Terahertz Imaging for Formalin Fixed Malignant Liver Tumors Using Two-Band Beamline at the Accelerator Facility of Nihon University
Abstract
1. Introduction
2. Materials and Methods
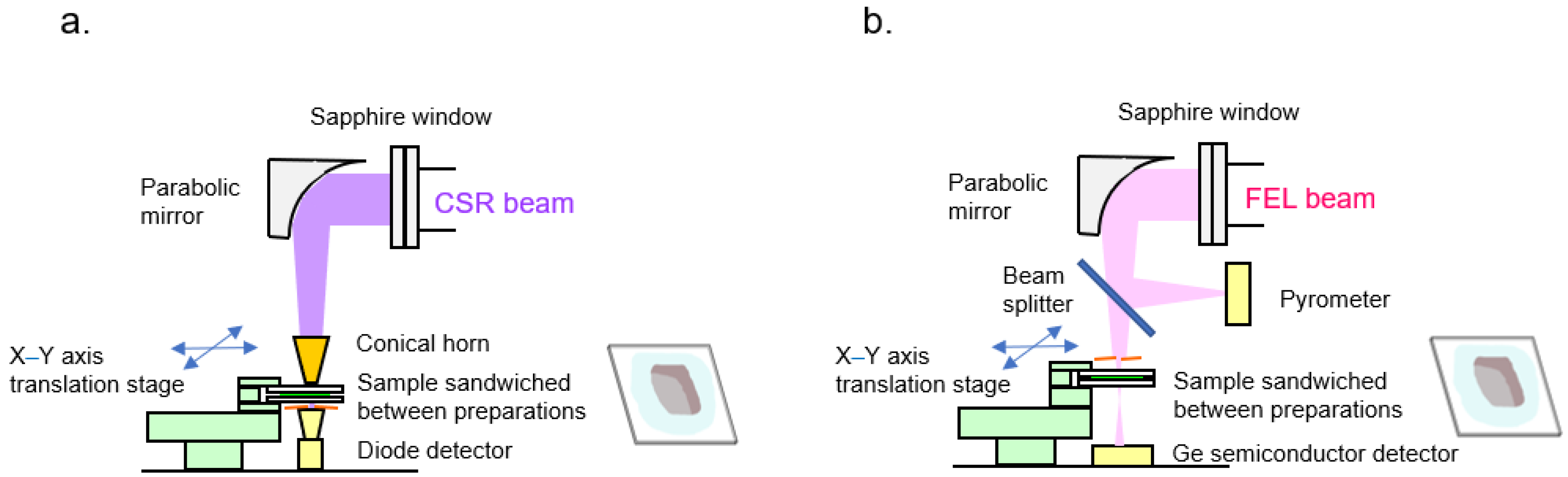
2.1. Infrared FEL/THz CSR Beamline
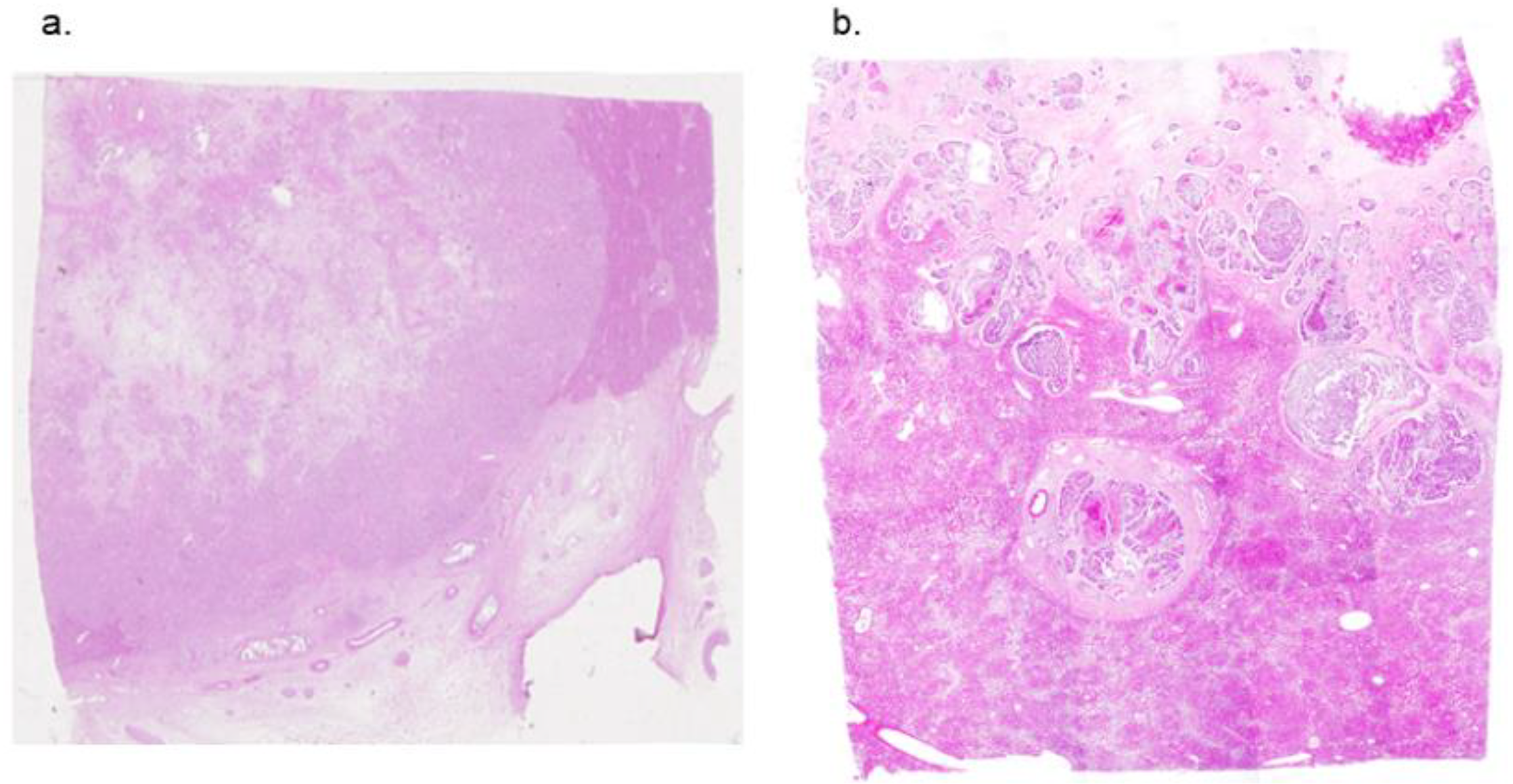
2.2. Samples of Formalin Fixed Human Liver Tumor
2.3. Image Analysis with THz CSR
2.4. Image Analysis with Infrared FEL Radiation
3. Results
3.1. Absorption Coefficient of the Formalin Fixed Normal Liver Tissue
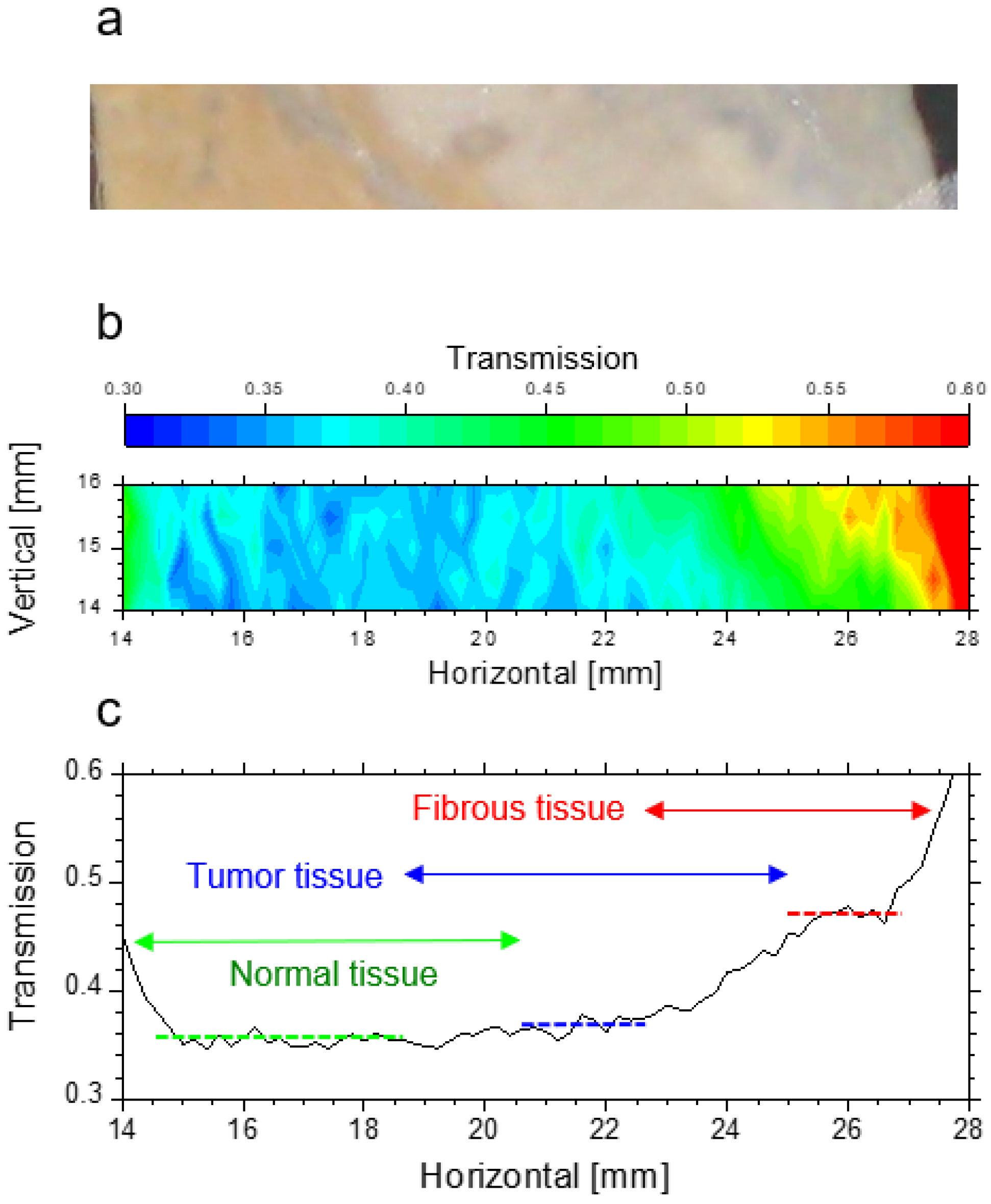
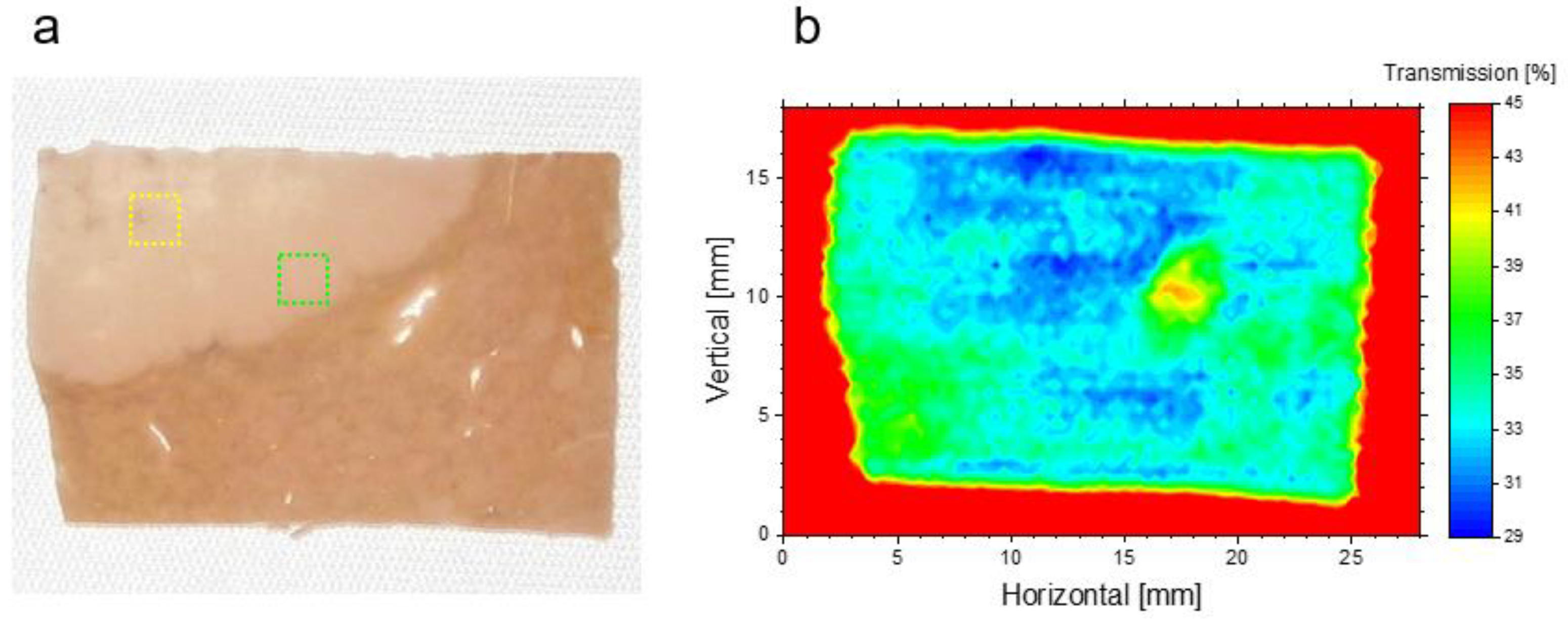
3.2. Transmission of Liver Normal Tissue and Malignant Tumor Tissue
3.3. Near-Infrared FEL Imaging
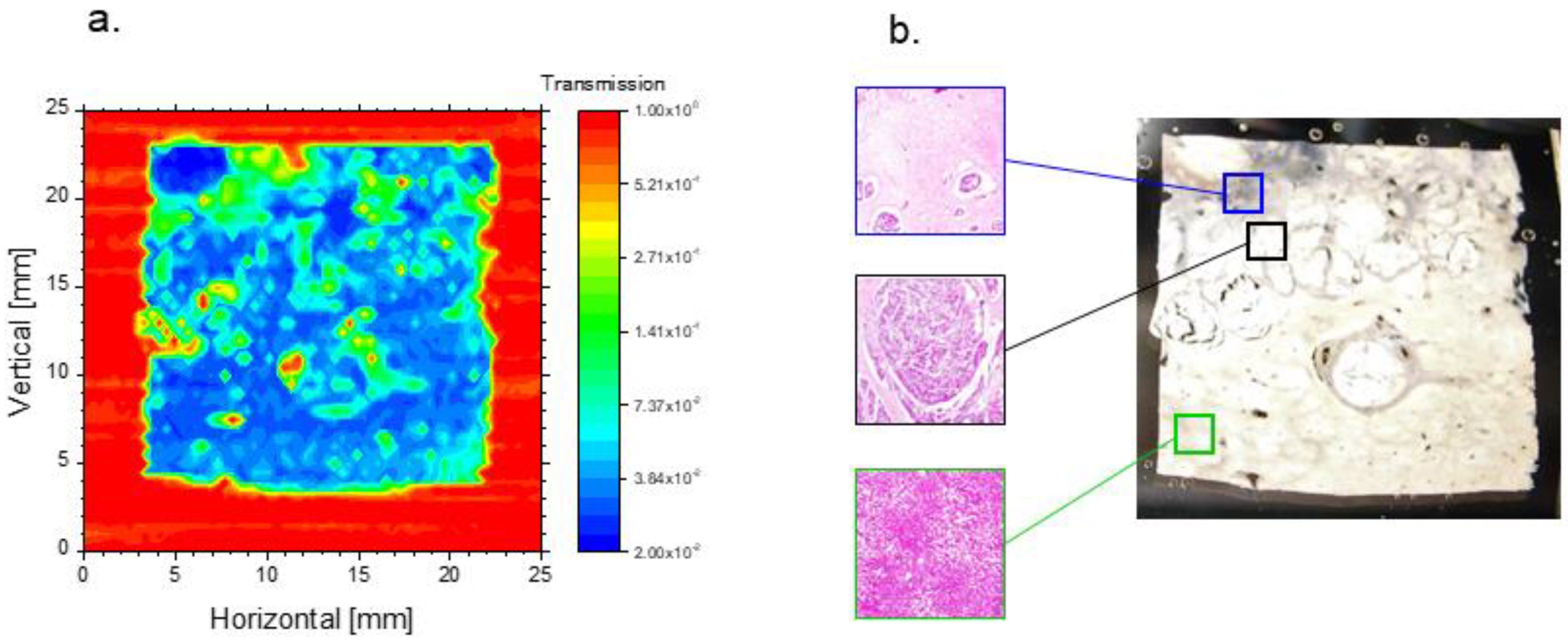
3.4. THz Imaging of Liver Tissues with Advanced Fibrosis
3.5. THz Imaging for Non-Fibrotic Formalin Fixed Liver Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marble, C.B.; Yakovlev, V.V. Biomedical optics applications of advanced lasers and nonlinear optics. J. Biomed. Opt. 2020, 25, 040902. [Google Scholar]
- Yu, C.; Fan, S.; Sun, Y.; Pickwell-MacPherson, E. The potential of terahertz imaging for cancer diagnosis: A review of investigations to date. Quant. Imaging Med. Surg. 2012, 2, 33. [Google Scholar] [PubMed]
- Son, J.H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- Cheon, H.; Paik, J.H.; Choi, M.; Yang, H.J.; Son, J.H. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 2019, 23, 6413. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Chen, H. A route to terahertz metamaterial biosensor integrated with microfluidics for liver cancer biomarker testing in early stage. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ian, M.; Patrick, T.; Vadimir, K.; Markelz, A.G.; George, D.K.; Peter, S. SPIE 10902 Nonlinear Frequency Generation and Conversion: Materials and Devices XVIII. In Proceedings of the SPIE, San Francisco, CA, USA, 4 March 2019; p. 1090218. [Google Scholar]
- Zhang, L.; Zhong, H.; Deng, C.; Zhang, C.; Zhao, Y. Terahertz wave reference-free phase imaging for identification of explosives. Appl. Phys. Lett. 2008, 92, 091117. [Google Scholar] [CrossRef]
- Kawase, K.; Ogawa, Y.; Watanabe, Y.; Inoue, H. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints. Opt. Express. 2003, 11, 2549–2554. [Google Scholar] [CrossRef]
- Ok, G.; Kim, H.J.; Chun, S.; Choi, S.W. Foreign-body detection in dry food using continuous sub-terahertz wave imaging. Food Control 2014, 42, 284–289. [Google Scholar] [CrossRef]
- Clothier, R.H.; Bourn, N. Effects of exposure on human primary keratinocyte differentiation and viability. J. Biol. Phys. 2003, 29, 179–185. [Google Scholar] [CrossRef]
- Sei, N.; Ogawa, H.; Hayakawa, K.; Tanaka, T.; Hayakawa, Y.; Nakao, K.; Sakai, T.; Nogami, K.; Inagaki, M. Complex light source composed from subterahertz-wave coherent synchrotron radiation and an infrared free-electron laser at the Laboratory for Electron Beam Research and Application. J. Opt. Soc. Am. 2014, B31, 2150–2156. [Google Scholar] [CrossRef]
- Sei, N.; Ogawa, H.; Hayakawa, K.; Tanaka, T.; Hayakawa, Y.; Nakao, K.; Sakai, T.; Nogami, K.; Inagaki, M. Observation of intense terahertz-wave coherent synchrotron radiation at LEBRA. J. Phys. D Appl. Phys. 2013, 46, 045104. [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Dolganova, I.N.; Chernomyrdin, N.V.; Katyba, G.M.; Gavdush, A.A.; Cherkasova, O.P.; Komandin, G.A.; Shchedrina, M.A.; Khodan, A.N.; Ponomarev, D.S.; et al. The progress and perspectives of terahertz technology for diagnosis of neoplasms: A review. J. Opt. 2019, 22, 013001. [Google Scholar] [CrossRef]
- Smolyanskaya, O.A.; Chernomyrdin, N.V.; Konovko, A.A.; Zaytsev, K.I.; Ozheredov, I.A.; Cherkasova, O.P.; Nazarov, M.M.; Guillet, J.P.; Kozlov, S.A.; Kistenevg, Y.V.; et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog. Quantum Electron. 2019, 62, 1–77. [Google Scholar] [CrossRef]
- Joseph, C.S.; Patel, R.; Neel, V.A.; Giles, R.H.; Yaroslavsky, A.N. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions optical and terahertz skin cancers imaging. J. Biophotonics 2014, 7, 295–303. [Google Scholar] [CrossRef]
- Nikitkina, A.I.; Bikmulina, P.Y.; Gafarova, E.R.; Kosheleva, N.V.; Efremov, Y.M.; Bezrukov, E.A.; Butnaru, D.; Dolganova, I.N.; Chernomyrdin, N.V.; Cherkasova, O.P.; et al. Terahertz radiation and the skin: A review. J. Biomed. Opt. 2021, 26, 043005. [Google Scholar] [CrossRef]
- Titova, L.V.; Ayesheshim, A.K.; Golubov, A.; Rodriguez-Juarez, R.; Woycicki, R.; Hegmann, F.A.; Kovalchuk, O. Intense THz pulses down-regulate genes associated with skin cancer and psoriasis: A new therapeutic avenue? Sci. Rep. 2013, 3, 2363. [Google Scholar] [CrossRef]
- Doradla, P.; Alavi, K.; Joseph, C.S.; Giles, R.H. Detection of colon cancer by continuous-wave terahertz polarization imaging technique. J. Biomed. Opt. 2013, 18, 090504. [Google Scholar] [CrossRef]
- Doradla, P.; Alavi, K.; Joseph, C.S.; Giles, R.H. Terahertz polarization imaging for colon cancer detection. In Proceedings of the International Society for Optics and Photonics (SPIE), San Francisco, CA, USA, 19–22 December 2014; Volume 8985, pp. 49–56. [Google Scholar]
- Sim, Y.C.; Park, J.Y.; Ahn, K.M.; Park, C.; Son, J.H. Terahertz imaging of excised oral cancer at frozen temperature. Biomed. Opt. Express 2013, 4, 1413–1421. [Google Scholar] [CrossRef]
- Fitzgerald, A.J.; Wallace, V.P.; Linan, M.J.; Bobrow, L.; Pye, R.J.; Purushotham, A.D.; Arnone, D.D. Terahertz pulsed imaging of human breast tumors. Radiology 2006, 239, 533–540. [Google Scholar] [CrossRef]
- Arbab, M.H.; Winebrenner, D.P.; Dickey, T.C.; Chen, A.; Klein, M.B.; Mourad, P.D. Terahertz spectroscopy for the assessment of burn injuries in vivo. J. Biomed. Opt. 2013, 18, 077004. [Google Scholar] [CrossRef]
- Bennett, D.; Taylor, Z.; Tewari, P.; Sung, S.; Maccabi, A.; Singh, R.; Culjat, M.; Grundfest, W.; Hubschman, J.P.; Brown, E. Assessment of corneal hydration sensing in the terahertz band: In vivo results at 100 GHz. J. Biomed. Opt. 2012, 17, 97008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Churchley, D.; Lynch, R.J.M.; Lippert, F.; Eder, J.S.O.; Alton, J.; Gonzalez-Cabezas, C. Terahertz pulsed imaging study to assess remineralization of artificial caries lesions. J. Biomed. Opt. 2001, 16, 026001. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Chen, T.N.; Chen, T.; Zhu, L.G.; Liu, Q.; Li, Z.; Li, F.; Zhong, S.C.; Li, Z.R.; Feng, H.; et al. Terahertz pulsed spectroscopy of paraffin-embedded brain glioma. J. Biomed. Opt. 2014, 19, 077001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Chen Shi, L.J.; Ma, K.; Tang, L.; Xu, D.; Yao, J.; Feng, H.; Chen, T. High-sensitivity terahertz imaging of traumatic brain injury in a rat model. J. Biomed. Opt. 2018, 23, 036015. [Google Scholar]
- Rong, L.; Latychevskaia, T.; Chen, C.; Wang, D.; Yu, Z.; Zhou, X.; Li, Z.; Huang, H.; Wang, Y.; Zhou, Z. Terahertz in-line digital holography of human hepatocellular carcinoma tissue. Sci. Rep. 2015, 5, 8445. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef]
- Pickwell-MacPherson, E.; Wallace, V.P. Terahertz pulsed imaging—A potential medical imaging modality? Photodiagnosis Photodyn. Ther. 2009, 6, 128–134. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sato, I.; Hayakawa, K.; Tanaka, T.; Nakazawa, H.; Yokoyama, K.; Kanno, K.; Sakai, T.; Ishiwata, K.; Enomoto, A.; et al. First lasing of LEBRA FEL at Nihon University at a wavelength of 1.5 μm. Nucl. Instrum. Meth. A 2002, 483, 29–33. [Google Scholar] [CrossRef]
- Danciu, M.; Alexa-Stratulat, T.; Stefanescu, C.; Dodi, G.; Tamba, B.I.; Mihai, C.T.; Stanciu, G.D.; Luca, A.; Spiridon, I.A.; Ungureanu, L.B.; et al. Terahertz spectroscopy and imaging: A cutting-edge method for diagnosing digestive cancers. Materials 2019, 12, 1519. [Google Scholar] [CrossRef]
- Parrott, E.P.J.; Sun, Y.; Pickwell-MacPherson, E. Terahertz spectroscopy: Its future role in medical diagnoses. J. Mol. Struct. 2011, 1006, 66–76. [Google Scholar] [CrossRef]
- Gong, A.; Qiu, Y.; Chen, X.; Zhao, Z.; Xia, L.; Shao, Y. Biomedical applications of terahertz technology. Appl. Spectrosc. Rev. 2020, 55, 418–438. [Google Scholar] [CrossRef]
- Storey, P. Introduction to magnetic resonance imaging and spectroscopy. Methods Mol. Med. 2006, 124, 3–57. [Google Scholar] [PubMed]
- Duan, F.; Wang, Y.Y.; Xu, D.G.; Shi, J.; Chen, L.Y.; Cui, L.; Bai, Y.H.; Xu, Y.; Yuan, J.; Chang, C. Feasibility of terahertz imaging for discrimination of human hepatocellular carcinoma. World J. Gastrointest. Oncol. 2019, 11, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Chernomyrdin, N.V.; Kucheryavenko, A.S.; Kolontaeva, G.S.; Katyba, G.M.; Dolganova, I.N.; Karalkin, P.A.; Ponomarev, D.S.; Kurlov, V.N.; Reshetov, I.V.; Skorobogatiy, M.; et al. Reflection-mode continuous-wave 0.15 λ-resolution terahertz solid immersion microscopy of soft biological tissues. Appl. Phys. Lett. 2018, 113, 111102. [Google Scholar] [CrossRef]
- Okada, K.; Serita, K.; Cassar, Q.; Murakami, H.; MacGrogan, G.; Guillet, J.P.; Mounaix, P.; Tonouchi, M. Terahertz near-field microscopy of ductal carcinoma in situ (DCIS) of the breast. J. Phys. Photonics 2020, 2, 044008. [Google Scholar] [CrossRef]

| Sample Number | Number of Data Point | Transmission of Normal Tissue | Transmission of Tumor Tissue | p-Value |
|---|---|---|---|---|
| #1 | 55 | 36.07% ± 2.30% | 37.88% ± 2.85% | <0.001 |
| #2 | 55 | 34.65% ± 1.34% | 31.11% ± 1.38% | <0.001 |
| #3 | 55 | 36.74% ± 2.08% | 35.88% ± 2.09% | 0.033 |
| #4 | 49 | 33.06% ± 1.07% | 31.89% ± 1.09% | <0.001 |
| #5 | 49 | 36.90% ± 1.37% | 38.35% ± 1.58% | <0.001 |
| #6 | 49 | 34.47% ± 0.86% | 37.45% ± 1.40% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawashima, Y.; Masaaki, S.; Kuyama, K.; Sakai, T.; Hayakawa, Y.; Kaneda, T.; Sei, N. Terahertz Imaging for Formalin Fixed Malignant Liver Tumors Using Two-Band Beamline at the Accelerator Facility of Nihon University. Appl. Sci. 2022, 12, 2229. https://doi.org/10.3390/app12042229
Kawashima Y, Masaaki S, Kuyama K, Sakai T, Hayakawa Y, Kaneda T, Sei N. Terahertz Imaging for Formalin Fixed Malignant Liver Tumors Using Two-Band Beamline at the Accelerator Facility of Nihon University. Applied Sciences. 2022; 12(4):2229. https://doi.org/10.3390/app12042229
Chicago/Turabian StyleKawashima, Yusuke, Suemitsu Masaaki, Kayo Kuyama, Takeshi Sakai, Yasushi Hayakawa, Takashi Kaneda, and Norihiro Sei. 2022. "Terahertz Imaging for Formalin Fixed Malignant Liver Tumors Using Two-Band Beamline at the Accelerator Facility of Nihon University" Applied Sciences 12, no. 4: 2229. https://doi.org/10.3390/app12042229
APA StyleKawashima, Y., Masaaki, S., Kuyama, K., Sakai, T., Hayakawa, Y., Kaneda, T., & Sei, N. (2022). Terahertz Imaging for Formalin Fixed Malignant Liver Tumors Using Two-Band Beamline at the Accelerator Facility of Nihon University. Applied Sciences, 12(4), 2229. https://doi.org/10.3390/app12042229






