Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece
Abstract
:1. Introduction
2. Experimental Details
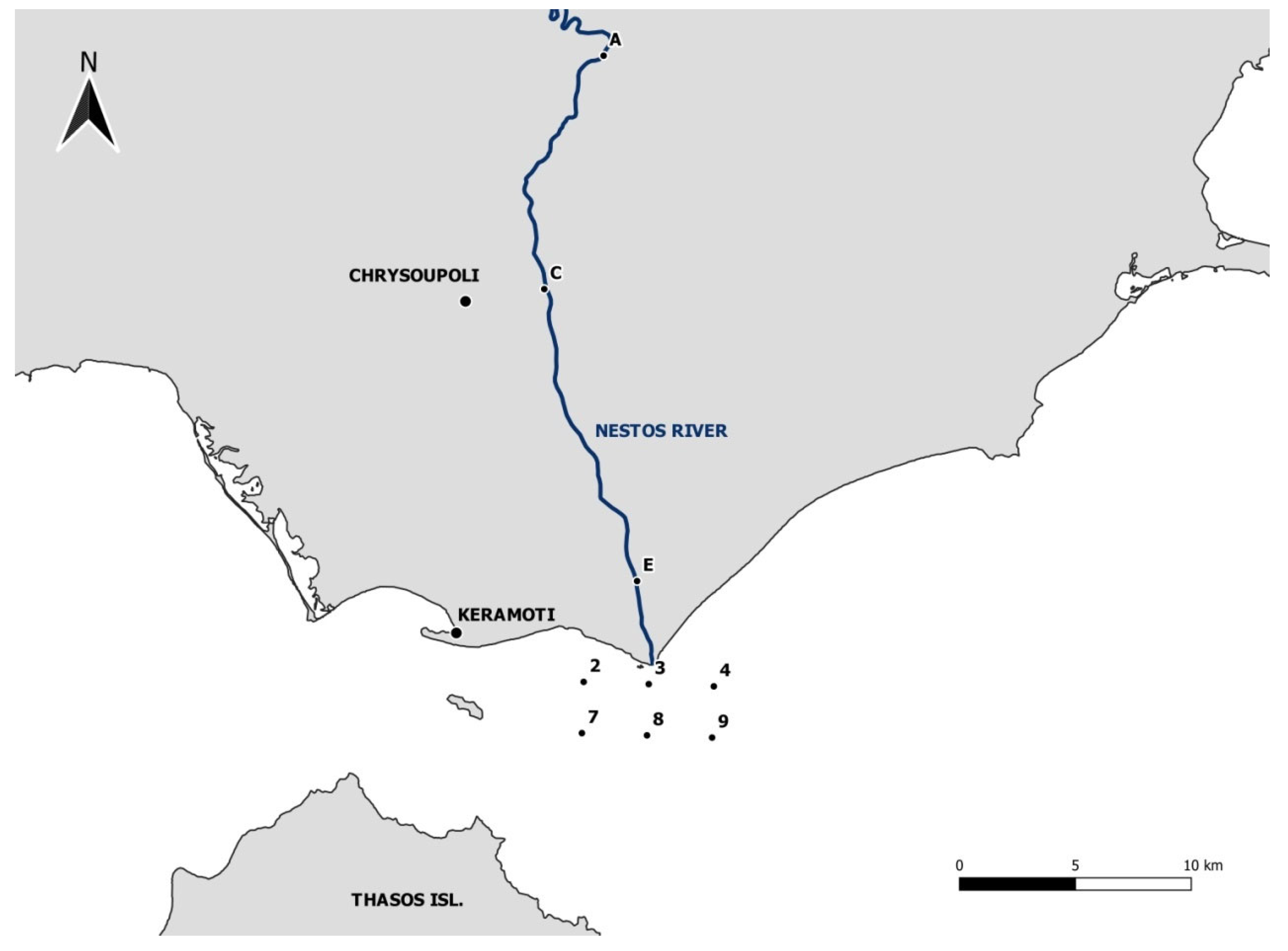
2.1. Study Area
2.2. Materials and Instrumentation
2.3. Sampling Method
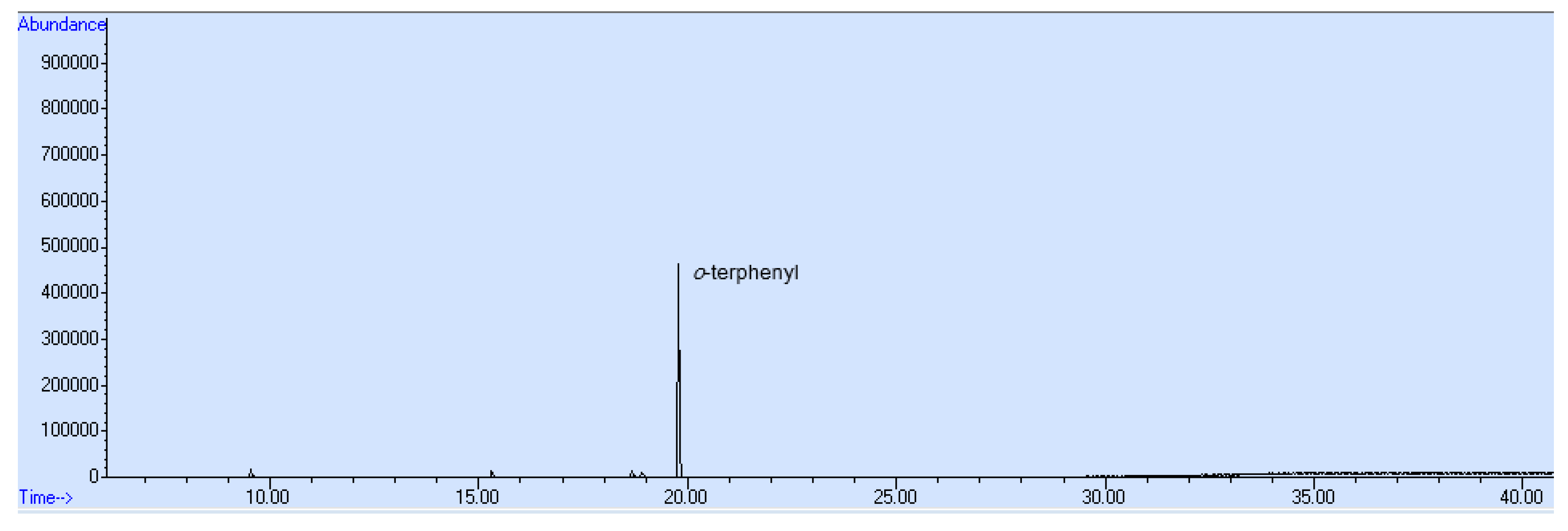
2.4. Recovery Control and Spiking Method
2.5. Organic Extraction from Water Samples
2.6. Organic Extraction from Sediment Samples
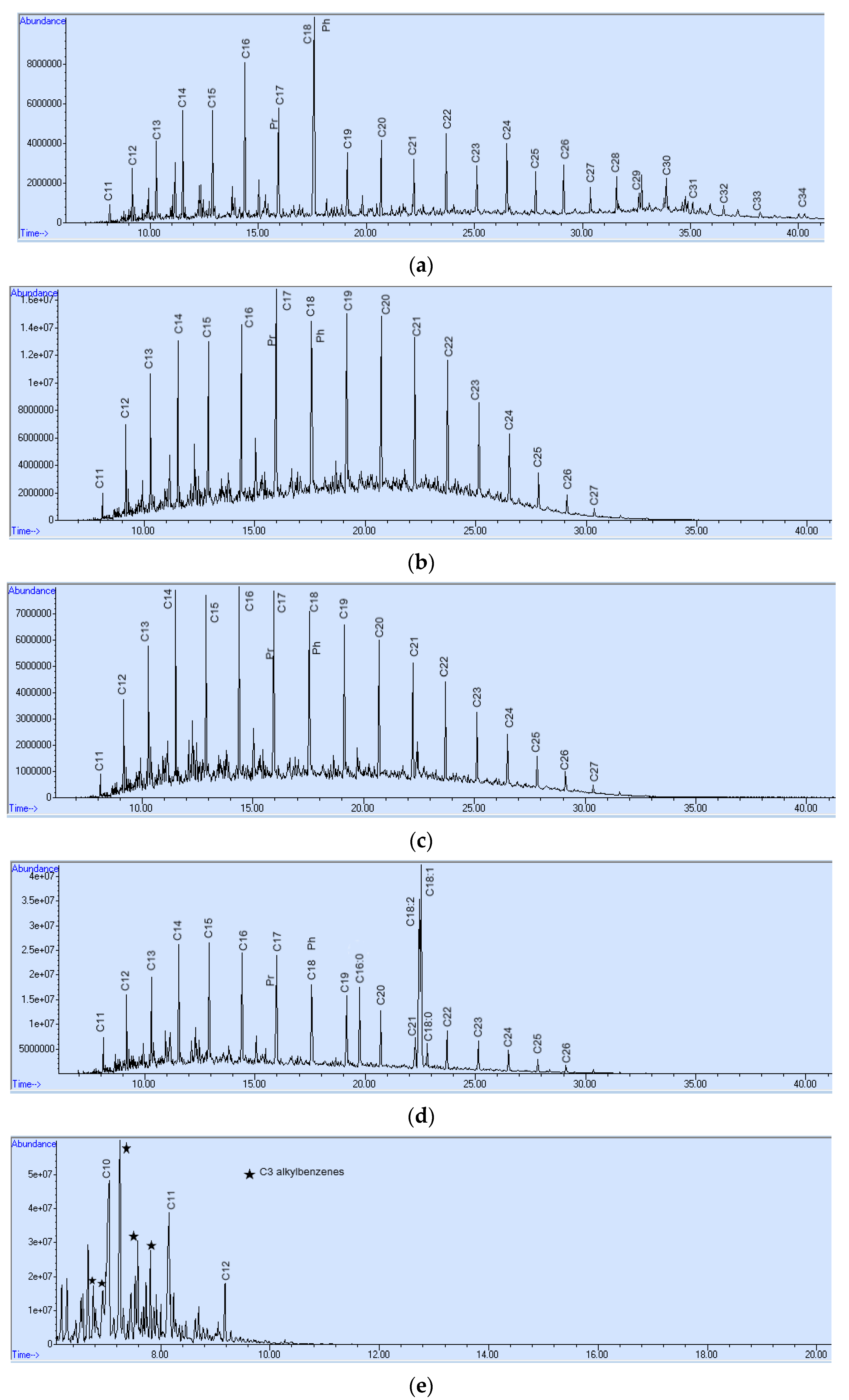
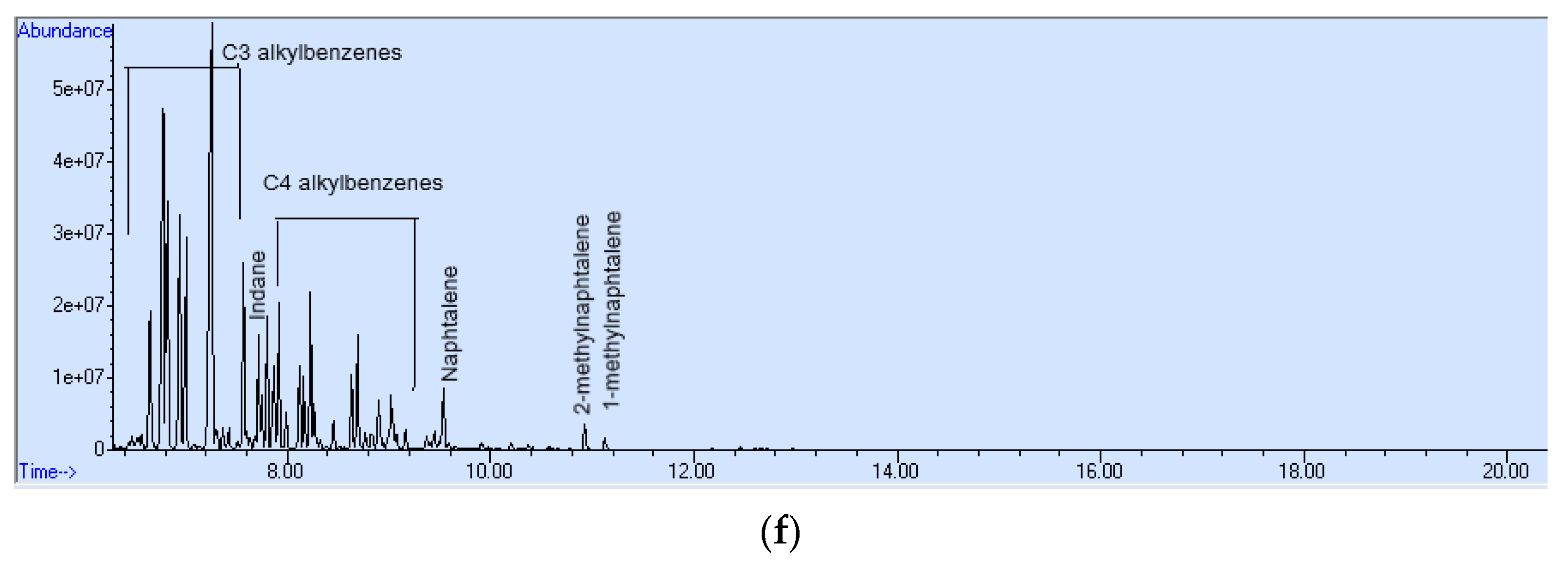
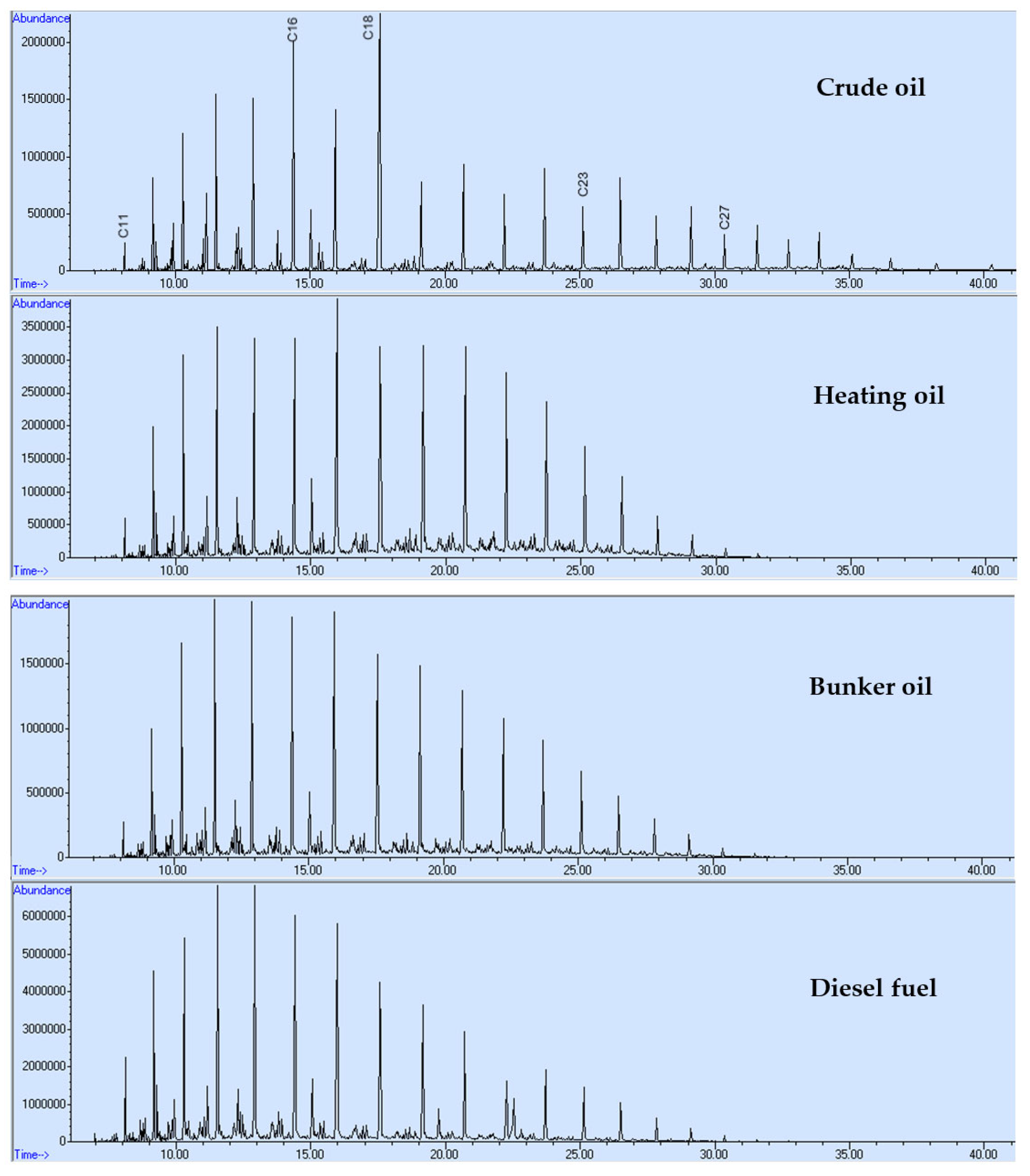
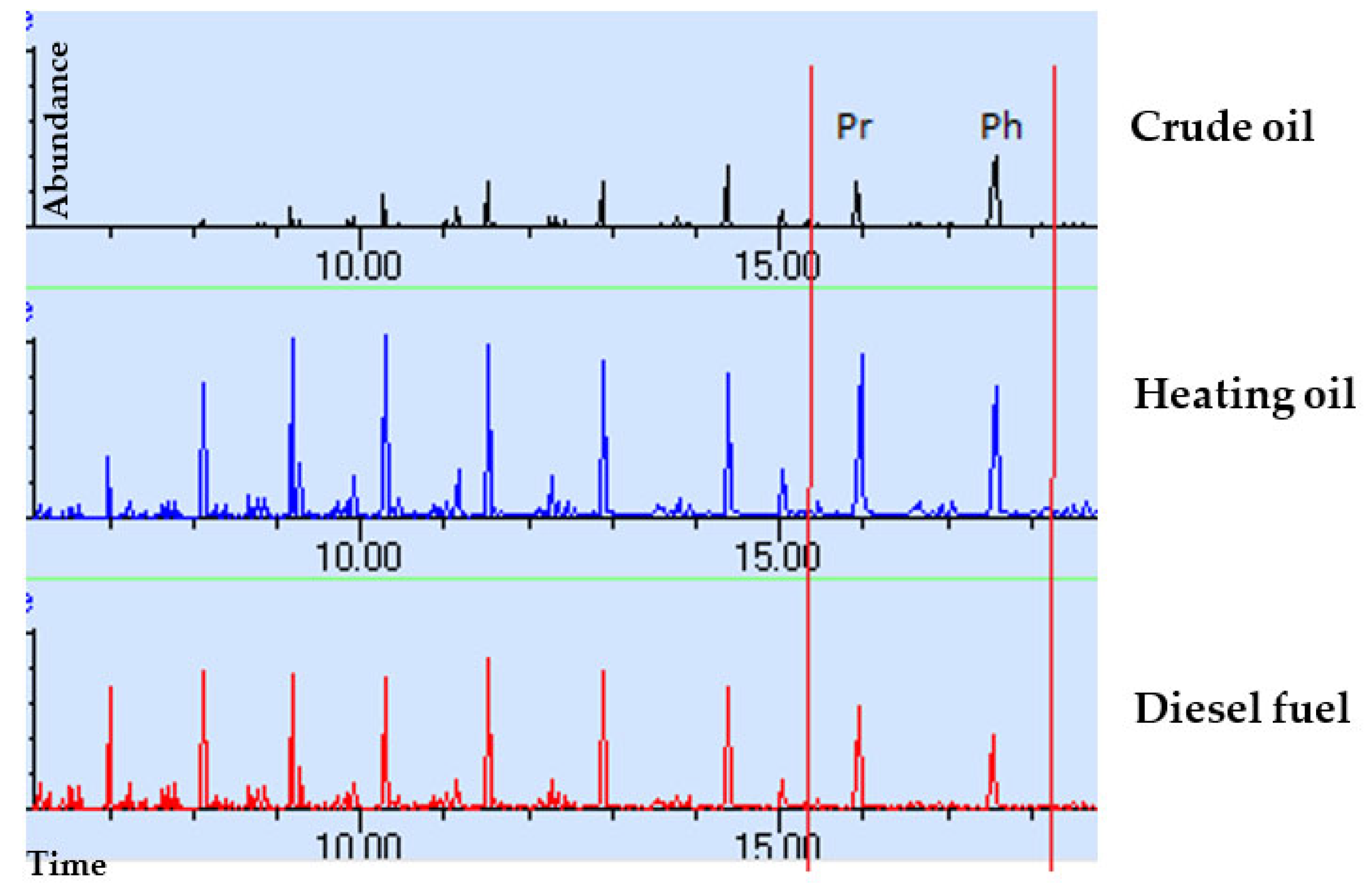
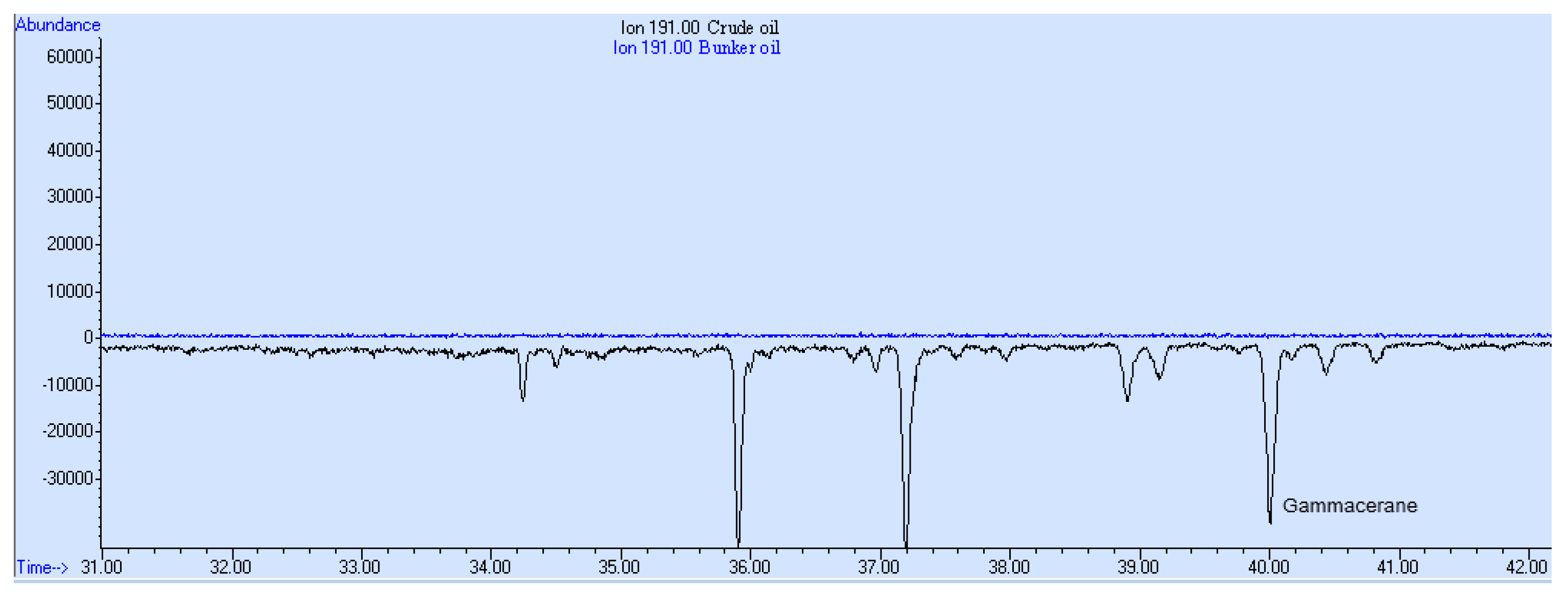
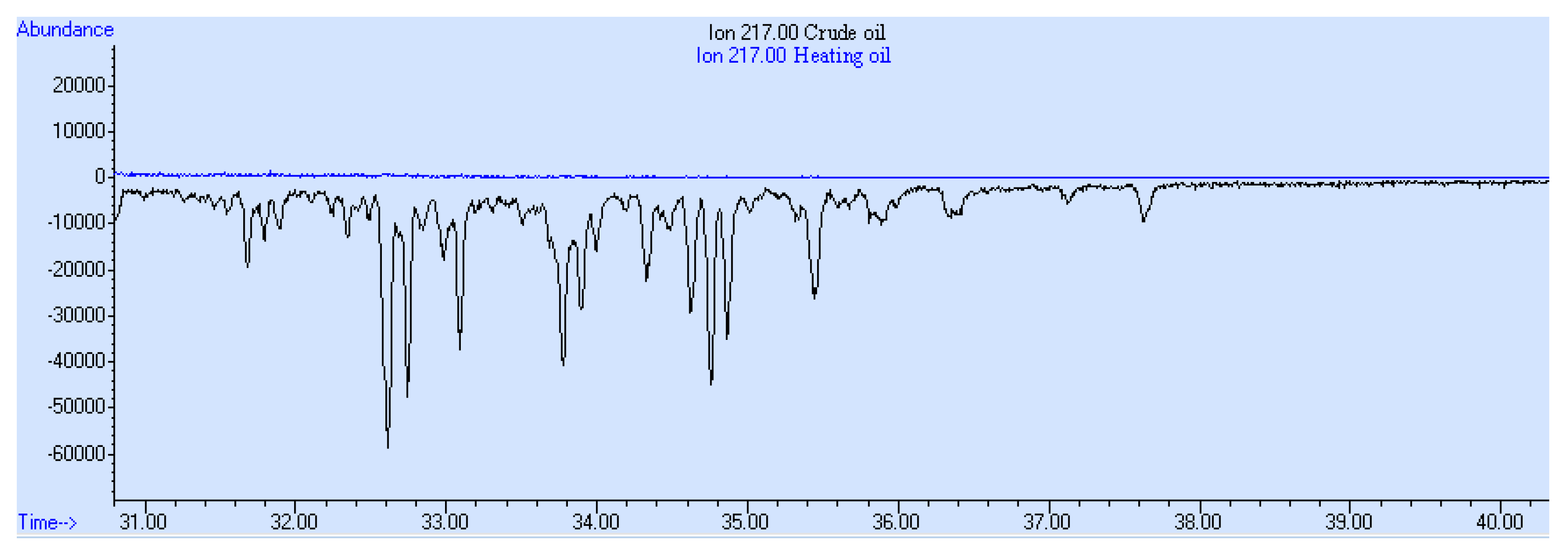
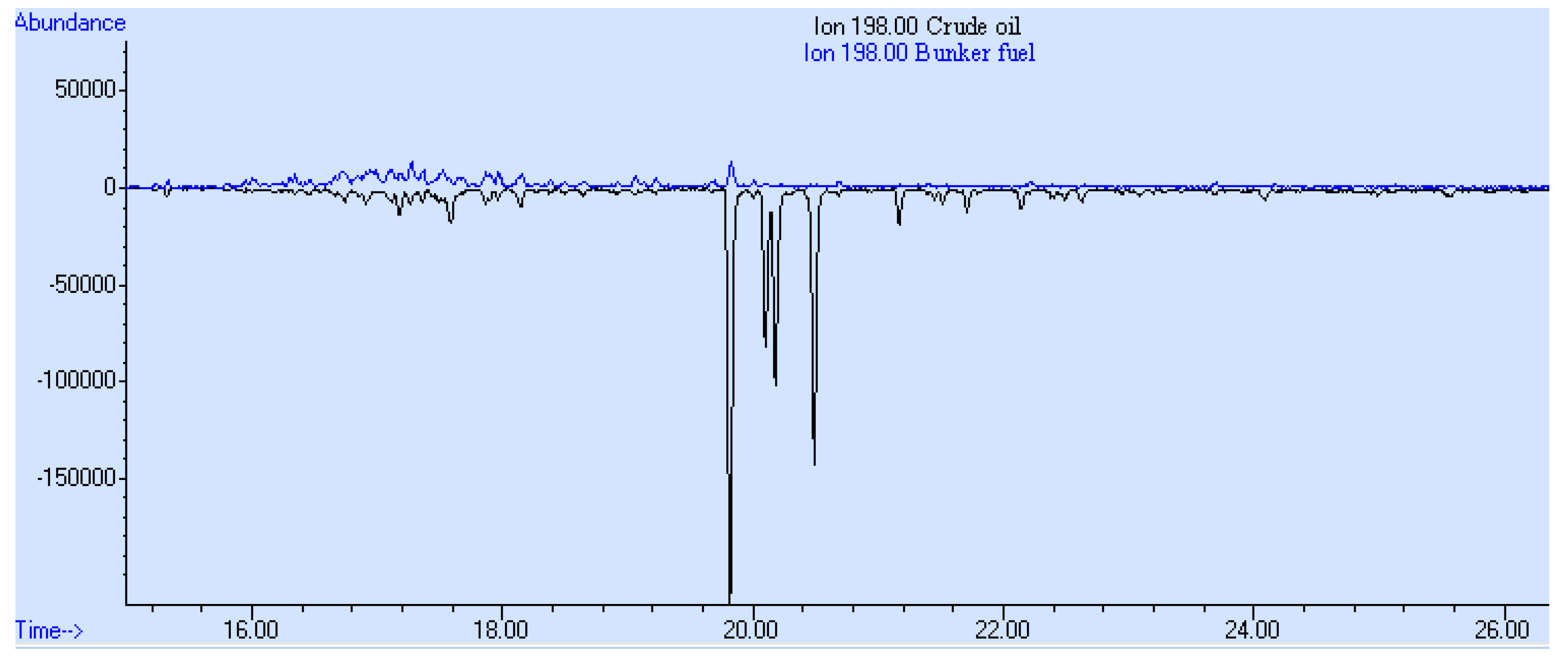
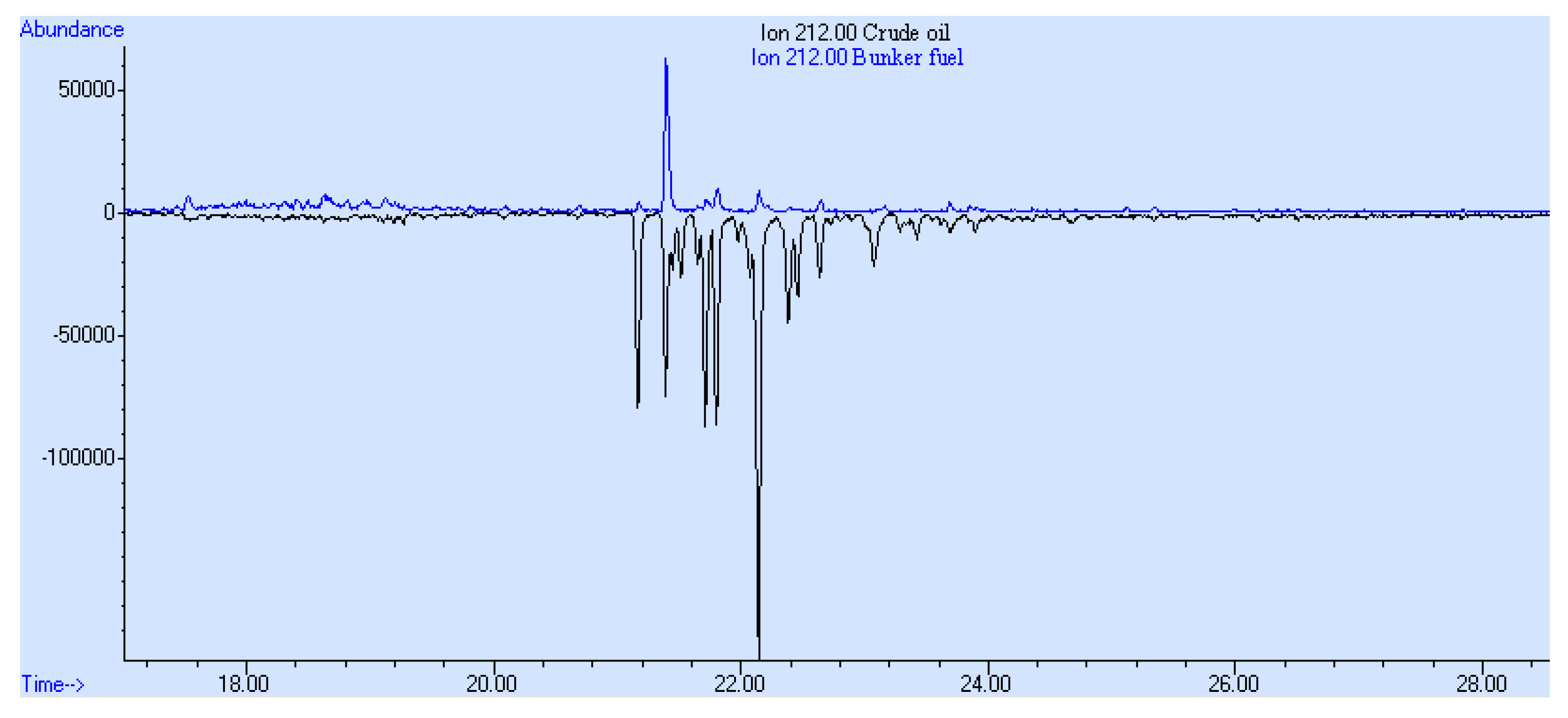
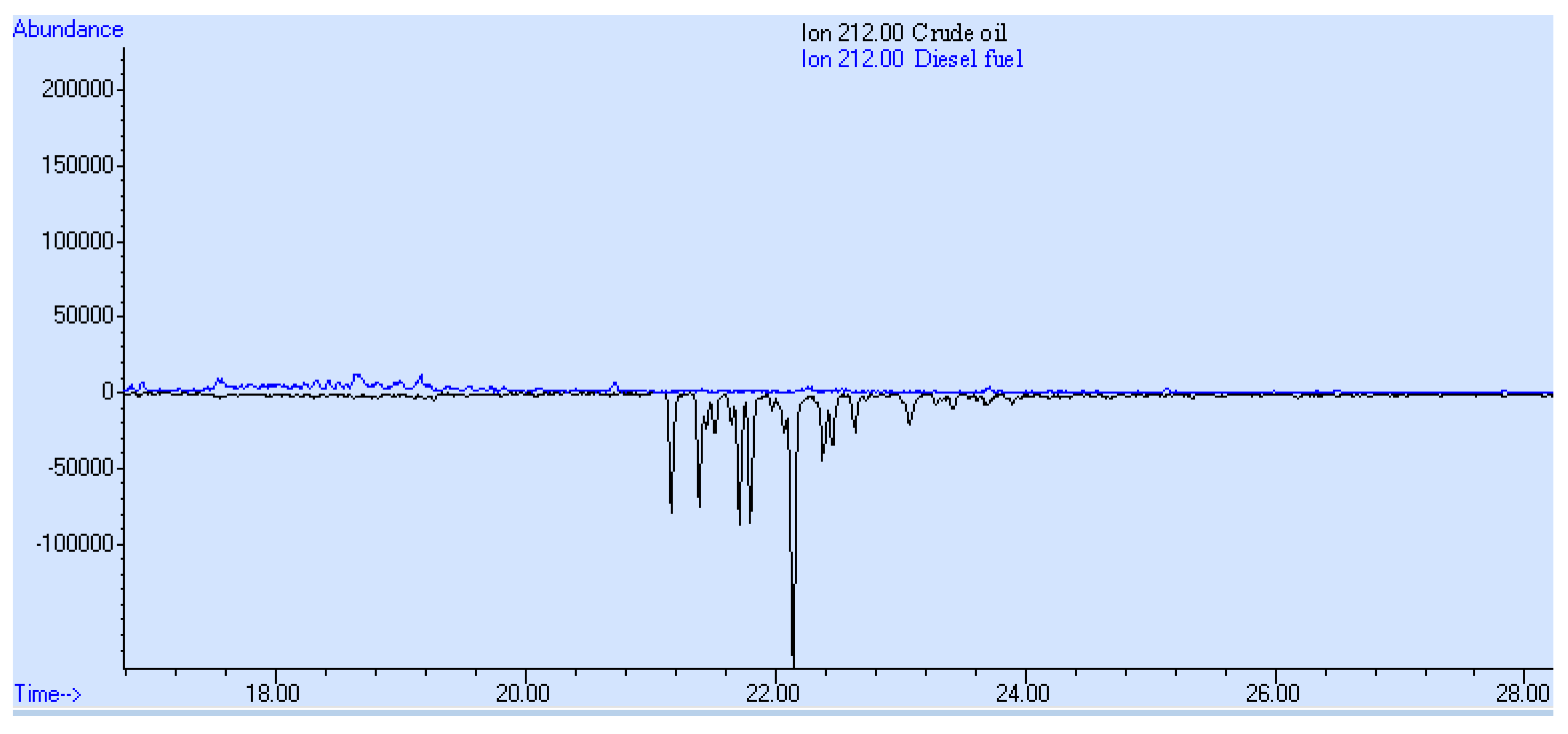
3. Results and Discussion
- The shape of chromatograms conforming to the standards peaks;
- The ratio between n-alkanes with even and odd numbers of carbon atoms;
- The ratio between phytane and pristine;
- The presence of parent and alkylated PAHs;
- The presence of alkyl-substituted and unsubstituted aromatic hydrocarbons;
- The presence of sulfur heterocyclic aromatic compounds.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cholakov, G. Control of pollution in the petroleum industry. Pollut. Control Technol. 2009, 3, 86–107. [Google Scholar]
- Mariano, J.B.; Rovere, E. Environmental Impacts of the Oil Industry; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2017. [Google Scholar]
- Wang, Z.; Fingas, M. Differentiation of the source of spilled oil and monitoring of the oil weathering process using gas chromatography-mass spectrometry. J. Chromatogr. A 1995, 712, 321–343. [Google Scholar] [CrossRef]
- Dincer Kırman, Z.; Sericano, J.L.; Wade, T.L.; Bianchi, T.S.; Marcantonio, F.; Kolker, A.S. Composition and depth distribution of hydrocarbons in barataria bay marsh sediments after the deepwater horizon oil spill. Environ. Pollut. 2016, 214, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Impact of total petroleum hydrocarbons on human health. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 139–165. [Google Scholar]
- Ahmed, F.; Fakhruddin, A.N.M. A review on environmental contamination of petroleum hydrocarbons and its biodegradation. Int. J. Environ. Sci. Nat. Res. 2018, 11, 63–69. [Google Scholar]
- Ramirez, M.I.; Arevalo, A.P.; Sotomayor, S.; Bailon-Moscoso, N. Contamination by oil crude extraction—Refinement and their effects on human health. Environ. Pollut. 2017, 231, 415–425. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Yang, Z.; Brown, C.E.; Hollebone, B.P.; Stout, S.A. 4-petroleum biomarker fingerprinting for oil spill characterization and source identification. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed.; Stout, S.A., Wang, Z., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 131–254. [Google Scholar]
- Wang, Z.; Fingas, M.; Yang, C.; Christensen, J.H. Crude oil and refined product fingerprinting: Principles. In Environmental Forensics; Elsevier: Amsterdam, The Netherlands, 1964; pp. 339–407. [Google Scholar]
- Blomberg, J.; Schoenmakers, P.J.; Brinkman, U.A.T. Gas chromatographic methods for oil analysis. J. Chromatogr. A 2002, 37, 137–173. [Google Scholar] [CrossRef]
- Hunt, J.M. Petroleum Geochemistry and Geology; W.H. Freeman: New York, NY, USA, 1996. [Google Scholar]
- Wang, Z.; Fingas, M.; Page, D. Oil spill identification. J. Chromatogr. A 1999, 843, 369–411. [Google Scholar] [CrossRef]
- Walters, C.C.; Moldowan, J.M.; Peters, K.E. The Biomarker Guide: Biomarkers and Isotopes in the Environment and Human History, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; Volume 1, pp. 1–2. [Google Scholar]
- Yang, C.; Brown, C.E.; Hollebone, B.; Yang, Z.; Lambert, P.; Fieldhouse, B.; Landriault, M.; Wang, Z. Chemical fingerprints of crude oils and petroleum products. In Oil Spill Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 209–304. [Google Scholar]
- Barakat, A.O.; Mostafa, A.R.; Qian, Y.; Kennicutt, M.C. Application of petroleum hydrocarbon chemical fingerprinting in oil spill investigations—Gulf of Suez, Egypt. Spill Sci. Technol. Bull. 2002, 7, 229–239. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Zhang, B.; He, S.; Zhao, M. Fingerprint and weathering characteristics of crude oils after dalian oil spill, China. Mar. Pollut. Bull. 2013, 71, 64–68. [Google Scholar] [CrossRef]
- Wang, C.; He, S.; Zhang, H.; Li, Y. Fingerprint and weathering characteristics of petroleum hydrocarbons in the coastal zone following the “7-16” Dalian crude oil spill, China. In Oil Spill Environmental Forensics Case Studies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 483–497. [Google Scholar]
- Wang, Z.; Fingas, M.; Sigouin, L. Characterization and source identification of an unknown spilled oil using fingerprinting techniques by gc-ms and gc-fid. LC GC N. Am. 2000, 18, 1058–1067. [Google Scholar]
- Kiepper, A.P.; Casilli, A.; Azevedo, D.A. Depositional paleoenvironment of brazilian crude oils from unusual biomarkers revealed using comprehensive two dimensional gas chromatography coupled to time of flight mass spectrometry. Org. Geochem. 2014, 70, 62–75. [Google Scholar] [CrossRef]
- Meyer, B.M.; Overton, E.B.; Turner, R.E. Oil source identification using diagnostic biomarker ratio analyses. Int. Oil Spill Conf. Proc. 2014, 2014, 2064–2073. [Google Scholar] [CrossRef]
- Yasser, M.M. Biomarkers. In Chromatography and Its Applications; Rania, E.M.E.D.S.D., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 9. [Google Scholar]
- Okparanma, R.N.; Mouazen, A.M. Determination of total petroleum hydrocarbon (tph) and polycyclic aromatic hydrocarbon (pah) in soils: A review of spectroscopic and nonspectroscopic techniques. Appl. Spectrosc. Rev. 2013, 48, 458–486. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, G.; Wang, Z.; Yang, Z.; Hollebone, B.; Landriault, M.; Shah, K.; Brown, C.E. Development of a methodology for accurate quantitation of alkylated polycyclic aromatic hydrocarbons in petroleum and oil contaminated environmental samples. Anal. Methods 2014, 6, 7760–7771. [Google Scholar] [CrossRef]
- Rocha, A.C.; Palma, C. Source identification of polycyclic aromatic hydrocarbons in soil sediments: Application of different methods. Sci. Total Environ. 2019, 652, 1077–1089. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, B.; Fan, Y.; Wen, M.; Han, X. Accurate analysis of polycyclic aromatic hydrocarbons (pahs) and alkylated pahs homologs in crude oil for improving the gas chromatography/mass spectrometry performance. Ecotoxicol. Environ. Saf. 2014, 100, 242–250. [Google Scholar] [CrossRef]
- Sørensen, L.; Meier, S.; Mjøs, S.A. Application of gas chromatography/tandem mass spectrometry to determine a wide range of petrogenic alkylated polycyclic aromatic hydrocarbons in biotic samples: Application of gc/ms/ms to determine a wide range of alkylated pahs. Rapid Commun. Mass Spectrom. 2016, 30, 2052–2058. [Google Scholar] [CrossRef]
- Chakhmakhchev, A.; Suzuki, M.; Takayama, K. Distribution of alkylated dibenzothiophenes in petroleum as a tool for maturity assessments. Org. Geochem. 1997, 26, 483–489. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Zhong, N.; Zhang, W.; Sadik, A.; Li, H. Ternary diagram of fluorenes, dibenzothiophenes and dibenzofurans: Indicating depositional environment of crude oil source rocks. Energy Explor. Exploit. 2013, 31, 569–588. [Google Scholar] [CrossRef]
- Zeigler, C.; Schantz, M.; Wise, S.; Robbat, A. Mass spectra and retention indexes for polycyclic aromatic sulfur heterocycles and some alkylated analogs. Polycycl. Aromat. Compd. 2012, 32, 154–176. [Google Scholar] [CrossRef]
- TAP. Integrated Esia Greece Annex 6.6.2—Groundwater Baseline Study; Trans Adriatic Pipeline: Athens, Greece, 2013. [Google Scholar]
- Christoforidis, A.; Stamatis, N.; Schmieder, K.; Tsachalidis, E. Organochlorine and mercury contamination in fish tissues from the river Nestos, Greece. Chemosphere 2008, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Petalas, C.; Pliakas, F.; Diamantis, I.; Kallioras, A. Development of an integrated conceptual model for the rational management of the transboundary nestos river, greece. Environ. Geol. 2005, 48, 941–954. [Google Scholar] [CrossRef]
- Zaimes, G.N.; Gounaridis, D.; Fotakis, D. Assessing riparian land-uses/vegetation cover along the Nestos river in Greece. Fresenius Environ. Bull. 2011, 20, 3217–3225. [Google Scholar]
- Zaimes, G.N.; Iakovoglou, V.; Syropoulos, D.; Kaltsas, D.; Avtzis, D. Article assessment of two adjacent mountainous riparian areas along Nestos river tributaries of Greece. Forests 2021, 12, 1284. [Google Scholar] [CrossRef]
- Zaimes, G.N.; Gounaridis, D.; Symeonakis, E. Assessing the impact of dams on riparian and deltaic vegetation using remotely-sensed vegetation indices and random forests modelling. Ecol. Indic. 2019, 103, 630–641. [Google Scholar] [CrossRef]
- Papastergios, G.; Fernández-Turiel, J.-L.; Georgakopoulos, A.; Gimeno, D. Natural and anthropogenic effects on the sediment geochemistry of Nestos river, northern Greece. Environ. Geol. 2009, 58, 1361–1370. [Google Scholar] [CrossRef]
- Kallioras, A.; Pliakas, F.; Diamantis, I. The legislative framework and policy for the water resources management of transboundary rivers in europe: The case of Nestos/Mesta river, between Greece and Bulgaria. Environ. Sci. Policy 2006, 9, 291–301. [Google Scholar] [CrossRef]
- Psilovikos, A.; Margoni, S.; Psilovikos, A. Simulation and trend analysis of the water quality monitoring daily data in Nestos river delta. Contribution to the sustainable management and results for the years 2000–2002. Environ. Monit. Assess. 2006, 116, 543–562. [Google Scholar] [CrossRef]
- Emmanouloudis, D.; Myronidis, D.; Panilas, S.; Efthimiou, G. The Role of Sediments in the Dynamics and Preservation of the Aquatic Forest in the Nestos Delta (Northern Greece); IAHS-AISH Publication: Wallingford, UK, 2006; pp. 214–222. [Google Scholar]
- Edgell, K.W.; Wesselman, R.J. USEPA (Environmental Protection Agency) Method Study 36 sw-846 Methods 8270/3510, gc/ms (Gas Chromatography/Mass Spectrometry) Method for Semivolatile Organics: Capillary-Column Technique Separatory-Funnel Liquid-Liquid Extraction. Final Report, September 1986–December 1987; United States Environmental Protection Agency: Washington, DC, USA, 1989.
- Hsu, C.S.; Walters, C.C.; Isaksen, G.H.; Schaps, M.E.; Peters, K.E. Biomarker analysis in petroleum exploration. In Analytical Advances for Hydrocarbon Research; Hsu, C.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 223–245. [Google Scholar]
- Stout, S.A.; Wang, Z. 3-chemical fingerprinting methods and factors affecting petroleum fingerprints in the environment. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed.; Stout, S.A., Wang, Z., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 61–129. [Google Scholar]
- Kiomourtzi, P.; Pasadakis, N.; Zelilidis, A. Source rock and depositional environment study of three hydrocarbon fields in Prinos–Kavala basin (north Aegean). Open Pet. Eng. J. 2008, 1, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.-S.; Lee, S. Discrimination methods for diesel origin by analyzing fatty acid methyl ester (fame) composition in diesel-contaminated soil. Sci. Rep. 2021, 11, 16245. [Google Scholar] [CrossRef] [PubMed]
- Suppajariyawat, P.; Andrade, A.F.B.d.; Elie, M.; Baron, M.; Gonzalez-Rodriguez, J. The use of chemical composition and additives to classify petrol and diesel using gas chromatography–mass spectrometry and chemometric analysis: A UK study. Open Chem. 2019, 17, 183–197. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Liu, Y.; Yang, Z.; Li, Y.; Shah, K.; Zhang, G.; Landriault, M.; Hollebone, B.; Brown, C.; et al. Aromatic steroids in crude oils and petroleum products and their applications in forensic oil spill identification. Environ. Forensics 2013, 14, 278–293. [Google Scholar] [CrossRef]
- Hegazi, A.H.; Andersson, J.T. 6-polycyclic aromatic sulfur heterocycles as source diagnostics of petroleum pollutants in the marine environment. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed.; Stout, S.A., Wang, Z., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 313–342. [Google Scholar]
- Zeigler, C.D.; Robbat, A. Comprehensive profiling of coal tar and crude oil to obtain mass spectra and retention indices for alkylated pah shows why current methods err. Environ. Sci. Technol. 2012, 46, 3935–3942. [Google Scholar] [CrossRef]
- Albanakis, K.; Psilovikos, A.; Margoni, S.; Styllas, M. Some recent observations on the nestos river sediment outflow and dispersion in the deltaic region. In Proceedings of the INTERREG Meeting—North Aegean System Functioning and Inter-Regional Pollution, Kavala, Greece, 28–30 May 2001; p. 18. [Google Scholar]
- Hatzianestis, I. The role of rivers in transporting organic contaminants in the marine environment of Greece. Geophys. Res. Abstr. 2013, 15, 11873. [Google Scholar]
- Hatzianestis, I.; Sklivagou, E. Dissolved and suspended polycyclic aromatic hydrocarbons (pah) in the north Aegean sea. Mediterr. Mar. Sci. 2002, 3, 89–98. [Google Scholar] [CrossRef]
| Reagents | Company |
|---|---|
| O-terphenyl | Merck |
| Dichloromethane | Carlo Erba |
| Acetone | Merck |
| n-Hexane | Merck |
| Naphthalene | Sigma-Aldrich |
| Anthracene | Sigma-Aldrich |
| Fluorene | Fluka |
| Phenanthrene | Fluka |
| Clean sediment | Sigma-Aldrich |
| Anhydrous sodium sulfate | Panreac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitkidou, S.; Kokkinos, N.; Emmanouilidou, E.; Yohannah, Y.; Spanos, T.; Chatzichristou, C.; Ene, A. Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece. Appl. Sci. 2022, 12, 1636. https://doi.org/10.3390/app12031636
Mitkidou S, Kokkinos N, Emmanouilidou E, Yohannah Y, Spanos T, Chatzichristou C, Ene A. Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece. Applied Sciences. 2022; 12(3):1636. https://doi.org/10.3390/app12031636
Chicago/Turabian StyleMitkidou, Sophia, Nikolaos Kokkinos, Elissavet Emmanouilidou, Yusuf Yohannah, Thomas Spanos, Christina Chatzichristou, and Antoaneta Ene. 2022. "Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece" Applied Sciences 12, no. 3: 1636. https://doi.org/10.3390/app12031636
APA StyleMitkidou, S., Kokkinos, N., Emmanouilidou, E., Yohannah, Y., Spanos, T., Chatzichristou, C., & Ene, A. (2022). Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece. Applied Sciences, 12(3), 1636. https://doi.org/10.3390/app12031636









