Abstract
Identifying species involved in biodeterioration processes is helpful, however further effort is needed to assess their ecological requirements and actual activity. Black fungi (BF) represent one of the most underestimated threats to stone cultural heritage in the Mediterranean basin; they are difficult to kill or remove due to their ability to grow inside the rock and cope with several stresses. Despite this, little is known about BF and factors favoring their growth on stone surfaces. Eighteen BF species were here investigated for temperature and salt tolerance, and metabolic traits by plate assays. The relation between some highly damaged monuments and their BF settlers was assessed using X-ray diffraction analysis, mercury intrusion porosimetry, and SEM. The sensitiveness to four commonly used traditional biocides was also tested. All strains were able to grow within the range of 5–25 °C and in the presence of 3.5% NaCl. Instrumental analyses were fundamental in discovering the relation between halophilic strains and weathered marble sculptures. The acid, cellulase, esterase, and protease production recorded proved BF’s potential to produce a chemical action on carbonate stones and likely affect other materials/historical artefacts. Besides, the use of carboxymethylcellulose and Tween 20 should be evaluated in restoration practice to prevent tertiary bioreceptivity. Agar diffusion tests helped identify the most resistant species to biocides, opening the perspective of its use as reference organisms in material testing procedures.
1. Introduction
All materials exposed to outdoor conditions are inevitably subject to deterioration processes leading to biotic and abiotic alterations. Surface colonization and the related biodeterioration phenomena greatly vary depending on the substrate’s bioreceptivity, macro- and microclimatic conditions (e.g., water availability, sun exposure, shading, and orientation), and nutrients supply [1,2,3,4].
Among the stone biodeteriogens, rock black fungi are considered one of the most remarkable issues for cultural heritage conservation, particularly in arid and semi-arid environments, where they live at their ecological optimum [5,6,7]. Black fungi (BF), also known as rock-inhabiting fungi (RIF), black yeasts, or microcolonial fungi (MCF), are a polyphyletic poikilotolerant morpho-ecological group with remarkable adaptations allowing them to cope with changes in extreme temperatures, drought, starvation, osmotic stress, and high solar and UV-radiation as it occurs on rock surfaces [8,9,10,11,12].
When colonizing rocks, BF can induce a chemical deterioration by secreting siderophore-like compounds, however the most relevant damages are believed to be due to a mechanical action. Hyphal morphology and the strong mechanical turgor pressure (up to 12.39 bar in penetrating silicate and carbonate rocks) allow them to dig cavities on the heritage stone surfaces at depths ranging from a few hundred microns to several millimeters [7,13,14]. Furthermore melanin, the most important factor in BF stress resistance, is also responsible of the aesthetic alterations imparting a dark, blackish-brown appearance to the lithic surface [15,16,17].
Knowledge of the deteriogens’ diversity is undoubtedly useful, however more efforts should be given to outline the ecological needs of the occurring species and their involvement in deterioration processes. The metabolic and ecological profiles, such as the environmental conditions, which favor the various taxonomic groups, are crucial in defining the risk for the artefacts and in designing both preventive/indirect and corrective/direct (e.g., biocide treatments) control measures [1,18]. In fact, the awareness on the tolerance range of the involved species for each environmental factor has a great practical relevance in preventing undesirable growth [1,19]. Such information is also relevant when considering biocide treatments, due to the need to customize the treatment selecting the most efficient product against specific microorganisms [20].
Corrective measures against fungal spreading are often required and such interventions are mainly carried out using biocidal products both to remove the biomass in the early stages of artwork treatment and later to prevent re-colonization [21,22,23]. In stone treatments, BF represent a challenge for restorers being able to survive and re-colonize artworks after restoration treatments with biocides or synthetic polymeric coatings [6,11,24,25,26,27]. To date, very little information is available on their sensitiveness to biocides and on their metabolic and eco-physiological profile, mainly limited to a few model fungi [28,29]. In this light, traditional culture dependent assays can be useful to deepen in detail their ecological traits and biodeteriorative potential [30].
Based on the multidisciplinary investigation surveys performed on the deteriogenic phenomena affecting the monumental Cemetery of Bonaria in Cagliari since 2010, we focused on a selection of 18 BF strains isolated from some of its marble monuments.
The goals of the present study were: (i) the selection and identification of the BF strains of interest; (ii) the assessment of the main fungal traits with respect to thermal and salt growth preferences; (iii) the possible relation between the recorded fungal traits and the substrate in selected monuments; (iv) the detrimental potential of the investigated species; and finally (v) their sensitiveness to a few traditional biocides. The achieved results will also give the opportunity of testing the best techniques of evaluation of metabolic evaluation and control. Data will also provide new insights to design more efficient preventive and control protocols, as well as on the leading forces driving the colonization processes.
2. Materials and Methods
2.1. Black Fungal Strains Selection and Identification
The ancient monumental cemetery of Bonaria (39°12′36.96″ N 9°07′26.13″ E) in Cagliari, Italy, has been object of multi-disciplinary studies aimed at favoring its conservation, rediscovery as urban historical heritage, and valorization [5,17,31,32,33,34]. The climatic conditions of the site are characterized by hot dry summers, very mild winters, and humidity may be high due the sea proximity. Over the period 2011–2014, we performed a few samplings from some Carrara marble funerary monuments (Figure 1), thanks to the collaboration of the Superintendence of Heritage Landscape, Historical, Artistic and Ethno-Anthropological Heritage for the metropolitan city of Cagliari and for the provinces of Oristano, Medio Campidano, and Municipality Cemeteries Direction of Cagliari.

Figure 1.
The sampled Carrara marble funerary monuments of the ancient monumental cemetery of Bonaria—Cagliari entitled to (A) Avv. Giuseppe Todde; (B) Antonio Viganigo; (C) A. M. Frau Carta; (D) Francesca Warzee; (E) Rossino Bolla spouse; (F) Giuseppina Ara dei conti Ciarella; (G) Zelina Ferrà Gastaldi Millelire; (H) Ignazio Ruda Roych conte di San Lorenzo; (I) Unknown Burial.
Forty BF strains were isolated and most of them were identified through phylogenetic analysis, and new species described [31]. Three strains not considered previously, namely CCFEE 5778, 5945, and 6327, were identified by internal transcribed spacer (ITS) and LSU sequencing (in case of poor ITS identities) followed by BLASTn comparison. The primer set used for amplifications were ITS4-ITS5 and LR0R-LR7, respectively. In short, the reactions were performed in a total volume of 25 μL using 5 pmol of each primer, Bioline BioMix (Bioline Reagents, London, UK), and about 30 ng of genomic DNA [35]. The PCR protocols consist of an initial denaturation and final extension and 35 cycles of amplification with annealing at 55 °C (ITS) and 52 °C (LSU). After identification, representative strains of the whole BF diversity found in the site have been subject to selection in order to give the wider representativeness possible with precedence to type strains (when available).
All strains are part of the Culture Collection of Fungi from Extreme Environments (CCFEE, Viterbo, Italy) and new sequences were deposited in GenBank.
2.2. Thermal Preferences, Salt Tolerance, and Metabolic Assays
Thermal preferences and salt tolerance were assessed by inoculating small fragments of fungal mycelia onto malt agar plates (MA: 30 g/L malt extract, 15 g/L bacteriological agar; VWR) and incubating them for a month within the range 0–40 °C with 5 °C intervals. The growth at 37 °C was also assessed.
Similarly, salt tolerance was determined inoculating small fragments of mycelia on MEA 2% plates (malt extract 20 g/L, 15 g/L bacteriological agar) with increasing concentration of NaCl (0, 3.5, 7, 10, 12.5, 15, 20% w/w), incubated at 20 °C for a month.
The detrimental potentials of isolates were assayed through plate trials for acid (CaCO3 Agar, ACID), amylase (AMY), lipase (LIP), and protease (namely caseinase; Skim Milk Agar, SM) production as previously described [36]. The cellulase and pectinase activities were assessed using CMC agar (CMC) and pectinase screening agar medium (PSAM), respectively. CMC was prepared using (NH4)H2PO4 1 g; KCl 0.2 g; MgSO4· 7H2O 1 g; yeast extract 1 g; carboxymethylcellulose low-density 26 g, and bacteriological agar 3 g per liter of solution. PSAM was instead prepared using pectin 1 g; (NH4)2 HPO4 0.3 g; KH2PO4 0.2 g; K2HPO4 0.3 g; MgSO4 0.01 g; and bacteriological agar 2.5 g per 100 mL of solution, pH 5.5. Plates were incubated at 20 °C for a month. To readability, for AMY, CMC, ACID, and PSAM plates were flooded with Lugols’ iodine solution and read after 15 min incubation and washed with saline (NaCl 0.9%). The colonies’ diameters were taken weekly and the halos, expressed as measure from the colony border, were taken at the end of the experiment. All measures were expressed as average of three replicas and standard deviation (SD).
2.3. Marble Substrate Investigations
To deepen the possible relation between the fungal traits recorded and the marble substrate, from which they were isolated, a selection of highly weathered monuments (e.g., Ruda Rojch and Viganigo) were analyzed by X-ray diffraction analysis (XRD), mercury intrusion porosimetry (MIP), light refraction optical microscopy and scanning electron microscopy (SEM).
In detail, detached and not replaceable scales were micronized in an agate jar, to obtain powders passing to 0.63 µm sieve and pressed in a glass holder. XRD spectra were acquired on powders using a diffractometer Rigaku Miniflex II, equipped with a graphite monochromator, Cukα wavelength in the following instrumental conditions: 15 kV, 30 mA, Ni filter, scan from 4 to 70°2θ, step sampling 0.01°θ. The identification of minerals was carried out using the search Crystal Impact MATCH! software v. 3.10, which uses, for comparison, both the JCPDS Database (Joint Committee on Powder Diffraction), and the COD Database (Crystallography Open Database) [37]. These results were compared to a quite unaffected old marble from a slab (Zelina Ferrà Gastaldi Millelire).
Total open porosity was measured by mercury intrusion porosimetry (MIP) on 1 cm3 (c.a.) degraded and control marble fragments oven-dried at 50 ± 5 °C until reaching constant mass. An AutoPore IV 9500 (Micromeritics Instrument Corporation, Norcross, GA, USA) operating at 2200 bar, and 10 s of equilibration time was used. Results were compared to unaffected-new Carrara white marble fragments (Statuarietto lithotype) used as control.
Specimens of about 0.5 × 0.5 cm, were preliminarily observed in light reflection mode using a MoticTM binocular microscope (Kowloon, Hong Kong) to evaluate the conservation conditions of samples and to select the portions of major interest for SEM analysis. These fragments were then glued in epoxy resin (Struers Ltd., Catcliffe, UK), cut in thin sections (30 µm thick) and finally polished. Other fragments were coated with a graphite layer (10 to 20 nm thick) and observed with a Zeiss EVO LS 15 environmental scanning electron microscope (Carl Zeiss SMT AG, Oberkochen, Germany), LaB6 cathode, EHD 5 kV, WD 13.5 mm.
2.4. Selected Chemicals
Among the available traditional biocidal formulations [20,21], we selected four products. Two of them contain quaternary ammonium compounds (QAC): Benzalkonium Chloride (CTS srl, Altavilla Vicentina, Italy; benzalkonium chloride 90%; BZC), and Preventol RI50 (Bresciani srl, Milano, Italy; benzalkonium chloride 50%; PREV). Otherwise, Lichenicida 264 (Bresciani srl, Milano, Italy; LICH) has N,N-dimethyl-N′-phenyl-N′-(fluoro-dichloromethyl)-sulphamide (also known as dichlofluanid) as active molecule, and Biotin R (CTS srl, Altavilla Vicentina, Italy; BioR) contains 3-iodo-2-propynyl butyl carbamate (IPBC), and 2-n-octyl-4-isothiazolin-3-one (OIT) dissolved in 2-(2-butoxyethoxy) ethanol. BZC and PREV were diluted in distillated water, LICH in alcohol [38], and BioR in white spirit.
2.5. Sensitiveness to Biocides
The sensitiveness of fungal isolates to biocides was measured using the agar diffusion tests (ADT) method. Due to their dimorphic clumped growth habit, the strong but variable melanization, and the absence of spores, the BF suspension was standardized as dry weight as follows: a MA slant culture was suspended scaping its surface in 3 mL of sterile saline solution (NaCl 0.9%), transferred into a 5 mL conical tube, homogenized by sterile pestle, and used to inoculate a 250-mL Erlenmeyer flask with malt extract broth (3% w/v; 30 mL). The incubation was performed at room temperature and 180 rpm on a rotary shaker for 1–3 days. ADTs were performed, in triplicate using 90 mm-Petri dishes. For each plate 200 μL of fungal suspension (8 ± 0.2 mg/mL dry weight) were added to 15 mL of MA by inclusion; a cellulose sterile disk (6 mm diam) was placed on the culture medium and soaked with 6 μL of biocide solution. Biocides were tested at the following concentrations: 0.5, 1, 1.5, 2, and 3% for BZC and PREV; 0.25, 0.5, and 1% for LICH, and 0.5, 1, 2, 3, 4, and 5% for BioR.
2.6. Statistical Analyses
PAST software (PAleontological STatistics, ver. 4.06b, [39]) was used to perform a Principal Component Analysis (PCA) assessing similarities/differences among the strains in study with respect of their salt tolerance, metabolic traits, and resistance to biocides.
Two-way ANOVA with Tukey post hoc test comparison (p < 0.05) was performed using GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA) to assess significance between biocides increasing doses.
3. Results
3.1. Identification and Selection of BF Species
Based on GenBank sequence match, the three strains were identified as follows. Strain CCFEE 5778 has been reported as Saccotheciaceae sp. due to the ITS (OM390240) best match with Saccothecium rubi MFLUCC 14-1171 (98.16%), and LSU (OM346745) match with Selenophoma linicola CBS 468.88 (98.90%) and Aurobasidium pullulans AFTOL-ID 912 (98.70%). Due to his very poor ITS match (OM568835) with Diaporthe longispora CBS 194.36 (coverage below 40%) and 97.21% LSU (OM346746) identity with Phaeotrichum benjaminii CBS 541.72, CCFEE 5945 was referred as “Unknown dothideomycete”. Differently, CCFEE 6327 (OM390241) was identified as Exophiala oligosperma due to its 99.83% identity with the species type strain CBS 725.88.
The selected strains were in total 18, one for each species found, and among them were 4 Knufia, 3 Neodevriesia, and 2 Exophiala species (Table 1).

Table 1.
The 18 strains in study, collection number (CCFEE), and monuments of isolation. (T) indicates type strain. In bold the new identified strains. All monuments were built in white Carrarese marble.
3.2. Main Ecological Traits Assessment: Thermal and Salt Tolerance
All the tested strains were able to grow within the range of 5–25 °C (Figure 2, Table S1); the majority can grow at 0 °C (13/18), less than a half (7/18) at 35 °C; only E. oligosperma CCFEE 6327 at 37 °C, and none at 40 °C. Most of the strains grew best at 20 °C, except K. petricola CCFEEE 5776, N. capensis CCFEE 6200, and E. oligosperma CCFEE 6327 having an optimum at 25 °C.
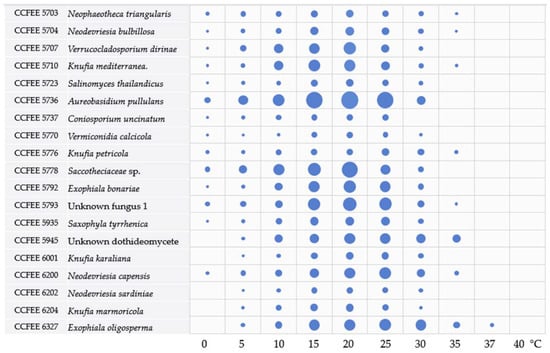
Figure 2.
Thermal preference chart. The bubble size is proportional to the average growth recorded at the end of the experiment (1 month). Table S1 reports the full average values and SD recorded.
Growth rates were extremely variable among the studied strains; for instance, after one month of incubation strains A. pullulans CCFEE 5736, Saccotheciaceae sp. 5778, E. bonariae CCFEE, 5792, and E. oligosperma CCFEE 6327 grew from about four (3.6) to seven times more than V. calcicola CCFEE 5770 and C. uncinatum CCFEE 5737.
Different degrees of salt tolerance were recorded (Figure 3, Table S2). Verr. dirinae CCFEE 5707, N. bulbillosa CCFEE 5704, Sal. thailandicus CCFEE 5723, and Neoph. triangularis CCFEE 5703 grow better in the presence of 3.5%. Five strains (namely the previous and A. pullulans) were able to grow at 20% NaCl, the highest concentration tested. A. pullulans CCFEE 5736, although able to grow in the presence of salt, drastically reduced its colony size up to 46% at 3.5% and 95.6% at 20% of NaCl. A high sensitivity to salt was instead found for the unknown dothideomycete CCFEE 5945 with a dramatic decrease in colony diameter of up to 74% in the presence of NaCl 3.5%, and no growth at 7%. Similarly, the slow-growing C. uncinatum CCFEE 5737 was unable to grow at 7%. The remaining strains showed instead an intermediate tolerance having K. petricola CCFEE 5776 as the most tolerant of the strains able to grow up to 10% NaCl.
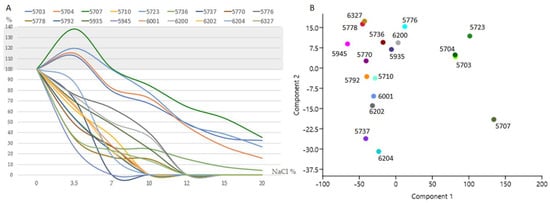
Figure 3.
Salt tolerance tests results. (A) The measures taken after one-month incubation at 20 °C are calculated as a percentage compared to the control plate (0% NaCl). (B) The PCA plot evidenced in the right side of the separated group of halophilic strains, and others (from right to left side) ordered according to their salt tolerance.
3.3. Mineralogical and Physical Investigations
Similar XRD diffraction patterns were recorded in the two deteriorated marbles affected by granular disintegration and crumbling (namely Antonio Viganigo, AV; and Ruda Roych, RR-data not shown). Significative differences were instead evidenced when the diffraction pattern of the AV deteriorated and soluble salt affected marble (Figure 4B) was compared to a gypsum film from an old compact marble monument (Ferrà Gastaldi Millelire; FGM, Figure 4A). Soluble salts and other decay phases were found in different amounts. In the grey film of FGM monument gypsum (CaSO4·2H2O) and calcite (CaCO3) prevailing on whewellite (CaC2O4·H2O), quartz (SiO2), halite (NaCl), sylvine (KCl) and titanite (CaTiSiO5). On a more weathered monument (AV), affected by granular disintegration and crumbling, the whitish crust shows mineralogical phases related to natural pollution (marine and soil particles) as halite, quartz, albitic plagioclase, as well gypsum and calcite.
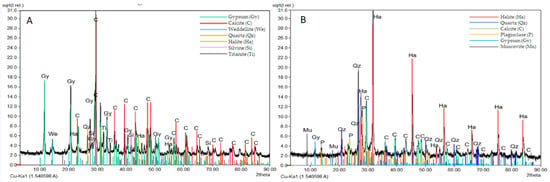
Figure 4.
XRD analysis results. XRD patterns of gypsum grey film on old compact marble (FGM) (A) and whitish crust on crumbling-granular disintegration marble (AV) (B).
MIP porograms (Figure 5A,B) evidenced high differences when comparing a new “Statuarietto” Carrara marble having a porosity of 0.7% ± 0.2 (black), and historical marble affected by granular decoesion 5% ± 0.2 (AV, gray) and high granular decoesion 8% ± 0.2 (RR, red). The porograms show macro- and micro-pores; the former are represented by microcracks or confluent calcitic crystal dislocation. The secondary porosity, acquired by physical and chemical decay processes, represents a key indicator of biodeterioration/bioreceptivity.
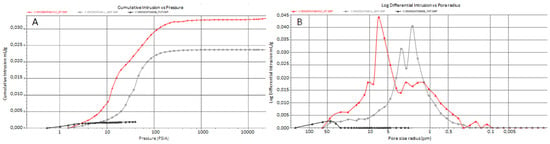
Figure 5.
MIP porosity results. MIP porosity of an unaffected-new “Statuarietto” marble (black), a decohesed (gray, AV), and highly decohesed marble (red, RR). (A) Cumulative Hg intrusion vs. pressure and (B) Log. differential intrusion vs. pore size distribution.
Light microscopy evidenced the presence of salts and other pollution materials in dark crusts (Figure 6B,D). SEM investigation confirmed the deep decohesion of calcitic crystals clasts, the presence of gypsum (desert rose twinning, Figure 6E), and halite crystals already evidenced in the previous analyses; these latter trials give an impacting measure of the phoenomena being marble crystals completely covered by salt (Figure 6H).
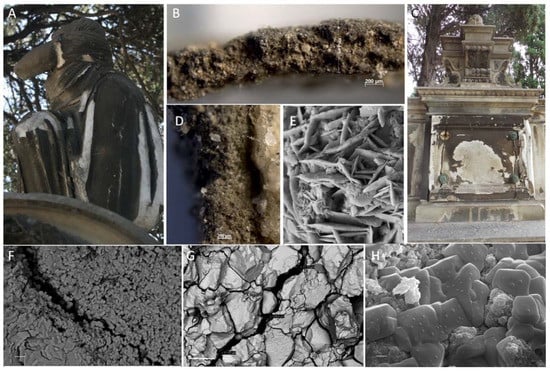
Figure 6.
Granular disintegration marble: a salty niche. Particular to the monuments affected by granular disintegration into sand and powder devoted to (A) Ruda Roych (RR) and (C) Antonio Viganigo (AV). (B,D) Dark sulfation crust of AV monument by light reflection optical microscopy: (C) cross section, (D) surface and fracture on dark crust and salt crystals (NaCl) deposition. (E–H) RR sample SEM observation. (E) gypsum crystals; (F,G) granular disintegration, (H) salt crystals (NaCl) on marble surface. The bar corresponds to 10 μm in (F), 200 μm in (G); and 2 μm in (H).
3.4. Detrimental Potential of the Investigated Species: The Metabolic Screenings
As reported in Table 2, acid production was observed after iodine pouring in nine out eighteen investigated strains. No production was recorded for the two Exophiala species, while positive records were found for all the Knufia species. All tested strains were positive for the esterase activity (LIP) being able to use Tween 20 as a sole carbon source and precipitate calcium salt crystals. The majority of the tested strains showed amylase (14/18), and cellulase (15/18) activity, while caseinase and pectinase activities were recorded in half of them (9/18).

Table 2.
Results of the metabolic assays performed on selected BF. The activities tested were for acid (ACID), lipase (LIP), amylase (AMY), protease (SM), pectinase (PSAM), and cellulase (CMC) production.
PCA analysis performed on halos occurred after metabolic assays evidenced in the right side the most responsive strains (Figure 7). All the halophilic strains take place within the most responsive strains. Interestingly K. mediterranea CCFEE 5710, due to the intense halos recorded, occupied quite a far position in respect to the other Knufia species in the study (namely K. petricola CCFEE 5776, K. karalitana CCFEE 6001, and K. marmoricola CCFEE 6204).
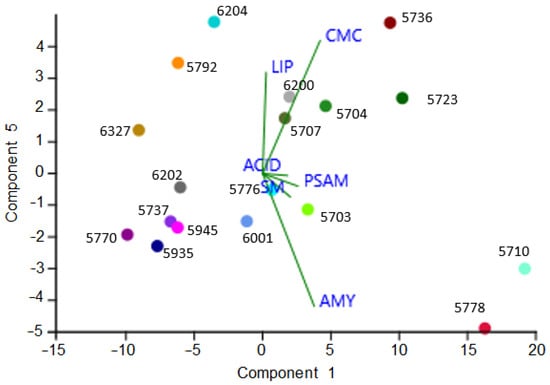
Figure 7.
PCA analysis performed on halos recorded for each plate test.
Moreover, during metabolic screenings it was possible to collect additional information such as the loss of melanization by the dothidealean fungi (namely A. pullulans CCFEE 5736 and Saccotheciaceae sp. CCFEE 5778) when grown in SM (Figure 8L), the volcano-like growth showed by K. mediterranea CCFEE 5710 in presence of high concentration of glucose (Figure 8B), and the inhibitory action of K. marmoricola and K. petricola against a cladosporiacean plate contaminant (Figure 8M,N, respectively).
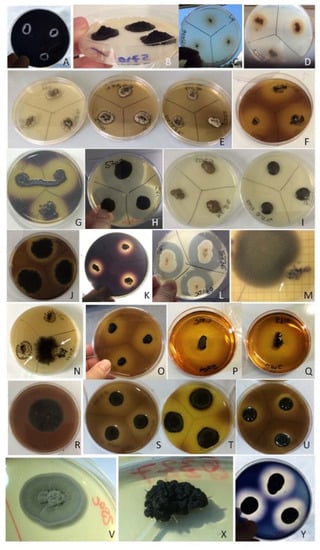
Figure 8.
Plate assays outcomes. (A) C. uncinatum CCFEE 5737 AMY test; (B) K. mediterranea CCFEE 5710 on CaCO3 agar: the colonies raise in the center and detach from plate; (C) K. petricola CCFEE 5776 esterase activity test (LIP); (D) E. oligosperma CCFEE 6327 esterase activity test (LIP); (E) N. bulbillosa CCFEE 5704 culture media darkening in presence of salt: from left to right side NaCl 0%, 3.5% and NaCl 7%; (F) K. mediterranea CCFEE 5710 PSAM test; (G) Neoph. triangularis CCFEE 5703 amylase test (AMY); (H) Neoph. triangularis CCFEE 5703 SM test; (I) Neoph. triangularis CCFEE 5703 colonies color shift in presence of salt from left to the right side NaCl 0% and 3.5%; (J) Verr. dirinae CCFEE 5707 PSAM test; (K) V. calcicola CCFEE 5770 AMY test; (L) A. pullulans CCFEE 5736 SM test; (M) Contaminated PSAM plate with K. marmoricola CCFEE 6204 creating inhibition halos (white arrow). (N) Contaminated PSAM plate with K. petricola CCFEE 5776 creating inhibition halos (white arrows); (O) K. petricola CCFEE 5776 acid production plate; (P) N. bulbillosa CCFEE 5704 cellulase test plate (CMC); (Q) K. mediterranea CCFEE 5710 cellulase test plate; (R) Verr. dirinae CCFEE 5707 negative acid production test; (S–U) K. karalitana CCFEE 6001, K. mediterranea CCFEE 5710, K. marmoricola CCFEEE 6204 positive acid production plate test; (V,X) E. oligosperma CCFEE 6327 colony morphology shifts from flat (V) grown at 25 °C to meristematic (X) grown at 35 °C; (Y) N. capensis CCFEE 6200 AMY test.
3.5. Sensitiveness to Traditional Biocides
The tested black fungal strains showed different degrees of sensitiveness to biocides (Table 3) and no linear response to incremental doses (Table S3). Two successive increasing biocide doses frequently did not significantly increase inhibitory halos (Table S4).

Table 3.
Inhibition halos recorded after the application of the highest dose suggested by suppliers. The different colors represent the different sensitiveness to biocides, as indicated in the legend below, where h is the halo recorded in millimeters. The complete results are shown in Table S3.
The majority of strains evidenced a low sensitiveness to both BZC and PREV, and others, such E. bonariae CCFEE 5792, proved quite tolerant to all the biocides tested.
PCA analysis performed considering all responses to the different doses of biocides applied (Table S3), evidenced a sort of gradient where N. bulbillosa CCFEE 5704, V. calcicola CCFEE 5770, and S. tyrrhenica CCFEE 5935 resulted the most sensitive species to the tested biocides (Figure 9A).
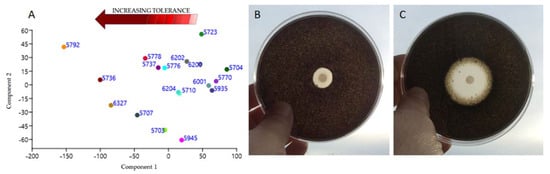
Figure 9.
Results of sensitiveness to traditional biocides. (A) PCA plot based on the ADT halos recorded using four different biocides at all the tested doses; from the left to the right there is an increasing sensitiveness, in the opposite sense an increasing tolerance to biocides. (B,C) ADT performed on E. oligosperma CCFEE 6327: (B) sharp inhibition halo obtained using BZC 3%, (C) double halo obtained when BioR 4% was used. There are notable zones of total (next to the disc), and partial inhibition (external) where growth is slowed.
4. Discussion
Biodeterioration processes are rarely caused by only one group of microorganisms, and more frequently are due to polymicrobic associations coexisting at the same time, in the same place, on a given artifact. To study them, two main approaches are available: the high throughput sequencing and the traditional culture-based approach. The biomolecular approach has become the dominant method of studying the microbial diversity in the last decade [41]. In fact, the main part of environmental microorganisms are unculturable and the behavior of a single species can frequently be explained in a community context [41]. However, beyond the exponentially increasing information, there is not an equally rapid increase in knowledge regarding the mechanisms and the main actors of the deterioration phenomena [42]. Moreover, the identification and characterization of monument settlers from a physiological and ecological point of view is of the utmost importance in order to design preventive control methods and biocide treatments [21,36,43,44,45]. From this perspective, an increasing number of papers propose traditional metabolic plate assays as a useful, easy and cost-effective tool to unveil the potential risk tied to some cultural heritage settlers [30,36,46,47,48,49,50]. Such a proposal is confirmed in this work.
4.1. Selection and Identification of the BF Strains of Interest
Even if they were all isolated from a single site, the eighteen strains considered in this study could represent other BF lithic communities even at different latitudes. Knufia, Neodevriesia, Exophiala, Coniosporium, and Vermiconidia are indeed well known genera due to their frequent finding and broad distribution on stone monuments in urbanized areas and natural crops.Their presence has been recorded in the Mediterranean countries as well as in Russia, Ukraine, Austria, Germany, Poland, China and, not least, Antarctica [51,52,53]. Information on their environmental preferences and metabolic traits could be helpful in the future to better understand their geographical distribution.
4.2. Temperature and Osmotic Tolerance as Indicators of Environmental Preferences
The thermal trials yielded important information on the optimum temperature of the studied strains, their growth range, and also on their growth rate. The majority of the strains grew within the range 5–30 °C, 39% of them instead within 0–35 °C, and only one, E. oligosperma CCFEE 6327, at 37 °C. With respect to their growth rate, the studied strains can be clustered into three groups: fast-; mid-fast-; and slow-growing black fungi. The majority of the slow-growing fungi (e.g., V. calcicola CCFEE 5770, C. uncinatum CCFEE 5737, and Knufia species) have been recorded from stone surfaces only; the others, namely the fast-growing (A. pullulans CCFEE 5736, and Saccotheciaceae sp. CCFEE 5778) and the mid-fast- growing species (e.g., E. bonariae and E. oligosperma, Unknown CCFEE 5945, Verr. dirinae CCFEE 5707, and N. capensis CCFEE 6200), can be considered at least in part to be occasional. A. pullulans is considered, indeed, a generalist that can inhabit different habitats without substantial specialization at the genomic level [54], and E. oligosperma is a well-known opportunist isolated from a number of household environments such as tap water and diesel car tanks [41,55,56]. This confirms the two survival strategies in fungi from extreme environments: they can be either widespread generalists, thriving in both extreme and moderate conditions, or specialists more or less confined to extremes [57,58,59].
Growth rate can even be drastically affected when salt is addicted to culture medium. All the tested strains were able to grow at 3.5% of NaCl. This trait increases their fitness for the site, being close to the sea with saltiness periodically deposited and washed away from surfaces with rains. Anyway, different degrees of tolerance were recorded, and strains tested grouped with respect to their response to increasing salt concentration and to their limits. The lowest tolerance has been recorded for C. uncinatum CCFEE 5737 and the unknown CCFEE 5945. The majority of the strains have their limits between 7 (6/18) and 10% (5/18), respectively. Five, instead, can grow up to 20%. While A. pullulans showed a broad-range tolerance to salt, Verr. dirinae CCFEE 5707, N. bulbillosa CCFEE 5704, Sal. thailandicus CCFEE 5723, and Neoph. triangularis CCFEE 5703 proved to be halophilic. The halophilic trait for a part of them was already reported [60,61], however, it was not for N. bulbillosa CCFEE 5704 and Verr. dirinae CCFEE 5707, which ability was suggested by indirect evidences only. For example, N. bulbillosa was isolated from limestone formations in Cala Saint Vincenꞔ in Mallorca Island, so possibly subjected to some extent to saltiness [62]. Otherwise, Verr. dirinae is worth a mention as even if described as a parasite of the lichen Dirina massiliensis [63], it has been isolated from other substrates/salted niches such as wood and marine macroalgae [64,65]. Moreover, the presence of some BF has been suggested to be in relation with the biodeterioration degree of tombstones [66].
4.3. Marble Deterioration Degree Selects the Resident Fungal Community
The instrumental stone investigations performed (namely XRD, light reflection microscopy, and SEM) on the two most deteriorated monuments evidenced the presence of gypsum and chloride crystals (mainly NaCl), the latter of which was found in high amounts. Their presence on marble stone is strictly related to the microclimatic condition, salt solubility and surface physical conditions (e.g., porosity, roughness). The severe alteration of the decayed marble has been shown by SEM and by MIP pore size distribution, where yields were up to 10 times higher than the new unaffected control specimen. These values can be explained with the time of outdoor exposition (1876 RR, 1890 AV, and 1900 FGM their years of built) and even more by microclimatic conditions. Previous studies showed that the most damaged marble pieces in the cemetery of Cagliari were all characterized by low values of solar radiation, high humidity and a reduced thermoforesis, favoring sulfatation and weathering along with the surfaces’ colonization [32,33,67,68]. Indeed, the nearby and dense vegetation, in addition to the low exposition to the dominant winds (Libeccio and Scirocco from south and Mistral from north-west), favored the stagnation of marine air and its penetration into damaged stones. Sea spray acts as a marine inoculum migrating between grains by capillarity; here the salt wet-dry cycles gradually and increasingly alter the materials’ structure [69,70,71,72]. Each crevice and intergrain space can become a salty micro-niche available for specialized microorganisms; along with the salt physical action, the selected community can contribute to stone structural destabilization [73,74,75,76]. The exclusive isolation of the halophilic fungi from sand disaggregated monuments only, highlighted as salt-attacked monuments constitute a suitable habitat for selected osmophilic/osmotolerant microorganisms [75,77,78]. However, this phenomenon seems to be progressive since Neoph. triangularis, N. bulbillosa, and Verr. dirinae were isolated from the RR monument (8% MIP porosity) only, while S. thailandicus and K. petricola from the AV monument (5% MIP porosity). On this basis, further studies are needed to investigate the possible use of the halophilic and the most halotolerant black fungal strains (e.g., K. petricola CCFEE 5776) as possible indicators of stone degradation in the early steps of salt-mediated decoesion or, by contrast, the use of low salt tolerant BF (e.g., C. uncinatum) as indicators of stone integrity in coastal environments.
4.4. Metabolic Assay, Ecological Traits, and Detrimental Potential of the Investigated Species
Knowledge on the relationship between microorganisms and colonized substrate drives the identification of the major conservation threats and may address the control strategies designed to face biodeterioration [79]. Plate assays were useful to outline some important metabolic traits in BF. Acid production has been recorded in half of the studied strains. Even if not leading to a complete dissolution of carbonates in the culture media, BF showed a detrimental potential well beyond their assessed biomechanical action [7]. A local decrease in the pH has been previously reported for K. petricola A95 and found here, for example, in all the Knufia species and in two of the three Neodevriesia [80]. Further studies are needed to asses if this trait is limitedly present in some selected genera or not. In any case more methods and/or protocol adjustments are needed to detect even weak responses.
The amylases, pectinases, esterases and cellulases activities recorded showed that these fungi can feed on vegetal sources and debris. According to the conceptual model for MCF proposed by Chertov and colleagues [81], the availability of organic nutrients is the dominant factor limiting their development on stone surfaces in European temperate and Mediterranean climates, while growth becomes very intensive in the presence of water. The metabolic features highlighted here support the well-known yet rarely applied practice of removing the organic matter from monuments to prevent fungal growth [82]. The production of esterases and caseinases activities suggests as these strains may also feed on wall paintings where oils and proteinaceous binders were used as already reported [83,84,85,86]. Furthermore, the inhibition halo (competition for space) produced by two Knufia species (namely K. petricola and K. mediterranea) against a cladosporiacean plate contaminant, provided evidence that some BF species in peculiar conditions (PSAM test) can compete, more than expected [87,88,89], against high sporulating fungi.
From a conservative point of view, the ability to metabolize carboxymethylcellulose (CMC) and Tween 20 (LIP) should be careful evaluated in restoration practice for their possible drawbacks. These chemicals, indeed, are commonly used as thickening agents and surfactants, respectively [90,91], and their residues on the treated artifact could lead to a tertiary bioreceptivity if not thoroughly removed.
4.5. Tolerance to Biocides
Despite the significant impact of the green revolution in the biocides market [92], traditional biocidal products are still widely used [93]. This is due to the consolidated protocols used on different materials, the wide knowledge on the pros and cons of their use, and the non-negligible economic aspects.
In situ trials are often used in restoration practice to customize treatments as they confidently reproduce, in a short time, the interaction of biocides with biota and materials, and provide a more reliable indication of the in situ persistence of the biocide [94,95,96,97]. However, several factors can negatively affect the efficiency of any restoration protocol. The metabolic state of the organism is important for their responsiveness to biocides treatments. Metabolically active colonies are, indeed, more sensitive to chemical and physical stresses [98]. BF, being poikilohydric organisms, withstand the unfavorable conditions in a dormant state mainly induced by desiccation (anhydrobiosis) [12,99]. In addition, fungi growing in stone cracks and fissures may be not easily reached by biocides applied on a monument surface during restoration treatments. Even the typical clump-like BF colonies, ensuring the optimal surface-volume ratio, minimizes the direct exposure to external stressors, including biocides; moreover, external cells in the colony shield the inner layers from external injuries (shadow effect) [100]. Conversely ADT plate assays have an undeniable advantage as they reproduce the best conditions to record the sensitivity to biocides of single metabolically active cells in a medium that cannot seize the biocide as stones frequently do. For these reasons, the tolerance/resistance recorded in the most responsive condition should warn about their possible survival after treatments, as well as their spread.
The used biocides have different formulations, mechanisms of action, and thus different outcomes. BZC (CAS No 8001-54-5) is a quaternary ammonium compound (QAC) routinely used for chemical antisepsis and disinfection and widely applied in cultural heritage preservation treatments. According to the concentration used, BZC acts as a highly active detergent causing cell membrane permeability alterations, irreversible damage to the barrier function and proteins denaturation [101]. Benzalkonium chloride is also the only active compound in the PREV patent, albeit in a lower concentration (50%) than in BZC (90%). Despite the lower concentration of QAC salt in PREV, the inhibition halos produced were frequently larger than BZC, such as for Sal. thailandicus CCFEE 5723, K. petricola CCFEE 5776, and S. tyrrhenica CCFEE 5935. This apparent incongruence, already evidenced in previous studies [36], has been explained with the possible different composition in terms of n-alkyl groups length and/or degree of C-C saturation in the two chemicals [102], and by additives, not specified in the composition of biocides but included in the patent of the commercial formulas [21,103]. The other two biocides tested have instead intracellular targets. As a matter of fact, N,N-dimethyl-N′-phenyl-N′-(fluoro-dichloromethylthio)-sulphamide (CAS No 1085-98-9), the active principle of LICH, is supposed to affect a number of enzymes by reacting with –SH bonds [104]. While the fungicidal and antimicrobial action played by IPBC (3-iodo-2-propynyl butyl carbamate, CAS No 55406-53-6) and OIT (2-n-octyl-4-isothiazolin-3-one; CAS No 26530-20-1), the active principles of BioR, are probably due to iodine toxicity, and inhibition of cell respiration/ATP production, respectively [105,106].
The achieved results evidenced an increasing biocidal power, in terms of inhibition halos and number of strains completely inhibited, as follows: BZC-PREV, LICH, and BioR. The thick multilayered and strongly melanized cell wall typical of these fungi serve as an efficient barrier for toxic substances with positive influence on resistance. Moreover, the presence of halos of total and partial inhibition recorded frequently when PREV, LICH, and BioR were applied (Figure 6C), could be explained by a certain ability to repair sub-lethal damages as resistance mechanism to biocides. Several strains, such as E. bonariae, A. pullulans, E. oligosperma, Verr. dirinae, and secondly K. petricola, and C. uncinatum, exhibited low responsiveness even to the most powerful biocide. This fact should warn against the use of extremely powerful biocides as a quick remedy in controlling whatever biological patina as the drastic decrease in competitors favors the spreading of the ones who survived. In the meanwhile, a stronger biocidal action is often backed to a higher toxicity for the operator and the environment. Conversely, the widespread use of ammonium quaternary salts (e.g., benzalkonium chloride), should be reduced to oppose the rapid recolonizations by serving as nutrients for micro- and macro-organisms [107]. The wide use of sub-inhibitory concentrations of benzalkonium chloride (and QACs), it has been suggested, may be responsible for a significant decrease in microorganisms’ sensitivity to biocides, driving the evolution of polyextremotolerant fungi towards the enhancement of their stress tolerance [108]. For this reason, the rotation of disinfectants is often recommended in practice, in order to kill the resistant biota [109]. In this context plate assays could be useful to test multiple chemicals. The ability to cope with different biocidal products shown by these strains also make them promising candidates as new reference organisms in material testing procedures [110].
5. Conclusions
This research gave an assessment of the main BF traits with respect to thermal and salt growth preferences, such as on the possible relation between the recorded fungal traits and the substrate. Plate assays resulted a fast, easy, cost-effective, and useful tool for such aims. To deepen the BF detrimental/colonizing potential, more plate tests should be designed or improved, and physical analyses were fundamental in proving the relation between halophilic strains and highly deteriorated marble surfaces. We also assessed the detrimental potential of the investigated species through metabolic assays, which were also useful to determine the conditions favoring their growth and substances that should be avoided to prevent tertiary bioreceptivity. We also evaluated the sensitivity to a few traditional biocides through ADT tests, which were useful to identify the most recalcitrant BF species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12042038/s1, Table S1: Thermal preferences recorded within the range 0–40 °C, Table S2: Salt tolerance recorded within the range 0–20% NaCl, Table S3: ADT results, Table S4: ADT two-way ANOVA Tuckey test row analysis.
Author Contributions
Conceptualization, D.I.; methodology, D.I., F.B. and P.M.; data curation, D.I., P.M. and F.B.; writing—original draft preparation, D.I.; writing—review and editing, D.I., L.Z., P.M., F.B. and G.C.; All authors have read and agreed to the published version of the manuscript.
Funding
The Ordine Nazionale dei Biologi for funding a scholarship for the project “Fungal deteriogens and stone monuments”.
Institutional Review Board Statement
No humans or animals are involved in this study.
Informed Consent Statement
No humans are involved in this study.
Data Availability Statement
All data resulting from this research are fully reported here or as Supplementary Material.
Acknowledgments
The authors wish to thank the Superintendence of Heritage Landscape, Historical, Artistic and Ethno-Anthropological Heritage for the metropolitan city of Cagliari and for the provinces of Oristano, Medio Campidano and, Municipality Cemeteries Direction of Cagliari for collaboration and sampling permission. Gianfranco Carcangiu is acknowledged for support in elaboration of XRD analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caneva, G.; Ceschin, S. Ecology of biodeterioration. In Plant Biology for Cultural Heritage; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; The Getty Conservation Institute: Los Angeles, CA, USA, 2009; pp. 35–58. [Google Scholar]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Sáiz-Jiménez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef]
- Tonon, C.; Favero-Longo, S.E.; Matteucci, E.; Piervittori, R.; Croveri, P.; Appolonia, L.; Meirano, V.; Serino, M.; Elia, D. Microenvironmental features drive the distribution of lichens in the House of the Ancient Hunt, Pompeii, Italy. Int. Biodeterior. Biodegrad. 2019, 136, 71–81. [Google Scholar] [CrossRef]
- Isola, D.; Selbmann, L.; Meloni, P.; Maracci, E.; Onofri, S.; Zucconi, L. Detrimental rock black fungi and biocides: A study on the Monumental Cemetery of Cagliari. In Science and Technology for the Conservation of Cultural Heritage; Rogerio-Candelera, M.A., Lazzari, M., Cano, E., Eds.; CRC Press: London, UK, 2013; pp. 83–86. [Google Scholar]
- Onofri, S.; Zucconi, L.; Isola, D.; Selbmann, L. Rock-inhabiting fungi and their role in deterioration of stone monuments in the Mediterranean area. Plant. Biosyst. 2014, 148, 384–391. [Google Scholar] [CrossRef]
- Tonon, C.; Breitenbach, R.; Voigt, O.; Turci, F.; Gorbushina, A.A.; Favero-Longo, S.E. Hyphal morphology and substrate porosity -rather than melanization-drive penetration of black fungi into carbonate substrates. J. Cult. Herit. 2021, 48, 244–253. [Google Scholar] [CrossRef]
- Selbmann, L.; Isola, D.; Zucconi, L.; Onofri, S. Resistance to UV-B induced DNA damage in extreme-tolerant cryptoendolithic Antarctic fungi: Detection by PCR assays. Fungal Biol. 2011, 115, 937–944. [Google Scholar] [CrossRef]
- Selbmann, L.; Grube, M.; Onofri, S.; Isola, D.; Zucconi, L. Antarctic epilithic lichens as niches for black meristematic fungi. Biology 2013, 2, 784–797. [Google Scholar] [CrossRef] [Green Version]
- Selbmann, L.; Isola, D.; Egidi, E.; Zucconi, L.; Gueidan, C.; de Hoog, G.S.; Onofri, S. Mountain tips as reservoirs for new rock-fungal entities: Saxomyces gen.nov. and four new species from Alps. Fungal Divers. 2014, 65, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Marvasi, M.; Donnarumma, F.; Frandi, A.; Mastromei, G.; Sterflinger, K.; Tiano, P.; Perito, B. Black microcolonial fungi as deteriogens of two famous marble statues in Florence, Italy. Int. Biodeterior. Biodegrad. 2012, 68, 36–44. [Google Scholar] [CrossRef]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2013, 175, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Dornieden, T.; Gorbushina, A.A.; Krumbein, W.E. Biodecay of cultural heritage as a space/time related ecological situation—An evaluation of a series of studies. Int. Biodeterior. Biodegrad. 2000, 46, 261–270. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Gazzano, C.; Girlanda, M.; Castelli, D.; Tretiach, M.; Baiocchi, C.; Piervittori, R. Physical and chemical deterioration of silicate and carbonate rocks by meristematic microcolonial fungi and endolithic lichens (Chaetothyriomycetidae). Geomicrobiol. J. 2011, 28, 732–744. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Krumbein, W.E.; Hamann, C.H.; Panina, L.; Soukharjevski, S.; Wollenzien, U. Role of black fungi in color change and biodeterioration of antique marbles. Geomicrobiol. J. 1993, 11, 205–222. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey). Antonie van Leeuwenhoek 2007, 91, 217–227. [Google Scholar] [CrossRef]
- Toreno, G.; Isola, D.; Meloni, P.; Carcangiu, G.; Selbmann, L.; Onofri, S.; Caneva, G.; Zucconi, L. Biological colonization on stone monuments: A new low impact cleaning method. J. Cult. Herit. 2018, 30, 100–109. [Google Scholar] [CrossRef]
- Caneva, G.; Isola, D.; Lee, H.J.; Chung, Y.J. Biological risk for hypogea: Shared data from Etruscan tombs in Italy and ancient tombs of the Baekje dynasty in Republic of Korea. Appl. Sci. 2020, 10, 6104. [Google Scholar] [CrossRef]
- Caneva, G.; Bartoli, F.; Savo, V.; Futagami, Y.; Strona, G. Combining statistical tools and ecological assessments in the study of biodeterioration patterns of stone temples in Angkor (Cambodia). Sci. Rep. 2016, 6, 32601. [Google Scholar] [CrossRef] [Green Version]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press CRC Press: Oakville, ON, Canada, 2017; 360p. [Google Scholar]
- Caneva, G.; Nugari, M.P.; Pinna, D.; Salvadori, O. Il Controllo del Degrado Biologico: I Biocidi nel Restauro dei Materiali Lapidei; Nardini: Firenze, Italy, 1996; pp. 1–200. [Google Scholar]
- Nugari, M.P.; Salvadori, O. Biocides and treatment of stone: Limitation and future prospects. In Art, Biology and Conservation: Biodeterioration of Works of Art; The Met Fifth Avenue: New York, NY, USA, 2003; pp. 518–535. [Google Scholar]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Cappitelli, F.; Nosanchuk, J.D.; Casadevall, A.; Toniolo, L.; Brusetti, L.; Florio, S.; Principi, P.; Borin, S.; Sorlini, C. Synthetic consolidants attacked by melanin-producing fungi: Case study of the biodeterioration of Milan (Italy) Cathedral marble treated with acrylics. Appl. Environ. Microbiol. 2007, 73, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Cappitelli, F.; Villa, F.; Sanmartín, P. Interactions of microorganisms and synthetic polymers in cultural heritage conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
- De Leo, F.; Urzì, C. Fungal colonization on treated and untreated stone surfaces. In Molecular Biology and Cultural Heritage; Saiz-Jimenez, C., Ed.; Swets & Zeitlinger BV: Lisse, The Netherlands, 2003; pp. 213–218. [Google Scholar]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Nai, C.; Wong, H.Y.; Pannenbecker, A.; Broughton, W.J.; Benoit, I.; de Vries, R.P.; Gueidan, C.; Gorbushina, A.A. Nutritional physiology of a rock-inhabiting, model microcolonial fungus from an ancestral lineage of the Chaetothyriales (Ascomycetes). Fungal Genet. Biol. 2013, 56, 54–66. [Google Scholar] [CrossRef]
- Voigt, O.; Knabe, N.; Nitsche, S.; Erdmann, E.A.; Schumacher, J.; Gorbushina, A.A. An advanced genetic toolkit for exploring the biology of the rock-inhabiting black fungus Knufia petricola. Sci. Rep. 2020, 10, 22021. [Google Scholar] [CrossRef]
- Trovão, J.; Portugal, A. Current knowledge on the fungal degradation abilities profiled through biodeteriorative plate essays. Appl. Sci. 2021, 11, 4196. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; De Hoog, G.S.; Selbmann, L. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar] [CrossRef]
- Meloni, P.; Vacca, G.; Massidda, L.; Carcangiu, G.; Mameli, P.; Cocco, O.; Toreno, G. Vulnerabilità dei beni culturali: Sistemi informatici finalizzati alla conservazione programmata e preventiva di monumenti in marmo. In Pensare la Prevenzione: Manufatti, Usi, Ambienti; Ricerche, A., Ed.; Parco Scientifico Tecnologico di Venezia: Marghera-Venezia, Italy, 2010; pp. 385–392. [Google Scholar]
- Meloni, P.; Vacca, G.; Massidda, L.; Carcangiu, G.; Mameli, P.; Toreno, G. Architettura in un Sistema GIS per la conservazione programmata delle opere all’aperto del cimitero storico di Bonaria in Cagliari (Sardegna). In Proceedings of the Atti del IV Convegno di Monitoraggio e Conservazione preventiva dei Beni Culturali, Fac. di Ingegneria, Cassino, Italy, 27–28 May 2010; pp. 76–82. [Google Scholar]
- Meloni, P.; Toreno, G.; Carcangiu, G.; Cocco, O.; Deiana, M.; Murru, A. Sinergie per la conservazione degli spazi della memoria: L’esperienza didattica dei cantieri pilota del cimitero monumentale di Bonaria in Cagliari. In Atti 2° Congresso Specialistico Internazionale sui Cimiteri Monumentali: Conoscenza, Conservazione e Restyling; Cicop Italia—Centro Internazionale per la Conservazione del Patrimonio Architettonico: Firenze, Italy, 2013; pp. 166–171. [Google Scholar]
- Isola, D.; Bartoli, F.; Langone, S.; Ceschin, S.; Zucconi, L.; Caneva, G. Plant DNA Barcode as a Tool for Root Identification in Hypogea: The Case of the Etruscan Tombs of Tarquinia (Central Italy). Plants 2021, 10, 1138. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Cecchini, A.; Caneva, G. Dark-pigmented biodeteriogenic fungi in Etruscan hypogeal tombs: New data on their culture-dependent diversity, favouring conditions, and resistance to biocidal treatments. Fungal Biol. 2021, 125, 609–620. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Czaja-Szewczak, B. Burial tunics from Naqlun. In Christianity and Monasticism in the Fayoum Oasis; Gabra, G., Ed.; Essays of the 2004 International Symposium of the Saint Mark Foundation and the Saint Shenouda the Archimandrite Coptic Society in Honor of Martin Krause; American University in Cairo Press: Cairo, Egypt, 2005; pp. 133–142. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 9 December 2021).
- Czachura, P.; Owczarek-Kościelniak, M.; Piątek, M. Salinomyces polonicus: A moderately halophilic kin of the most extremely halotolerant fungus Hortaea werneckii. Fungal Biol. 2021, 125, 459–468. [Google Scholar] [CrossRef]
- Isola, D.; Scano, A.; Orrù, G.; Prenafeta-Boldú, F.X.; Zucconi, L. Hydrocarbon-contaminated sites: Is there something more than Exophiala xenobiotica? New insights into black fungal diversity using the long cold incubation method. J. Fungi 2021, 7, 817. [Google Scholar] [CrossRef]
- Sterflinger, K.; Little, B.; Pinar, G.; Pinzari, F.; de los Rios, A.; Gu, J.D. Future directions and challenges in biodeterioration research on historic materials and cultural properties. Int. Biodeterior. Biodegrad. 2018, 129, 10–12. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Miller, A.Z.; Saiz-Jimenez, C. Lascaux Cave: An example of fragile ecological balance in subterranean environments. In Microbial Life in Cave Systems; Engel, A.S., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2015; pp. 279–301. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Krakova, L.; Pangallo, D.; Bruno, L. Effects of biocide treatments on the biofilm community in Domitilla’s catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: “Find the guilty bug—Microorganisms involved in the biodeterioration of archeological and historical artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef]
- Jurado, V.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Saiz-Jimenez, C. Biodeterioration of Salón de Reinos, Museo Nacional del Prado, Madrid, Spain. Appl. Sci. 2021, 11, 8858. [Google Scholar] [CrossRef]
- Ma, W.; Wu, F.; Tian, T.; He, D.; Zhang, Q.; Gu, J.-D.; Duan, Y.; Ma, D.; Wang, W.; Feng, H. Fungal diversity and its contribution to the biodeterioration of mural paintings in two 1700-Year-Old Tombs of China. Int. Biodeterior. Biodegrad. 2020, 152, 104972. [Google Scholar] [CrossRef]
- Savković, Ž.; Unković, N.; Stupar, M.; Franković, M.; Jovanović, M.; Erić, S.; Šarić, K.; Stanković, S.; Dimkić, I.; Vukojević, J.; et al. Diversity and biodeteriorative potential of fungal dwellers on ancient stone stela. Int. Biodeterior. Biodegrad. 2016, 115, 212–223. [Google Scholar] [CrossRef]
- Trovão, J.; Tiago, I.; Catarino, L.; Gil, F.; Portugal, A. In vitro analyses of fungi and dolomitic limestone interactions: Bioreceptivity and biodeterioration assessment. Int. Biodeterior. Biodegrad. 2020, 155, 105107. [Google Scholar] [CrossRef]
- Unković, N.; Erić, S.; Šarić, K.; Stupar, M.; Savković, Ž.; Stanković, S.; Dimkić, I.; Vukojević, J.; Grbić, M.L. Biogenesis of secondary mycogenic minerals related to wall paintings deterioration process. Micron 2017, 100, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Su, L.; Yang, S.; Sun, J.; Liu, B.; Fu, R.; Wu, B.; Liu, X.; Cai, L.; Guo, L.; et al. Unveiling the hidden diversity of rock-inhabiting fungi: Chaetothyriales from China. J. Fungi 2020, 6, 187. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Zelenskaya, M.S.; Vlasov, A.D.; Bobir, S.Y.; Yakkonen, K.L.; Vlasov, D.Y. Microorganisms in superficial deposits on the stone monuments in Saint Petersburg. Microorganisms 2022, 10, 316. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Zucconi, L.; Isola, D.; Rottigni, M.; Ghiglione, C.; Piazza, P.; Alvaro, M.C.; Schiaparelli, S. Distributional records of Antarctic fungi based on strains preserved in the Culture Collection of Fungi from Extreme Environments (CCFEE) Mycological Section associated with the Italian National Antarctic Museum (MNA). MycoKeys 2015, 10, 57. [Google Scholar] [CrossRef]
- Gostinčar, C.; Turk, M.; Zajc, J.; Gunde-Cimerman, N. Fifty Aureobasidium pullulans genomes reveal a recombining polyextremotolerant generalist. Environ. Microbiol. 2019, 21, 3638–3652. [Google Scholar] [CrossRef] [Green Version]
- Isola, D.; Selbmann, L.; de Hoog, G.S.; Fenice, M.; Onofri, S.; Prenafeta-Boldú, F.X.; Zucconi, L. Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia 2013, 175, 369–379. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Armjio-Medina, C.; Isola, D. Black fungi in the built environment—The good, the bad, and the ugly. In Viruses, Bacteria, and Fungi in the Built Environment. Designing Healthy Indoor Environments; Pacheco-Torgal, F., Ivanov, V., Falkinham, J.O., Eds.; Woodhead Publishing: Duxford, UK, 2021; pp. 65–99. [Google Scholar]
- Gostinčar, C.; Stajich, J.E.; Zupancic, J.; Zalar, P.; Gunde-Cimerman, N. Genomic evidence for intraspecific hybridization in a clonal and extremely halotolerant yeast. BMC Genom. 2018, 19, 364. [Google Scholar] [CrossRef]
- Gostinčar, C.; Zajc, J.; Lenassi, M.; Plemenitas, A.; de Hoog, S.; Al Hatmi, A.M.S.; Gunde-Cimerman, N. Fungi between extremotolerance and opportunistic pathogenicity on humans. Fungal Divers. 2018, 93, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Gostinčar, C.; Zalar, P.; Gunde-Cimerman, N. No need for speed: Slow development of fungi in extreme environments. Fungal Biol. Rev. 2022, 39, 1–14. [Google Scholar] [CrossRef]
- Kogej, T.; Ramos, J.; Plemenitas, A.; Gunde-Cimerman, N. The halophilic fungus Hortaea werneckii and the halotolerant fungus Aureobasidium pullulans maintain low intracellular cation concentrations in hypersaline environments. Appl. Environ. Microbiol. 2005, 71, 6600–6605. [Google Scholar] [CrossRef] [Green Version]
- Butinar, L.; Sonjak, S.; Zalar, P.; Plemenitaš, A.; Gunde-Cimerman, N. Melanized halophilic fungi are eukaryotic members of microbial communities in hypersaline waters of solar salterns. Botanica Marina 2005, 48, 73–79. [Google Scholar] [CrossRef]
- Egidi, E.; De Hoog, G.S.; Isola, D.; Onofri, S.; Quaedvlieg, W.; De Vries, M.; Verkley, G.J.M.; Stielow, J.B.; Zucconi, L.; Selbmann, L. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Divers. 2014, 65, 127–165. [Google Scholar] [CrossRef]
- Crous, P.W.; Braun, U.; Schubert, K.; Groenewald, J.Z. Delimiting Cladosporium from morphologically similar genera. Stud. Mycol. 2007, 58, 33–56. [Google Scholar] [CrossRef]
- Piñar, G.; Dalnodar, D.; Voitl, C.; Reschreiter, H.; Sterflinger, K. Biodeterioration risk threatens the 3100 year old staircase of Hallstatt (Austria): Possible involvement of halophilic microorganisms. PLoS ONE 2016, 11, e0148279. [Google Scholar] [CrossRef]
- Gnavi, G.; Garzoli, L.; Poli, A.; Prigione, V.; Burgaud, G.; Varese, G.C. The culturable mycobiota of Flabellia petiolata: First survey of marine fungi associated to a Mediterranean green alga. PLoS ONE 2017, 12, e0175941. [Google Scholar] [CrossRef]
- Owczarek-Kościelniak, M.; Krzewicka, B.; Piątek, J.; Kołodziejczyk, Ł.M.; Kapusta, P. Is there a link between the biological colonization of the gravestone and its deterioration? Int. Biodeterior. Biodegrad. 2020, 148, 104879. [Google Scholar] [CrossRef]
- Casti, M.; Meloni, P.; Pia, G.; Palomba, M. Differential damage in the semi-confined Munazio Ireneo cubicle in Cagliari (Sardinia): A correlation between damage and microclimate. Environ. Earth Sci. 2017, 76, 529. [Google Scholar] [CrossRef]
- Murru, A.; Freire-Lista, D.M.; Fort, R.; Varas-Muriel, M.J.; Meloni, P. Evaluation of post-thermal shock effects in Carrara marble and Santa Caterina di Pittinuri limestone. Constr. Build. Mater. 2018, 186, 1200–1211. [Google Scholar] [CrossRef]
- Silva, B.; Rivas, T.; Prieto, B. Relation between type of soluble salt and decay forms in granitic coastal churches in Galicia (NW Spain). In Proceedings of the Origin, Mechanisms and Effects of Salts on Degradation of Monuments in Marine and Continental Environments, Bari, Italy, 25–27 March 1996; Zezza, Ed.; pp. 181–190. [Google Scholar]
- Stryszewska, T. The change in selected properties of ceramic materials obtained from ceramic brick treated by the sulphate and chloride ions. Constr. Build. Mater. 2014, 66, 268–274. [Google Scholar] [CrossRef]
- Vázquez-Nion, D.; Troiano, F.; Sanmartín, P.; Valagussa, C.; Cappitelli, F.; Prieto, B. Secondary bioreceptivity of granite: Effect of salt weathering on subaerial biofilm growth. Mater. Struct. 2018, 51, 158. [Google Scholar] [CrossRef]
- Zanardini, E.; May, E.; Purdy, K.J.; Murrell, J.C. Nutrient cycling potential within microbial communities on culturally important stoneworks. Environ. Microbiol. Rep. 2019, 11, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Saiz-Jimenez, C.; Laiz, L. Occurrence of halotolerant/halophilic bacterial communities in deteriorated monuments. Int. Biodeterior. Biodegrad. 2000, 46, 319–326. [Google Scholar] [CrossRef]
- Piñar, G.; Ripka, K.; Weber, J.; Sterflinger, K. The micro-biota of a sub-surface monument the medival chapel of St. Virgil (Vienna, Austria). Int. Biodeterior. Biodegrad. 2009, 63, 851–859. [Google Scholar] [CrossRef]
- Adamiak, J.; Otlewska, A.; Gutarowska, B. Halophilic microbial communities in deteriorated buildings. World J. Microbiol. Biotechnol. 2015, 31, 1489–1499. [Google Scholar] [CrossRef]
- Adamiak, J.; Bonifay, V.; Otlewska, A.; Sunner, J.A.; Beech, I.B.; Stryszewska, T.; Kańka, S.; Oracz, J.; Żyżelewicz, D.; Gutarowska, B. Untargeted metabolomics approach in halophiles: Understanding the biodeterioration process of building materials. Front. Microbiol. 2017, 8, 2448. [Google Scholar] [CrossRef]
- Piñar, G.; Ettenauer, J.; Sterflinger, K. La vie en rose: A review of the rosy discoloration of subsurface monuments. In The Conservation of Subterranean Cultural Heritage; Saiz-Jimenez, C., Ed.; CRC Press/Balkema: Leiden, The Netherlands, 2014; pp. 113–124. [Google Scholar]
- Otlewska, A.; Adamiak, J.; Stryszewska, T.; Kanka, S.; Gutarowska, B. Factors determining biodiversity of halophilic microorganisms on historic masonry buildings. Microbes Environ. 2017, 32, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A. Melding the old with the new: Trends in methods used to identify, monitor, and control microorganisms on cultural heritage materials. Microb Ecol. 2018, 76, 64–80. [Google Scholar] [CrossRef]
- Gerrits, R.; Pokharel, R.; Breitenbach, R.; Radnik, J.; Feldmann, I.; Schuessler, J.A.; von Blanckenburg, F.; Gorbushina, A.A.; Schott, J. How the rock-inhabiting fungus K. petricola A95 enhances olivine dissolution through attachment. Geochim. Cosmochim. Acta 2020, 282, 76–97. [Google Scholar] [CrossRef]
- Chertov, O.; Gorbushina, A.; Deventer, B. A model for microcolonial fungi growth on rock surfaces. Ecol. Modell 2004, 177, 415–426. [Google Scholar] [CrossRef]
- Salvadori, O.; Municchia, A.C. The role of fungi and lichens in the biodeterioration of stone monuments. Open Conf. Proc. J. 2016, 7, 39–54. [Google Scholar] [CrossRef]
- He, D.; Wu, F.; Ma, W.; Zhang, Y.; Gu, J.D.; Duan, Y.; Xu, R.; Feng, H.; Wang, W.; Li, S.W. Insights into the bacterial and fungal communities and microbiome that causes a microbe outbreak on ancient wall paintings in the Maijishan Grottoes. Int. Biodeterior. Biodegrad. 2021, 163, 105250. [Google Scholar] [CrossRef]
- Zucconi, L.; Gagliardi, M.; Isola, D.; Onofri, S.; Andaloro, M.C.; Pelosi, C.; Pogliani, P.; Selbmann, L. Biodeterioration agents dwelling in or on the wall paintings of the Holy Saviour’s cave (Vallerano, Italy). Int. Biodeterior. Biodegrad. 2012, 70, 40–46. [Google Scholar] [CrossRef]
- Zucconi, L.; Canini, F.; Isola, D.; Caneva, G. Fungi affecting wall paintings: A meta-analysis of their diversity. Appl. Sci. 2022; under review. [Google Scholar]
- Suphaphimol, N.; Suwannarach, N.; Purahong, W.; Jaikang, C.; Pengpat, K.; Semakul, N.; Yimklan, S.; Jongjitngam, S.; Jindasu, S.; Thiangtham, S.; et al. Identification of microorganisms dwelling on the 19th century Lanna mural paintings from Northern Thailand using culture-dependent and-independent approaches. Biology 2022, 11, 228. [Google Scholar] [CrossRef]
- Selbmann, L.; Isola, D.; Fenice, M.; Zucconi, L.; Sterflinger, K.; Onofri, S. Potential extinction of Antarctic endemic fungal species as a consequence of global warming. Sci. Total Environ. 2012, 438, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Muggia, L.; Moreno, L.F.; Wang, M.; Al-Hatmi, A.M.; da Silva Menezes, N.; Shi, D.; Deng, S.; Ahmed, S.; Hyde, K.D.; et al. A re-evaluation of the Chaetothyriales using criteria of comparative biology. Fungal Divers. 2020, 103, 47–85. [Google Scholar] [CrossRef]
- Quan, Y.; van den Ende, B.G.; Shi, D.; Prenafeta-Boldú, F.X.; Liu, Z.; Al-Hatmi, A.M.; Ahmed, S.A.; Verweij, P.E.; Kang, Y.; de Hoog, S. A comparison of isolation methods for black fungi degrading aromatic toxins. Mycopathologia 2019, 184, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The essential oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cultl. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and future perspectives for biocides and antifouling products for stone-built cultural heritage: Ionic liquids as a challenging alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Galeotti, M. Monitoring the performance of innovative and traditional biocides mixed with consolidants and water-repellents for the prevention of biological growth on stone. Sci. Total Environ. 2012, 423, 132–141. [Google Scholar] [CrossRef]
- Salvadori, O.; Charola, A.E. Methods to Prevent Biocolonization and Recolonization: An Overview of Current Research for Architectural and Archaeological Heritage; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011; pp. 37–50. [Google Scholar]
- Urzì, C.; De Leo, F.; Galletta, M.; Salamone, P.; Balzarotti, R. Efficiency of biocide in “in situ” and “in vitro” treatment. Study case of the “Templete de Mudejar”, Guadalupe, Spain. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone Venice June 19–24; Elsevier Science BV: Amsterdam, The Netherlands, 2000; pp. 531–539. [Google Scholar]
- Delgado Rodrigues, J.; Valero Congil, J.; Wakefield, R.; Brechet, E.; Larrañaga, I. Monitoraggio della biocolonizzazione e valutazione dell’efficacia di un biocida. In Arkos: Scienza e Restauro Dell’architettura; Nardini: Firenze, Italy, 2004; Volume 5, pp. 52–58. [Google Scholar]
- Tesei, D. Black fungi research: Out-of-this-world implications. Encyclopedia 2022, 2, 212–229. [Google Scholar] [CrossRef]
- Onofri, S.; Selbmann, L.; Barreca, D.; Isola, D.; Zucconi, L. Do fungi survive under actual space conditions? Searching for evidence in favor of the lithopansperimia. Plant. Biosyst. 2009, 143, S85–S87. [Google Scholar] [CrossRef]
- Onofri, S.; de la Torre, R.; de Vera, J.P.; Ott, S.; Zucconi, L.; Selbmann, L.; Scalzi, G.; Venkateswaran, K.J.; Rabbow, E.; Sánchez Iñigo, F.J.; et al. Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiol 2012, 12, 508–516. [Google Scholar] [CrossRef]
- Acero, F.J.; Carbú, M.; El-Akhal, M.R.; Garrido, C.; González-Rodríguez, V.E.; Cantoral, J.M. Development of proteomics-based fungicides: New strategies for environmentally friendly control of fungal plant diseases. Int. J. Mol. Sci. 2011, 12, 795–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant. Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, CA, USA, 2008. [Google Scholar]
- Corbett, J.R.; Wright, K.; Baillie, A.C. The Biochemical Mode of Action of Pesticides, 2nd ed.; Academic Press: London, UK, 1984. [Google Scholar]
- Juergensen, L.; Busnarda, J.; Caux, P.Y.; Kent, R. Fate, behavior, and aquatic toxicity of the fungicide IPCB in the Canadian environment. Environ. Toxicol. 2000, 15, 201–213. [Google Scholar] [CrossRef]
- Williams, T.M. The mechanism of action of isothiazolone biocides. Power Plant. Chem. 2007, 9, 14–22. [Google Scholar]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Gostinčar, C.; Grube, M.; Gunde-Cimerman, N. Evolution of fungal pathogens in domestic environments? Fungal Biol. 2011, 115, 1008–1018. [Google Scholar] [CrossRef]
- Langsrud, S.; Sundheim, G.; Borgmann-Strahsen, R. Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp. J. Appl. Microbiol. 2003, 95, 874–882. [Google Scholar] [CrossRef]
- Ruibal, C.; Selbmann, L.; Avci, S.; Martin-Sanchez, P.M.; Gorbushina, A.A. Roof-inhabiting cousins of rock-inhabiting fungi: Novel melanized microcolonial fungal species from photocatalytically reactive subaerial surfaces. Life 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).