Comparative Study of Roxarsone Reduction by Shewanella oneidensis MR-1 and Cellulomonas sp. Strain Cellu-2a
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Enrichment Media
2.2. Direct Roxarsone Reduction Experiment in Anaerobic Conditions
2.3. Indirect Roxarsone Reduction Experiment in Anaerobic Conditions Using Solid Iron (HFO)
2.4. Analytical Measurements
3. Results and Discussion
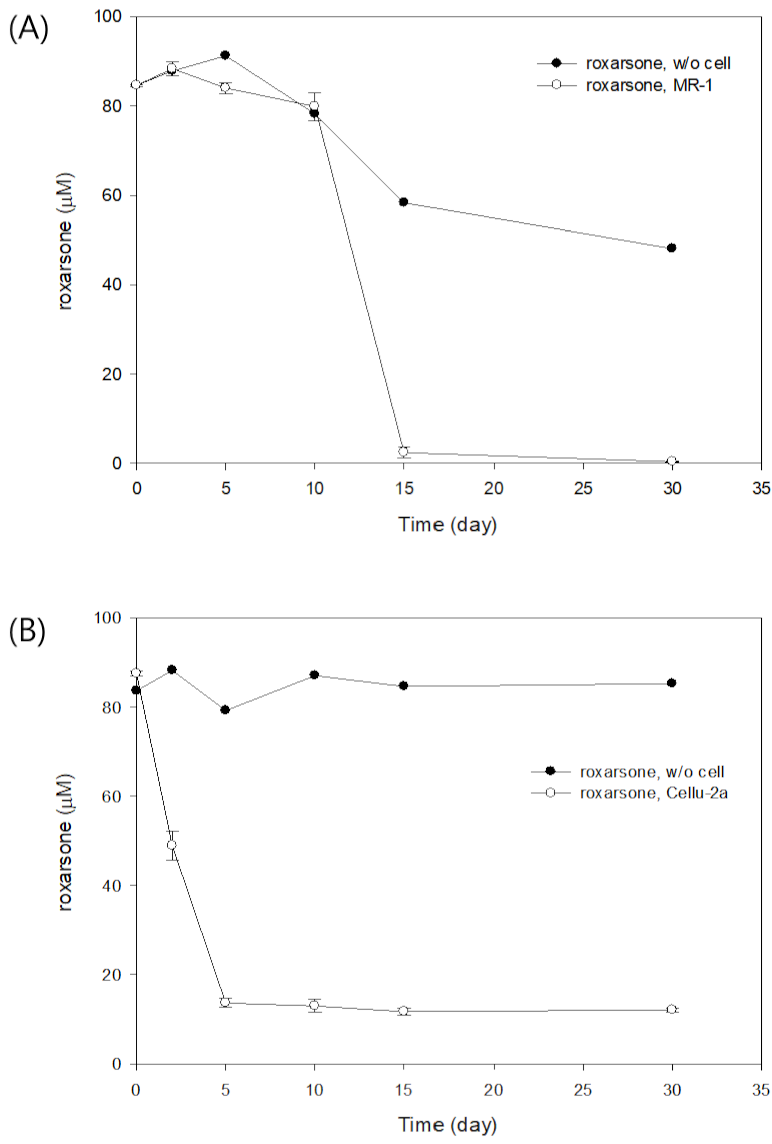
3.1. Direct Roxarsone Reduction Experiment
3.2. Indirect Roxarsone Reduction Experiment at Different Time Intervals
3.3. Environmental Aspects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, H.D.; Johnson, Z.B. Use of antibiotics and roxarsone in broiler chickens in the USA: Analysis for the years 1995 to 2000. Poult. Sci. 2002, 81, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Lanzirotti, A.; Sutton, S.; Davis, J.A.; Sparks, D.L. Arsenic speciation and reactivity in poultry litter. Environ. Sci. Technol. 2003, 37, 4083–4090. [Google Scholar] [CrossRef]
- Garbarino, J.R.; Bednar, A.J.; Rutherford, D.W.; Beyer, R.S.; Wershaw, R.L. Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ. Sci. Technol. 2003, 37, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachman, K.E.; Raber, G.; Francesconi, K.A.; Navas-Acien, A.; Love, D.C. Arsenic species in poultry feather meal. Sci. Total Environ. 2012, 417–418. [Google Scholar] [CrossRef]
- D’Angelo, E.; Zeigler, G.; Beck, E.G.; Grove, J.; Sikora, F. Arsenic species in broiler (Gallus gallus domesticus) litter, soils, maize (Zea mays L.), and groundwater from litter-amended fields. Sci. Total Environ. 2012, 438, 286–292. [Google Scholar] [CrossRef]
- Stolz, J.F.; Perera, E.; Kilonzo, B.; Kail, B.; Crable, B.; Fisher, E.; Ranganathan, M.; Wormer, L.; Basu, P. Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (roxarsone) and release of inorganic arsenic by Clostridium species. Environ. Sci. Technol. 2007, 41, 818–823. [Google Scholar] [CrossRef]
- Adak, A.; Mangalgiri, K.P.; Lee, J.; Blaney, L. UV irradiation and UV-H2O2 advanced oxidation of the roxarsone and nitarsone organoarsenicals. Water Res. 2015, 70, 74–85. [Google Scholar] [CrossRef]
- Fisher, D.J.; Yonkos, L.T.; Staver, K.W. Environmental concerns of roxarsone in broiler poultry feed and litter in Maryland, USA. Environ. Sci. Technol. 2015, 49, 1999–2012. [Google Scholar] [CrossRef]
- Liang, T.; Ke, Z.; Chen, Q.; Liu, L.; Chen, G. Degradation of roxarsone in a silt loam soil and its toxicity assessment. Chemosphere 2014, 112, 128–133. [Google Scholar] [CrossRef]
- Bednar, A.J.; Garbarino, J.R.; Ferrer, I.; Rutherford, D.W.; Wershaw, R.L.; Ranville, J.F.; Wildeman, T.R. Photodegradation of roxarsone in poultry litter leachates. Sci. Total Environ. 2003, 302, 237–245. [Google Scholar] [CrossRef]
- Chen, W.-R.; Huang, C.-H. Surface adsorption of organoarsenic roxarsone and arsanilic acid on iron and aluminum oxides. J. Hazard. Mater. 2012, 227–228, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Wilson, L.D.; Sammynaiken, R. Sorptive uptake studies of an aryl-arsenical with iron oxide composites on an activated carbon support. Materials 2014, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Tong, Z.; Chen, G.; Zhan, X.; Hu, Z. Adsorption of roxarsone by iron (hydr)oxide-modified multiwalled carbon nanotubes from aqueous solution and its mechanisms. Int. J. Environ. Sci. Technol. 2014, 11, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Ke, Z.; Liang, T.; Liu, L.; Wang, G. Shewanella oneidensis MR-1-induced Fe(III) reduction facilitates roxarsone transformation. PLoS ONE 2016, 11, e0154017. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, Y.; Wen, Y.; Fei, Y. Response of soil microbial communities to roxarsone pollution along a concentration gradient. J. Environ. Sci. Health Part A 2017, 52, 819–827. [Google Scholar] [CrossRef]
- Huang, K.; Peng, H.; Gao, F.; Liu, Q.; Lu, X.; Shen, Q.; Le, X.C.; Zhao, F.-J. Biotransformation of arsenic-containing roxarsone by an aerobic soil bacterium Enterobacter sp. CZ-1. Environ. Pollut. 2019, 247, 482–487. [Google Scholar] [CrossRef]
- Ehrenreich, A.; Widdel, F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 1994, 60, 4517–4526. [Google Scholar] [CrossRef] [Green Version]
- Hegler, F.; Posth, N.R.; Jiang, J.; Kappler, A. Physiology of phototrophic iron(II)-oxidizing bacteria: Implications for modern and ancient environments. FEMS Microbiol. Ecol. 2008, 66, 250–260. [Google Scholar] [CrossRef]
- Widdel, F.; Pfennig, N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch. Microbiol. 1981, 129, 395–400. [Google Scholar] [CrossRef]
- Khanal, A.; Hur, H.-G.; Fredrickson, J.K.; Lee, J.-H. Direct and indirect reduction of Cr(VI) by fermentative Fe(III)-reducing Cellulomonas sp. strain Cellu-2a. J. Microbiol. Biotechnol. 2021, 31, 1519–1525. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Frensemeier, L.M.; Büter, L.; Vogel, M.; Karst, U. Investigation of the oxidative transformation of roxarsone by electrochemistry coupled to hydrophilic interaction liquid chromatography/mass spectrometry. J. Anal. Atoms. Spectrom. 2017, 32, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Peretyazhko, T.; Zachara, J.M.; Heald, S.M.; Kukkadapu, R.K.; Liu, C.; Plymale, A.E.; Resch, C.T. Reduction of Tc(VII) by Fe(II) sorbed on Al (hydr)oxides. Environ. Sci. Technol. 2008, 42, 5499–5506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortinas, I.; Field, J.A.; Kopplin, M.; Garbarino, J.R.; Gandolfi, A.J.; Sierra-Alvarez, R. Anaerobic biotransformation of roxarsone and related N-substituted phenylarsonic acids. Environ. Sci. Technol. 2006, 40, 2951–2957. [Google Scholar] [CrossRef]
- Moody, J.P.; Williams, R.T. The metabolism of 4-hydroxy-3-nitrophenylarsonic acid in hens. Food Cosmet. Toxicol. 1964, 2, 707–715. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Lu, X.; Liu, Q.; Huang, R.; Hu, B.; Kachanoski, G.; Zuidhof, M.J.; Le, X.C. Arsenic metabolites, including N-acetyl-4-hydroxy-m-arsanilic acid, in chicken litter from a roxarsone-feeding study involving 1600 chickens. Environ. Sci. Technol. 2016, 50, 6737–6743. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, A.; Ho, C.T.; Hur, H.-G.; Lee, J.-H. Comparative Study of Roxarsone Reduction by Shewanella oneidensis MR-1 and Cellulomonas sp. Strain Cellu-2a. Appl. Sci. 2022, 12, 1839. https://doi.org/10.3390/app12041839
Khanal A, Ho CT, Hur H-G, Lee J-H. Comparative Study of Roxarsone Reduction by Shewanella oneidensis MR-1 and Cellulomonas sp. Strain Cellu-2a. Applied Sciences. 2022; 12(4):1839. https://doi.org/10.3390/app12041839
Chicago/Turabian StyleKhanal, Anamika, Cuong Tu Ho, Hor-Gil Hur, and Ji-Hoon Lee. 2022. "Comparative Study of Roxarsone Reduction by Shewanella oneidensis MR-1 and Cellulomonas sp. Strain Cellu-2a" Applied Sciences 12, no. 4: 1839. https://doi.org/10.3390/app12041839
APA StyleKhanal, A., Ho, C. T., Hur, H.-G., & Lee, J.-H. (2022). Comparative Study of Roxarsone Reduction by Shewanella oneidensis MR-1 and Cellulomonas sp. Strain Cellu-2a. Applied Sciences, 12(4), 1839. https://doi.org/10.3390/app12041839






