegetation | Planting Year | Basal Area (m2 ha–1) | Elevation (m) | Slope (%) | Rain (mm) | T (°C) | N dep kg ha−1y−1 | S dep kg ha−1y−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alice Holt | Oak | 1996–2019 | Woodland | 1935 | 27.3 | 80 | 0 | 662 | 11.6 | 9.8 | 7.6 |
| Savernake | Oak | 1996–2005 | Woodland | 1950 | 18.0 | 107 | 1 | 796 | 11.3 | 12.7 | 10.9 |
| Grizedale | Oak | 1996–2006 | Woodland | 1920 | 23.5 | 115 | 30 | 1800 | 9.5 | 13.9 | 14.2 |
| Thetford | Scots pine | 1996–2019 | Heathland | 1967 | 35.7 | 20 | 1 | 600 | 11.3 | 13.6 | 7.5 |
| Sherwood | Scots pine | 1996–2005 | Grassland | 1952 | 38.1 | 265 | 22 | 1200 | 9.8 | 18.6 | 17.6 |
| Rannoch | Scots pine | 1996–2010 | Moorland | 1965 | 47.8 | 470 | 25–30 | 1161 | 8.5 | 4.8 | 4.8 |
| Rogate | Scots pine | 2010–2019 | Heathland | 1950 | 37.8 | 80 | 0 | 662 | 11.4 | 10.7 | 4.1 |
| Coalburn | Sitka spruce | 1996–2019 | Moorland | 1974 | 91.1 | 300 | 1 | 1400 | 9.0 | 10.2 | 8.3 |
| Tummel | Sitka spruce | 1996–2006 | Grassland | 1969 | 65.0 | 400 | 6–10 | 1100 | 7.5 | 4.8 | 6.4 |
| Llyn Brianne | Sitka spruce | 2010–2014 | Moorland | 1973 | 85.4 | 450 | 20 | 2100 | 10.1 | 8.9 | 9.7 |
| Site Name | Soil Type (FAO) | Soil Horizon | Soil pH (H2O) | C% | N% | Soil CEC cmol c kg–1 | Soil BS% |
|---|---|---|---|---|---|---|---|
| Alice Holt | Eutric vertisol | Ah | 5.4 | 2.69 | 0.56 | 23.10 | 95 |
| BCg | 6.2 | 1.08 | 0.027 | 25.83 | 96 | ||
| Savernake | Eutric vertisol | E | 4.7 | 3.47 | 0.138 | 15.73 | 22 |
| 2BCtg | 6.2 | 1.21 | 0.068 | 23.15 | 99 | ||
| Grizedale | Cambic podzol | Ah | 4.3 | 8.27 | 0.456 | 12.92 | 9 |
| Bs | 5.1 | 5.01 | 0.279 | 6.71 | 7 | ||
| Thetford | Ferralic arenosol | Ah | 5.3 | 1.98 | 0.482 | 4.93 | 92 |
| Bw | 7.0 | 0.31 | 0.285 | 3.23 | 99 | ||
| Sherwood | Cabric podzol | Ah | 4.1 | 2.69 | 0.161 | 6.51 | 4 |
| BC | 4.5 | 1.37 | 0.064 | 2.58 | 7 | ||
| Rannoch | Gleyic podzol | E | 4.2 | 3.87 | 0.111 | 10.03 | 9 |
| Bs | 4.8 | 3.42 | 0.082 | 2.35 | 13 | ||
| Rogate | Humic Podzol | E | 4.3 | 2.90 | 0.103 | 2.57 | 36 |
| Bs | 4.4 | 1.30 | 0.041 | 1.36 | 16 | ||
| Coalburn | Umbric gleysol | Ah(g) | 4.0 | 6.52 | 0.357 | 17.46 | 6 |
| Bg | 3.8 | 1.66 | 0.060 | 12.41 | 33 | ||
| Tummel | Ferric podzol | Ah | 5.1 | 2.5 | 0.185 | 6.3 | 52 |
| Bs | 5.9 | 0.4 | 0.027 | 0.9 | 54 | ||
| Llyn Brianne | Umbric gleysol | A | 4.0 | 4.44 | 0.29 | 16.36 | 3 |
| Bg | 4.3 | 3.51 | 0.05 | 13.18 | 2 |
| (3a) | |||||||||
| Deposition Ca kg ha−1y−1 | Weathering Ca kg ha−1y−1 | Leaching Ca kg ha−1y−1 | Tree Uptake Ca kg ha−1y−1 | Deposition Mg kg ha−1y−1 | Weathering Mg kg ha−1y−1 | Leaching Mg kg ha−1y−1 | Tree Uptake Mg kg ha−1y−1 | ||
| Site (Tree species) | Soil type (WRB, 2016) | ||||||||
| Alice Holt (Oak) | Eutric Vertisol | 5.75 | 199.77 | 27.71 | 10.74 | 2.53 | 63.73 | 7.89 | 1.15 |
| Savernake (Oak) | Eutric Vertisol | 5.89 | 199.77 | 38.45 | 9.11 | 2.15 | 91.11 | 13.73 | 0.81 |
| Grizedale (Oak) | Cambic Podzol | 5.76 | 13.71 | 8.53 | 4.89 | 6.03 | 81.13 | 10.12 | 0.70 |
| Thetford (Scots pine) | Ferralic Arenosol | 3.97 | 185.14 | 159.72 | 5.54 | 1.99 | 2.60 | 3.84 | 1.12 |
| Sherwood (Scots pine) | Cambic Podzol | 7.77 | 2.85 | 8.39 | 4.06 | 4.27 | 13.27 | 4.04 | 0.61 |
| Rannoch (Scots pine) | Gleyic Podzol | 2.20 | 2.03 | 3.60 | 4.08 | 2.42 | 2.60 | 3.58 | 1.17 |
| Rogate (Scots pine) | Podzol | 4.39 | 2.54 | 5.29 | 3.28 | 3.55 | 2.96 | 3.52 | 0.55 |
| Coalburn (Sitka spruce) | Umbric gleysol | 4.01 | 102.20 | 38.55 | 7.99 | 2.94 | 54.45 | 6.08 | 1.25 |
| Tummel (Sitka spruce) | Ferric Podzol | 2.54 | 92.50 | 39.88 | 6.16 | 2.01 | 13.27 | 8.26 | 0.96 |
| Llyn Brianne (Sitka spruce) | Umbric gleysol | 5.98 | 4.98 | 0.72 | 4.58 | 7.67 | 54.45 | 4.31 | 1.28 |
| Deposition K kg ha−1y−1 | Weathering K kg ha−1y−1 | Leaching K kg ha−1y−1 | Tree Uptake K kg ha−1y−1 | ||||||
| Site (Tree species) | Soil type (WRB, 2016) | ||||||||
| Alice Holt (Oak) | Eutric Vertisol | 4.44 | 211.50 | 1.25 | 6.00 | ||||
| Savernake (Oak) | Eutric Vertisol | 4.86 | 287.71 | 1.36 | 4.67 | ||||
| Grizedale (Oak) | Cambic Podzol | 6.84 | 125.28 | 8.03 | 3.60 | ||||
| Thetford (Scots pine) | Ferralic Arenosol | 3.62 | 1.45 | 0.78 | 3.44 | ||||
| Sherwood (Scots pine) | Cambic Podzol | 6.35 | 44.01 | 2.98 | 2.47 | ||||
| Rannoch (Scots pine) | Gleyic Podzol | 2.69 | 1.39 | 1.53 | 3.01 | ||||
| Rogate (Scots pine) | Podzol | 4.47 | 1.69 | 6.54 | 2.35 | ||||
| Coalburn (Sitka spruce) | Umbric gleysol | 4.38 | 103.74 | 0.78 | 6.82 | ||||
| Tummel (Sitka spruce) | Ferric Podzol | 3.07 | 44.01 | 0.86 | 5.97 | ||||
| Llyn Brianne (Sitka spruce) | Umbric gleysol | 6.92 | 103.74 | 0.72 | 6.75 | ||||
| (3b) | |||||||||
| % of Inputs Deposition Ca | % of Inputs Weathering Ca | % Outputs Leaching Ca | % Outputs Tree Uptake Ca | % of Inputs Deposition Mg | % of Inputs Weathering Mg | % Outputs Leaching Mg | % Outputs Tree Uptake Mg | ||
| Site (Tree species) | Soil type (WRB, 2016) | ||||||||
| Alice Holt (Oak) | Eutric Vertisol | 3 | 97 | 72 | 28 | 4 | 96 | 87 | 13 |
| Savernake (Oak) | Eutric Vertisol | 3 | 97 | 81 | 19 | 2 | 98 | 94 | 6 |
| Grizedale (Oak) | Cambic Podzol | 30 | 70 | 64 | 36 | 7 | 93 | 94 | 6 |
| Thetford (Scots pine) | Ferralic Arenosol | 2 | 98 | 97 | 3 | 43 | 57 | 77 | 23 |
| Sherwood (Scots pine) | Cambic Podzol | 73 | 27 | 67 | 33 | 24 | 76 | 87 | 13 |
| Rannoch (Scots pine) | Gleyic Podzol | 52 | 48 | 47 | 53 | 48 | 52 | 75 | 25 |
| Rogate (Scots pine) | Podzol | 63 | 37 | 62 | 38 | 55 | 45 | 86 | 14 |
| Coalburn (Sitka spruce) | Umbric gleysol | 4 | 96 | 83 | 17 | 5 | 95 | 83 | 17 |
| Tummel (Sitka spruce) | Ferric Podzol | 3 | 97 | 87 | 13 | 13 | 87 | 90 | 10 |
| Llyn Brianne (Sitka spruce) | Umbric gleysol | 55 | 45 | 14 | 86 | 12 | 88 | 77 | 23 |
| average (%) | 29 | 71 | 67 | 33 | 21 | 79 | 85 | 15 | |
| % of Inputs Deposition K | % of Inputs Weathering K | % Outputs Leaching K | % Outputs Tree Uptake K | ||||||
| Site (Tree species) | Soil type (WRB, 2016) | ||||||||
| Alice Holt (Oak) | Eutric Vertisol | 2 | 98 | 17 | 83 | ||||
| Savernake (Oak) | Eutric Vertisol | 2 | 98 | 23 | 77 | ||||
| Grizedale (Oak) | Cambic Podzol | 5 | 95 | 69 | 31 | ||||
| Thetford (Scots pine) | Ferralic Arenosol | 71 | 29 | 18 | 82 | ||||
| Sherwood (Scots pine) | Cambic Podzol | 13 | 87 | 55 | 45 | ||||
| Rannoch (Scots pine) | Gleyic Podzol | 66 | 34 | 34 | 66 | ||||
| Rogate (Scots pine) | Podzol | 73 | 27 | 74 | 26 | ||||
| Coalburn (Sitka spruce) | Umbric gleysol | 4 | 96 | 10 | 90 | ||||
| Tummel (Sitka spruce) | Ferric Podzol | 7 | 93 | 13 | 87 | ||||
| Llyn Brianne (Sitka spruce) | Umbric gleysol | 6 | 94 | 10 | 90 | ||||
| average (%) | 25 | 75 | 32 | 68 | |||||
| Long Term Base Cation Mass Balance | ||||||
|---|---|---|---|---|---|---|
| Ca | Mg | K | Total (Ca + Mg + K) | |||
| Site | Tree Species | Soil Type (WRB, 2016) | kg ha−1y−1 | kg ha−1y−1 | kg ha−1y−1 | kg ha−1y−1 |
| Alice Holt | Oak | Eutric Vertisol | 167.07 | 57.22 | 208.68 | 432.96 |
| Savernake | Oak | Eutric Vertisol | 158.09 | 78.72 | 286.54 | 523.35 |
| Grizedale | Oak | Cambic Podzol | 6.05 | 76.33 | 120.49 | 202.87 |
| Thetford | Scots pine | Ferralic Arenosol | 23.84 | –0.38 | 0.85 | 24.32 |
| Sherwood | Scots pine | Cambic Podzol | –1.84 | 12.89 | 44.91 | 55.96 |
| Rannoch | Scots pine | Gleyic Podzol | –3.44 | 0.27 | –0.46 | –3.64 |
| Rogate | Scots pine | Podzol | –1.64 | 2.44 | –2.73 | –1.93 |
| Coalburn | Sitka spruce | Umbric gleysol | 59.69 | 50.05 | 100.51 | 210.26 |
| Tummel | Sitka spruce | Ferric Podzol | 48.99 | 6.05 | 40.25 | 95.30 |
| Llyn Brianne | Sitka spruce | Umbric gleysol | 5.66 | 56.52 | 103.19 | 165.37 |
| Decreasing Trend | Long Term Linear Trends | |||
|---|---|---|---|---|
| Increasing Trend | Ca | Mg | K | |
| Site (Tree Species) (Years of Monitoring) | Soil Type (WRB, 2016) | kg ha−1y−1 | kg ha−1y−1 | kg ha−1y−1 |
| Alice Holt (Oak) (24 years) | Eutric Vertisol | r2 = 0.24 (p < 0.01) | r2 = 0.14 (p = 0.068) | r2 = 0.03 (p = 0.392) |
| Savernake (Oak) (10 years) | Eutric Vertisol | r2 = 0.01 (p = 0.906) | r2 = 0.01 (p = 0.915) | r2 = 0.34 (p = 0.051) |
| Grizedale (Oak) (11 years) | Cambic Podzol | r2 = 0.03 (p = 0.281) | r2 = 0.08 (p = 0.383) | r2 = 0.17 (p = 0.213) |
| Thetford (Scots pine) (24 years) | Ferralic Arenosol | r2 = 0.01 (p = 0.802) | r2 = 0.04 (p = 0.441) | r2 = 0.05 (p = 0.293) |
| Sherwood (Scots pine) (10 years) | Cambic Podzol | r2 = 0.25 (p = 0.09) | r2 = 0.30 (p = 0.076) | r2 = 0.15 (p = 0.231) |
| Rannoch (Scots pine) (10 years) | Gleyic Podzol | r2 = 0.09 (p = 0.689) | r2 = 0.04 (p = 0.787) | r2 = 0.09 (p = 0.689) |
| Rogate (Scots pine) (10 years) | Podzol | r2 = 0.16 (p = 0.246) | r2 = 0.02 (p = 0.676) | r2 = 0.60 (p < 0.001) |
| Coalburn (Sitka spruce) (15 years) | Umbric gleysol | r2 = 0.61 (p < 0.001) | r2 = 0.64 (p < 0.001) | r2 = 0.015 (p = 0.637) |
| Tummel (Sitka spruce) (10 years) | Ferric Podzol | r2 = 0.02 (p = 0.791) | r2 = 0.12 (p = 0.361) | r2 = 0.35 (p = 0.072) |
| Llyn Brianne (Sitka spruce) (24 years) | Umbric gleysol | r2 = 0.04 (p = 0.409) | r2 = 0.02 (p = 0.500) | r2 = 0.28 (p = 0.020) |
| Nutrient Budgets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | K | |||||||
| Harvesting regime | CH | WTHB | WTHG | CH | WTHB | WTHG | CH | WTHB | WTHG |
| Soil type | |||||||||
| Gleys (surface and ground water) | |||||||||
| Brown Earths | ≤20 | ||||||||
| Podzols/Ironpans | <12 | ≤8 | |||||||
| Peaty gleys on Ca-rich geology | |||||||||
| Peaty gleys on acid geology | |||||||||
| Deep peats | <18 | ≤18 | <16 | <8 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanguelova, E.; Benham, S.; Nisbet, T. Long Term Trends of Base Cation Budgets of Forests in the UK to Inform Sustainable Harvesting Practices. Appl. Sci. 2022, 12, 2411. https://doi.org/10.3390/app12052411
Vanguelova E, Benham S, Nisbet T. Long Term Trends of Base Cation Budgets of Forests in the UK to Inform Sustainable Harvesting Practices. Applied Sciences. 2022; 12(5):2411. https://doi.org/10.3390/app12052411
Chicago/Turabian StyleVanguelova, Elena, Sue Benham, and Tom Nisbet. 2022. "Long Term Trends of Base Cation Budgets of Forests in the UK to Inform Sustainable Harvesting Practices" Applied Sciences 12, no. 5: 2411. https://doi.org/10.3390/app12052411
APA StyleVanguelova, E., Benham, S., & Nisbet, T. (2022). Long Term Trends of Base Cation Budgets of Forests in the UK to Inform Sustainable Harvesting Practices. Applied Sciences, 12(5), 2411. https://doi.org/10.3390/app12052411





