Featured Application
Personalized medicine.
Abstract
A comprehensive detection of a wide variety of diagnostic markers is required for the realization of personalized medicine. As a sensor to realize such personalized medicine, a single molecule electrical measurement method using nanodevices is currently attracting interest for its comprehensive simultaneous detection of various target markers for use in biological and medical application. Single-molecule electrical measurement using nanodevices, such as nanopore, nanogap, or nanopipette devices, has the following features:; high sensitivity, low-cost, high-throughput detection, easy-portability, low-cost availability by mass production technologies, and the possibility of integration of various functions and multiple sensors. In this review, I focus on the medical applications of single- molecule electrical measurement using nanodevices. This review provides information on the current status and future prospects of nanodevice-based single-molecule electrical measurement technology, which is making a full-scale contribution to realizing personalized medicine in the future. Future prospects include some discussion on of the current issues on the expansion of the application requirements for single-mole-cule measurement.
1. Introduction
Personalized medicine is known as tailor-made medical care and healthy life system for each person by providing appropriate treatment and medication. In order to realize these personalized medication systems, the development of sensing technologies, which can detect detailed personal health status and information by monitoring various medical sensors, is essential. Single-molecule electrical measurement using nanostructure integrated devices, called “nanodevice”, is one of the candidates to addressing the issues in this realization of personalized medicine (Figure 1). Compared to conventional analytical techniques, there are several advantages of single-molecule electrical measurements using nanodevices: high sensitivity, which enables early diagnosis and monitoring, the simplicity and compactness of the measurement system, which enables easy-portability in field works and research; and the possibility of the integration of various functions and multiple sensors such as separation and purification.
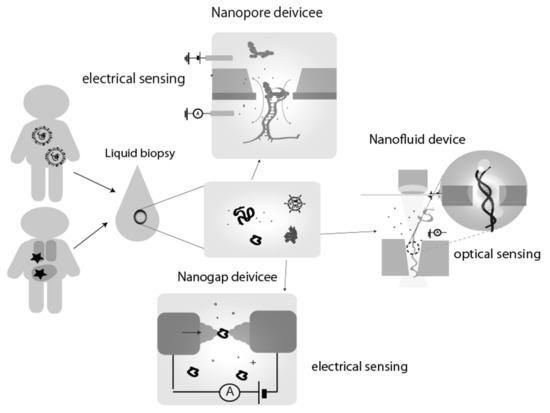
Figure 1.
Schematics of single-molecule detection by nanodevices for biological/medical application. In personalized medicine, non-invasive liquid biopsy (urine, blood, etc.) is expected to be used for the application of sampling. The samples include miRNAs and proteins released from the liposomes of cells, as well as the virus itself in case of infection. The target markers in these samples are electrically measured at the single molecule level using nanodevices, such as nanopores, nanogap electrodes, and nano fluid/channels. As single-molecule detection methods, electrical and optical and its combination measurements have been reported.
Nanopore based single molecule measurement is one of the most attractive single molecule measurements using nanodevices [1,2]. The detection principle of nanopore based single molecule measurement methods is the same as the Coulter counter detection principle. The principles are herein briefly described. The nanopore device has two solution chambers separated by a membrane with a single nanopore, and the chambers are filled with an electrolyte solution. When a single molecule/particle flows into the nanopore, the electrical conductivity of the electrolyte solution in the pore decreases due to the excluded volume of the particle, resulting in a decrease in ionic current. From the magnitude of the electrical signal of the current decrements during the pore translocation, it is possible to analyze physical quantity such as the volume of the sample.
Nanopipette based single molecule measurement is also one of the important methods for single molecule electrical measurements. A nanopipette device is defined as a pipette with a very fine tip that has a nanoscale opening. Similar to nanopore, the detection principle of nanopipettes is done by blockage of the ionic current through the pipette by the passage of the target samples. Nanopipettes have been widely applied from cells to ions due to their ease of preparation, which allows the size of the pipette to be controlled according to the target sample [3,4,5].
Nanogap based single molecule measurement is also used for nanodevices for single molecule electrical measurement [6]. The measurement principle is to measure the electrical conductivity of individual molecules by using around 1 nm gaps, which are formed by using a mechanically controllable or electrical break junction method. Sample molecules enter and translocate through the nanogap-electrodes, ensuring the facilitation of the tunneling current via the nucleotide molecules. The tunnel-current intensity is related to the molecular energy level so that signal intensity represents the electrical conductivity of individual molecules. This method enables the analysis of chemical bonds, steric structure, molecular interactions between target molecules and gold electrodes, and the polarity of sample molecules.
With the recent development of the nanodevice fabrication by semiconductor mass-production technique, the fabrication cost of the devices has rapidly decreased so that the nanodevice sensors are able to be utilized for biological/medical applications. In this review, we introduce the cutting-edge medical applications that have been made possible by using nanodevices.
2. Single Molecule Electrical Measurements: Biomolecule Detection
The first step for disease diagnosis and health status is the detection of biomolecules. The detection methods are roughly separated into two categories: direct sensing and indirect sensing for single molecule measurement using nanodevices.
2.1. Direct Sensing by Single Molecule Electrical Measurements
Direct sensing involves directly reading physical properties from the target molecule. Physical properties include the electronic state, the physical volume, which include the solvation shell of the molecule/ions, and structural differences, which are induced by conformational change of the molecule via inter/intra molecular interaction (Figure 2). These direct sensing methods are applied to single-molecule sequencing for biological polymers such as DNA, RNA, and peptides. These direct sensing methods potentially contribute to the detection of rare target markers and the discovery of new target markers.
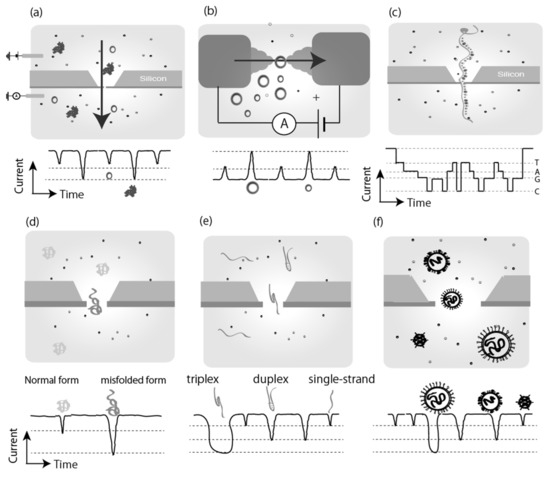
Figure 2.
Direct sensing of target molecule/particle by using nanodevices. In single molecule/particle measurement using nanodevices, an electrical signal for a single molecule is observed as a current-time profile when the molecule passes through the sensor device. In the case of nanopore devices, the intensity of decrement in ion current during passage represents the volume of the molecule (a), and in the case of nanogap devices, the signal intensity of the tunnel-current represents the electronic conductivity of the molecule (b). When a single biopolymer passes through the sensor, each unit of the polymer (nucleotide for DNA and RNA, amino acid for peptide and protein) passes through the sensor, and the electrical signal reflects the sequence of the biopolymer (c). Detection of different molecular signals occurs even for the same target molecule. If a structural change has occurred, different electrical signals reflecting the different structures would be observed (d–f). In fact, signals reflecting differences in the intramolecular hydrogen bond morphology of proteins (d:prion) and nucleic acid base chains (DNA single-strand, duplex, and triplex) have been observed. In addition, differences of shapes in the nanoparticles and viruses have also been identified by electrical signals reflecting the shape of the particles (f).
2.2. Indirect Sensing by Single Molecule Electrical Measurements
Indirect sensing involves detecting the specific molecular marker by using analytical probes, which are designed to selectively bind to the target marker and serve to amplify the electrical signal by nanodevices (Figure 3). By using probe molecules, target marker molecules specifically form a host (probe)—guest (target) conjugate, resulting in the signal amplification of target molecules so that they are easily discriminated from the host only (probe) and no-target other molecules. In addition to signal amplification, some probe molecules work as chemical scavengers in order to purify or exclude molecules that interfere with detection other than guests. The use of these probe molecules enables the specific and selective detection of guests, resulting in an improvement of the sensing accuracy and sensitivity.
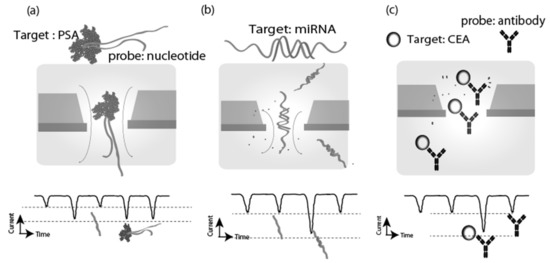
Figure 3.
Indirect sensing of target molecule/particle by nanodevices. In this sensing mode, a probe molecule that selectively binds to the target molecule is required. DNA aptamer (a) and complementary nucleotides (b) are used as probe molecules. In addition to nucleotide probe molecules, peptide chains and monoclonal antibodies (c) are used to measure the binding form of target molecules and particles using nanodevices, and identify the differences in electrical signals.
Up to now, there have been the reports on identifications of various chemical species, including nucleobases and nucleotides such as DNA and RNA [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24], amino acids and peptides [25,26,27,28,29], proteins [30,31,32,33], carbohydrates [34,35,36], biocompatible polymers [37,38,39,40], second messenger molecules and organic molecules [41,42,43,44,45], ions [46,47,48,49,50,51,52], toxic nanoparticles [53], and viruses [54,55]. Among these bimolecular sensing, based on the DNA and amino-acid identification method, single-molecule electrical sequencing methods are developed [56,57,58,59,60,61,62] (Table 1). These molecules are important target biomolecules for medical applications and thus detailed studies on these target groups are described in the following sections.

Table 1.
Biomolecule-sensing species: device, target, and tools for single-molecule detection.
2.3. Nucleotide Sensing
Nucleic acid and nucleotides, such as DNA and RNA, are biologically important targets for understanding diseases and health conditions caused by genetic abnormalities and abnormal expression, as DNA is the main body of genes, and RNA is a substance that is translated from DNA during gene expression. Recently, in addition to the natural nucleobases [7,8,9,10,11,12,16,17,18,19,24], the detection of post-translational modifications has become important and interesting as epi-transcriptome research fields because these nucleotide and nucleobase modifications play roles in gene expression and suppression, which are closely related to various biological malfunction such as disease and aging. Since there are about over one hundred types of known base modifications in eukaryotic cells, some of the epi-modification, such as methylated nucleobases (e.g., methyl cytosine, methyl adenine) and oxidized guanine are detected by these nanodevice detection methods [13,14,15,20,21,22,23].
2.4. Amino Acids, Peptides and Proteins Sensing
Amino acids, peptides and proteins are also important target molecules because they perform various biological functions such as catalyzing metabolic reactions, DNA replication, signal transduction and metabolism thus abnormalities are closely related to diseases. Up to now, there have been reports on the detection of the twenty amino acids, and post-translational modifications of amino acids such as methylation, acetylation, and phosphorylation have also been detected [25,26,27,28]. Peptide and proteins, which are polymers of amino acids, are also important targets. Besides reading sequences or direct sensings by nanodevices, the indirect sensing method is often utilized for the detection of proteins such as streptavidin, maltose-binding protein (MBP), etc., by using probes such as nucleic acid aptamers that bind to the host molecules of specific target molecules [29,30,31,32,33].
2.5. Glycans, and Biocompatible Polymer (PEG) Sensing
Glycans are an important biopolymer, belong to group of compounds consisting of various sugars connected by glycosidic bonds. Glycans can bind not only to other sugars but also to proteins, lipids, and other small molecules to produce a variety of molecules. These glycoproteins and glycolipids are important biopolymers that play important physiological roles in living organisms, and their detection has attracted much attention. Such glycosides and its related molecules have been reported by single-molecule detection [34,35,36]. Besides this, biocompatible polymers have attracted interest as nanodevice modification substances. Among them, polyethylene glycol (PEG) is often used in the chemical modification of nanodevices for avoiding the non-specific sticking of protein on the devices. In the reports of single-molecule measurements, the degree of polymerization of PEGs have been detected [37,38,39].
2.6. Second Messengers, Ion Sensing
Second messengers and ions, which play an important role in the transmission of information in the body, are also important targets. Among the second messengers, the detection of cAMP and neurotransmitters such as adrenaline, a neurotransmitter are reported [41,42,43,44,45]. In these reports, direct sensing of these molecules were successfully achieved by optimizing the size of nanopores and nanogap for nanodevices because the nanodevice sensors are sensitive to the size of molecular volume, three-dimensional structure, and hydration radius of the targets. In the case of indirect sensing, probe molecules, which selectively interact with the target molecules, are utilized for detection of the target ion [46,47,48,49,50,51,52]. For instance, this method has successfully detected harmful ions such as uranium [49] and mercury ions [50]. The strategy of size optimization for nanodevices also works for detection of viruses, cells, particulate matter (PM), etc. [53,54,55].
2.7. Single-Molecule Electrical Detection Based Sequencing
Based on the sequential identification of chemical species such as nucleotides and amino acids by single molecule electrical measurements, single-molecule electrical sequencing was developed to read sequences for biopolymers of DNA, RNA, and peptides (Figure 4) [56,57,58,59,60,61,62,63,64]. Nucleic acid sequencing [56,57,58,59,60] and peptide sequencing [61,62] methods have been proposed and recently developed. Compared to conventional sequencers, this single molecule electrical sequencer has the following features: first, this sequencing methodology does not need any amplification process, thus it can reduce the analytical time and cost of reagents for amplification, and allows the equipment to become smaller; second, this electrical sequencing methodology can read DNA information faster than the conventional optical probe based sequencing technologies as optical probe-based sequencing needs a DNA elongation reaction; third, epigenetic information can be detected as the native sample molecules are directly observed without amplification. Particularly, single-molecule DNA sequencing is increasingly utilized for on-site portable sequencing because it is cheaper and more compact than conventional DNA sequencers.
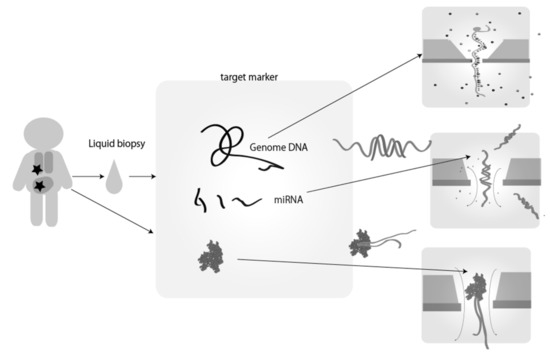
Figure 4.
Cancer diagnosis by single-molecule electrical detection of cancer marker molecules and counting of miRNA molecules. Tumor-specific marker molecules in liquid biopsy samples, such as blood, urine, and saliva obtained from patients, are measured. After these samples are pretreated by purification, amplification, and chemical treatment, single-molecule measurements are performed using nanodevices to distinguish between patients and healthy individuals. There are several reports of the detection of genes and miRNAs by sequencing, and/or detection using probe molecules that selectively bind to the target.
3. Applications of Single Molecule Measurement: Disease Diagnosis, Monitoring
As examples of single-molecule measurement applications (Table 2), we focus on the diagnosis of cancer diseases [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88], viral infections [55,64,89,90,91,92,93,94,95], drug detection and screening [96,97,98,99,100,101], dementia, brain and nervous system diagnoses, and monitoring of other dangerous substance epidemics [102,103,104,105,106,107,108,109,110,111].

Table 2.
Biological/medical application by single-molecule electrical measurement using nanodevices.
3.1. Cancer Diagnosis
One of the most widely reported studies is cancer diagnosis. It is generally known that cancer is caused by genetic abnormalities in cells, but there are many possible causes, and the corresponding diagnostic markers are different for each of the causes. In the application of cancer diagnosis for personal medical care, it is necessary to find the cause of each cancer individually and to administer the corresponding drug appropriately. Therefore, the simultaneous detection of various diagnostic marker molecules is required.
Diagnostic marker molecules for cancer diagnosis include specific DNA gene sequences, specific genomic sequence duplications such as specific short tandem repeats (STRs), overexpression or suppression of miRNAs, CpG methylation, and tumor marker proteins and so on.
Sequencing methods by nanodevices are utilized for the detection of cancer related gene sequences such as genetic mutations and polymorphisms [63,64,65,66,67]. For example, the genetic abnormality of TP53, a specific oncogene, was detected in blood samples [67]. It is possible to perform the detection by the Illumina and Sanger sequencing method but the nanodevice based single-molecule sequencer can detect them cheaper and faster.
Indirect detection methods are utilized for the detection of cancer markers by using nucleotide probes, which are designed to selectively bind to the target cancer marker. For instance, by using a nucleic acid probe with complementary base pairs and a target miRNA, the detection of cancer-related miRNAs was successfully achieved [68,69,70,71,72,73,74,75]. In this method, a specific miRNA is detected by the probe molecule bound to the miRNA conjugates in blood. In addition, by using multi targeting probes, which can be hybridized with several types of miRNAs, multiple target miRNAs were detected simultaneously [70,71]. For no nucleotide type target markers, detection by using the nucleotide probe, which can interact with target markers, was successfully achieved [79,81]. For instance, using a nucleic acid aptamer probe, prostate-specific antigen (PSA) was detected [79]. The development of the evolutionary engineering method using nucleic acids and peptides can produce probe molecules with high selectivity so that the nanodevice detection method using probe molecules can become more accurate in the future. CpG methylation has been reported in various cancers such as colorectal cancer and lung cancer, and has attracted attention as target cancer markers. By using single-molecule detection with nanodevices, such epi-genetic detection was also reported [83].
3.2. Alzheimer’s Disease, Huntington’s Disease, and Prion Diseases Detection
In addition to cancer diagnoses, there have been reports on the detection of Alzheimer’s disease, Huntington’s disease, and prion diseases related to target markers [101,102,103,104,105,106]. These diseases are known to be induced by abnormal protein aggregation, thus the detection of the protein aggregation is the first step for the disease diagnosis. For instance, single-molecule detection method by nanodevice succeeded in detecting differences in the aggregation structure of amyloid-β [103,104] and prion [105]. Therefore, these methods are expected to contribute to the understanding of the cause of the disease, early detection, and the development of drugs.
3.3. Virus Detection
It has become an important target to diagnose the presence or absence of viral infection cheaply, quickly and accurately (Figure 5) due to the recent COVID-19 pandemic having raised interest in on-site sequencing to monitor mutant strains. Up to now, there have been several reports on virus detection by single-molecule detection methods [53,64,88,89,90,91,92,93,94]. They are mainly categorized into two methods. The first is the detection of viral RNA sequences, and the second is the detection of viral particle shapes. For instance, the first method successfully detected influenza A [89], HPV [64], Lhasa fever [110], and Ebola [85] by on-site sequencing with Minion. The second method is the direct detection of differences in the three-dimensional structure and shape of viruses. Using this method, various types of viruses, such as Influenza A and B, Coronavirus, Adenovirus, and Respiratory Syncytial virus [88], along with HCoV-229E, SARS-CoV, MERS-CoV, and SARS-CoV-2 [53], have been successfully identified.
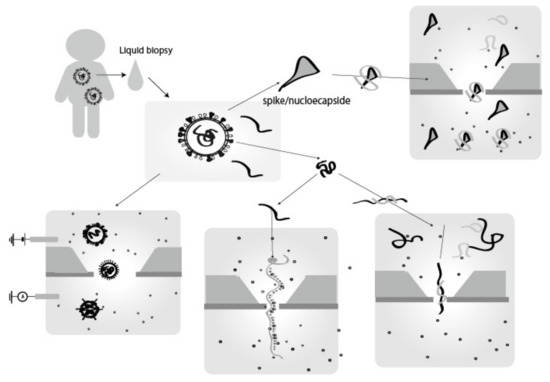
Figure 5.
Virus detection by single-molecule/particle electrical detection with a nanopore device. Sample viruses are collected from liquid biopsy samples, such as nasal swabs, saliva, blood, and urine, etc. As targets, there are virus whole-particles, RNA and/or its cDNA converted from the virus RNA, and parts of the virus particles and its proteins such as spikes that constitute the virus. The presence of viruses has been detected by identifying the shape of the virus, sequencing nucleic acid base chains, detecting known target nucleic acid base sequences by complementary strand sequences, and detecting target markers such as proteins by using probe molecules that selectively bind to them.
3.4. Drug Screening and Environmental Monitoring
Single-molecule drug screening is one of the major targets due to its featuring inexpensive measurement systems and rapid evaluation of various candidate chemical substances. The detection of drugs by single-molecule detection methods has been reported [89,90,91,92,93,94]. For example, drugs such as ibuprofen [95], doxorubicin [99], and trifluridine (FTD) [100] were detected. It is expected that further integration of these single-molecule detection technologies enables comprehensive drug screening by using the Total Analysis System (TAS), which consists of various kinds of probe molecules immobilized sensor array.
In the application of environmental monitoring, these single-molecule measurements by nanodevices are expected to provide stable, long-term measurements of a wide variety of target molecules with low costs. The application of environmental monitoring includes the constant monitoring of pollutants in the air, aqueous solutions, and soil, such as toxic and hazardous substances. Among them, metagenomic sequencing has recently become interesting [107,110]. Metagenomic sequencing does not target a single microorganism or bacterium, but a mixture of genomes from various microorganisms or bacteria. For example, it can be used to classify bacterial populations in clinical samples of patients without purification. In addition to such medical applications, the environmental impact of genetically modified organisms (GMOs) by the monitoring of the rate of genetic modification in plants is a potential important target. The monitoring of hazardous substances such as bacteria, viruses, and explosives are also important targets. There have been reports on the detection of harmful ions [44,47,48] and explosives such as TNT [112].
4. Discussion and Future Prospects
In order to increase applications of single-molecule measurement by nanodevice, further integration of functional nanostructures and improvements and the simplification of analysis methods are required. Some approaches to these issues are described in the following paragraphs.
The first approach is the integration of various functional nanostructures on nanodevice sensors. For instance, nanochannels have made great achievements in sample control and transport by electrophoresis in sensor devices [113,114,115] and nanopillar structure serve as purification, separation, and transportation [116,117]. Along with the integration of nanodevices, the development of fabrication techniques for integrated nanodevices is an important issue. In nanodevices, if the shape of the fabricated device can be parallelized with high accuracy, the throughput of measurement can be expected to be dramatically improved. So far, parallelization has been reported for nanopore and nanogap devices [118,119].
The second approach is to use nanochannel or nanowell structures for optical sensor detection. For example, a single molecule in a nanochannel can be detected by optical microscopy with high sensitivity [120,121,122,123]. There are also zero-mode waveguides using the near-field effect and Raman spectroscopy using the plasmon phenomenon in the nanogap [124,125,126,127,128]. These nano-optics/electronics hybrid devices would improve the sensing selectivity.
The third approach is the development of bioinformatics analysis methods using artificial intelligence (AI). In single-molecule measurements, the data volume is exponentially increased because of the high-speed data acquisition and large number of detected molecular signals in sample solutions, compared to conventional analytical methods. Moreover, the detected signal shape, i.e., electrical current-time profile, become more complex. Therefore, AI-based informatics analysis methods are suitable for sample identifications as the method enables the extraction of characteristic parameters from complex signals. In recent years, AI-based analysis has made it possible to identify target molecules among similar structural molecules [10,111,129,130,131]. Thus, it can be said that the evolution of bioinformatics analysis is essential for single-molecule measurement by nanodevice.
In addition to these developments of nanodevice based technologies and methods, there are several steps that precede real clinical practice use. The first step is the standardization of measurement systems, the nanodevices, and the data-analysis methods. The next step is to obtain medical approval from the government in each country. In the current stage, nanodevice methods are currently taking these steps towards application in the medical field. For example, the Food and Drug Administration (FDA) has issued guidelines for obtaining medical approval for next-generation sequencing nanodevices such as MinION [132,133,134]. Furthermore, the recent COVID-19 pandemic has accelerated the movement to detect viruses (SARS-CoV-2) and virus mutants by nanodevices. It is expected that the application of this technology will expand as this trend becomes more active in the future.
In summary, the single-molecule electrical measurement by nanodevices is an interdisciplinary field that is currently undergoing development while incorporating new technologies. I believe that single-molecule measurement methods using nanodevices will greatly develop as a key technology for realizing personal medical care that enables point-of-care, which is considered a dream come true, while expanding the range of its applications.
Funding
This research was funded by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (21H01741).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, G.; Wang, L.; Han, Y.; Zhou, S.; Guan, X. Nanopore Stochastic Detection: Diversity, Sensitivity, and Beyond. Acc. Chem. Res. 2013, 46, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Howorka, S.; Siwy, Z. Nanopore analytics: Sensing of single molecules. Chem. Soc. Rev. 2009, 38, 2360–2384. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, G.; Chaves, G.; Olivier, J.; Ozel, R.E.; Pourmand, N. Nanopipettes as Monitoring Probes for the Single Living Cell: State of the art and future directions in molecular biology. Cells 2018, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, J.; Pourmand, N. Nanopipettes—The past and the present, N Pourmand. APL Mater. 2020, 8, 100902. [Google Scholar] [CrossRef]
- Actis, P.; Mak, A.C.; Pourmand, N. Functionalized nanopipettes: Toward label-free, single cell biosensors. Bioanal. Rev. 2010, 1, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Zwolak, M.; Di Ventra, M. Colloquium: Physical approaches to DNA sequencing and detection. Rev. Mod. Phys. 2008, 80, 141–165. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, B.M.; Bashir, R. Nanopore sensors for nucleic acid analysis. Nat. Nanotech. 2011, 6, 615–624. [Google Scholar] [CrossRef]
- Deamer, D.W.; Branton, D. Characterization of Nucleic Acids by Nanopore Analysis. Acc. Chem. Res. 2002, 35, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.L.; Cao, C.; Long, Y.T. Single molecule analysis by biological nanopore sensors. Analyst 2014, 139, 3826–3835. [Google Scholar] [CrossRef]
- Kono, N.; Arakawa, K. Nanopore sequencing: Review of potential applications in functional genomics. Dev. Growth Differ. 2019, 61, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotech. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Benner, S.; Chen, R.J.A.; Wilson, N.A.; Abu-Shumays, R.; Hurt, N.; Lieberman, K.R.; Deamer, D.W.; Dunbar, W.B.; Akeson, M. Sequence-specific detection of individual DNA polymerase complexes in real time using a nanopore. Nat. Nanotechnol. 2007, 2, 718–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatz, M.C. Nanopore sequencing meets epigenetics. Nat. Methods 2017, 14, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.V.B.; Stoddart, D.; Heron, A.J.; Mikhailova, E.; Maglia, G.; Donohoe, T.J.; Bayley, H. Identification of epigenetic DNA modifications with a protein nanopore. Chem. Commun. 2010, 46, 8195–8197. [Google Scholar] [CrossRef]
- Schreiber, J.; Wescoe, Z.L.; Abu-Shumays, R.; Vivian, J.T.; Baatar, B.; Karplus, K.; Akeson, M. Error rates for nanopore discrimination among cytosine, methylcytosine, and hydroxymethylcytosine along individual DNA strands. Proc. Natl. Acad. Sci. USA 2013, 110, 18910–18915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, M.; Taniguchi, M.; Yokota, K.; Kawai, T. Identifying single nucleotides by tunnelling current. Nat. Nanotechnol. 2010, 5, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A.; Huang, S.; He, J.; Liang, F.; Zhang, P.M.; Li, S.Q.; Chen, X.; Sankey, O.; Lindsay, S. Electronic Signatures of all Four DNA Nucleosides in a Tunneling Gap. Nano Lett. 2010, 10, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Prasongkit, J.; Grigoriev, A.; Pathak, B.; Ahuja, R.; Scheicher, R.H. Transverse Conductance of DNA Nucleotides in a Graphene Nanogap from First Principles. Nano Lett. 2011, 11, 1941–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; He, J.; Chang, S.A.; Zhang, P.M.; Liang, F.; Li, S.Q.; Tuchband, M.; Fuhrmann, A.; Ros, R.; Lindsay, S. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010, 5, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Sen, S.; Lindsay, S.; Zhang, P.M. Recognition Tunneling of Canonical and Modified RNA Nucleotides for Their Identification with the Aid of Machine Learning. ACS Nano 2018, 12, 7067–7075. [Google Scholar] [CrossRef]
- Tsutsui, M.; Matsubara, K.; Ohshiro, T.; Furuhashi, M.; Taniguchi, M.; Kawai, T. Electrical detection of single methylcytosines in a DNA oligomer. J. Am. Chem Soc. 2011, 133, 9124–9128. [Google Scholar] [CrossRef]
- Ohshiro, T.; Konno, M.; Asai, A.; Komoto, Y.; Yamagata, A.; Doki, Y.; Eguchi, H.; Ofusa, K.; Taniguchi, M.; Ishii, H. Single-Molecule RNA Sequencing for Simultaneous Detection of m6A and 5mC. Sci. Rep. 2021, 11, 19304. [Google Scholar] [CrossRef]
- Komoto, Y.; Ohshiro, T.; Taniguchi, M. Detection of an alcohol-associated cancer marker by single-molecule quantum sequencing. Chem. Comm. 2020, 56, 14299–14302. [Google Scholar] [CrossRef] [PubMed]
- Karhanek, M.; Kemp, J.T.; Pourmand, N.; Davis, R.W.; Webb, C.D. Single DNA molecule detection using nanopipettes and nanoparticles. Nano Lett. 2005, 5, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, H.; Sarthak, K.; Ensslen, T.; Piguet, F.; Manivet, P.; Pelta, J.; Behrends, J.C.; Aksimentiev, A.; Oukhaled, A. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 2019, 38, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Larrea, D. Single-amino acid discrimination in proteins with homogeneous nanopore sensors and neural networks. Biosens. Bioelectron. 2021, 180, 7. [Google Scholar] [CrossRef]
- Zhao, Y.A.; Ashcroft, B.; Zhang, P.M.; Liu, H.; Sen, S.M.; Song, W.; Im, J.; Gyarfas, B.; Manna, S.; Biswas, S.; et al. Single-molecule spectroscopy of amino acids and peptides by recognition tunnelling. Nat. Nanotechnol. 2014, 9, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Tsutsui, M.; Yokota, K.; Furuhashi, M.; Taniguchi, M.; Kawai, T. Detection of post-translational modifications in single peptides using electron tunnelling currents. Nat. Nanotechnol. 2014, 9, 835–840. [Google Scholar] [CrossRef]
- Piguet, F.; Ouldali, H.; Pastoriza-Gallego, M.; Manivet, P.; Pelta, J.; Oukhaled, A. Identification of single amino acid differences in uniformly charged homopolymeric peptides with aerolysin nanopore. Nat. Commun. 2018, 9, 966. [Google Scholar] [CrossRef]
- Oukhaled, A.; Bacri, L.; Pastoriza-Gallego, M.; Betton, J.M.; Pelta, J. Sensing Proteins through Nanopores: Fundamental to Applications. ACS Chem. Biol. 2012, 7, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Movileanu, L.; Howorka, S.; Braha, O.; Bayley, H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nat. Biotechnol. 2000, 18, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Oukhaled, G.; Mathe, J.; Biance, A.L.; Bacri, L.; Betton, J.M.; Lairez, D.; Pelta, J.; Auvray, L. Unfolding of Proteins and Long Transient Conformations Detected by Single Nanopore Recording. Phys. Rev. Lett. 2007, 98, 158101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.L.; Tsai, C.Y.; Sun, C.C.; Chen, C.C.; Kuo, L.S.; Chen, P.H. Ultrasensitive electrical detection of protein using nanogap electrodes and nanoparticle-based DNA amplification. Biosens. Bioelectron. 2007, 22, 3139–3145. [Google Scholar] [CrossRef]
- Fennouri, A.; Przybylski, C.; Pastoriza-Gallego, M.; Bacri, L.; Auvray, L.; Daniel, R. Single Molecule Detection of Glycosaminoglycan Hyaluronic Acid Oligosaccharides and Depolymerization Enzyme Activity Using a Protein Nanopore. ACS Nano 2012, 6, 9672–9678. [Google Scholar] [CrossRef] [PubMed]
- Kullman, L.; Winterhalter, M.; Bezrukov, S.M. Transport of Maltodextrins through Maltoporin: A Single-Channel Study. Biophys. J. 2002, 82, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Bacri, L.; Oukhaled, A.; Hemon, E.; Bassafoula, F.B.; Auvray, L.; Daniel, R. Discrimination of Neutral Oligosaccharides through a Nanopore. Biochem. Biophys. Res. Commun. 2011, 412, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.W.F.; Rodrigues, C.G.; Stanford, V.M.; Rubinson, K.A.; Krasilnikov, O.V.; Kasianowicz, J.J. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl. Acad. Sci. USA 2007, 104, 8207–8211. [Google Scholar] [CrossRef] [Green Version]
- Movileanu, L.; Cheley, S.; Bayley, H. Partitioning of Individual Flexible Polymers into a Nanoscopic Protein Pore. Biophys. J. 2003, 8, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Oukhaled, A.G.; Biance, A.L.; Pelta, J.; Auvray, L.; Bacri, L. Transport of Long Neutral Polymers in the Semidilute Regime through a Protein Nanopore. Phys. Rev. Lett. 2012, 108, 088104. [Google Scholar] [CrossRef]
- Umehara, S.; Karhanek, M.; Davis, R.W.; Pourmand, N. Label-free biosensing with functionalized nanopipette probes. Proc. Natl. Acad. Sci. USA 2009, 106, 4611–4616. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.L.; Wang, H.Y.; Sutherland, T.C.; Long, Y.T. Monitoring of an ATP-Binding Aptamer and its Conformational Changes Using an α-Hemolysin Nanopore. Small 2011, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Ohshiro, T.; Taniguchi, M. Development of Single-Molecule Electrical Identification Method for Cyclic Adenosine Monophosphate Signaling Pathway. Nanomaterials 2021, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Ohshiro, T.; Yoshida, T.; Tarusawa, E.; Yagi, T.; Washio, T.; Taniguchi, M. Time-resolved neurotransmitter detection in mouse brain tissue using an artificial intelligence nanogap. Sci. Rep. 2020, 10, 11244. [Google Scholar] [CrossRef] [PubMed]
- Cheley, S.; Gu, L.Q.; Bayley, H. Stochastic sensing of nanomolar inositol 1,4,5-trisphosphate with an engineered pore. Chem. Biol. 2002, 9, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Braha, O.; Gu, L.Q.; Zhou, L.; Lu, X.F.; Cheley, S.; Bayley, H. Simultaneous stochastic sensing of divalent metal ions. Nat. Biotechnol. 2000, 18, 1005–1007. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Wang, D.Y.; Tian, Y.; Jiang, L. Ion/Molecule Transportation in Nanopores and Nanochannels: From Critical Principles to Diverse Functions. J. Am. Chem. Soc. 2019, 141, 8658–8669. [Google Scholar] [CrossRef]
- Liu, L.; You, Y.; Zhou, K.; Guo, B.Y.; Cao, Z.; Zhao, Y.L.; Wu, H.C. A Dual-Response DNA Probe for Simultaneously Monitoring Enzymatic Activity and Environmental pH Using a Nanopore. Angew. Chem. Int. Ed. 2019, 131, 15071–15076. [Google Scholar] [CrossRef]
- Roozbahani, G.M.; Chen, X.H.; Zhang, Y.W.; Xie, R.Q.; Ma, R.; Li, D.E.; Li, H.Z.; Guan, X.Y. Peptide-Mediated Nanopore Detection of Uranyl Ions in Aqueous Media. ACS Sens. 2017, 2, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Zeng, T.; Liu, L.; Zhao, K.; Zhao, Y.L.; Liu, X.J.; Wu, H.C. Highly Sensitive and Selective DNA-Based Detection of Mercury(II) with α-Hemolysin Nanopore. J. Am. Chem. Soc. 2011, 133, 18312–18317. [Google Scholar] [CrossRef]
- Umehara, S.; Pourmand, N.; Webb, C.D.; Davis, R.W.; Yasuda, K.; Karhanek, M. Current rectification with poly-L-lysine-coated quartz nanopipettes. Nano Lett. 2006, 6, 2486–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozel, R.E.; Bulbul, G.; Perez, J.; Pourmand, N. Functionalized Quartz Nanopipette for Intracellular Superoxide Sensing: A Tool for Monitoring Reactive Oxygen Species Levels in Single Living Cell. ACS Sens. 2018, 3, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Yokota, K.; Yoshida, T.; Hotehama, C.; Kowada, H.; Esaki, Y.; Taniguchi, M.; Washio, T.; Kawai, T. Identifying Single Particles in Air Using a 3D-Integrated Solid-State Pore. ACS Sens. 2019, 4, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Arima, A.; Tsutsui, M.; Washio, T.; Baba, Y.; Kawai, T. Solid-State Nanopore Platform Integrated with Machine Learning for Digital Diagnosis of Virus Infection. Anal. Chem. 2021, 93, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Minami, S.; Ono, C.; Hamajima, R.; Morimura, A.; Hamaguchi, S.; Akeda, Y.; Kanai, Y.; Kobayashi, T.; Kamitani, W.; et al. Combining machine learning and nanopore construction creates an artificial intelligence nanopore for coronavirus detection. Nat. Commun. 2021, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Garalde, D.R.; Snell, E.A.; Jachimowicz, D.; Sipos, B.; Lloyd, J.H.; Bruce, M.; Pantic, N.; Admassu, T.; James, P.; Warland, A.; et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Method. 2018, 15, 201. [Google Scholar] [CrossRef]
- Di Ventra, M.; Taniguchi, M. Decoding DNA, RNA and peptides with quantum tunnelling. Nat. Nanotechnol. 2016, 11, 117–126. [Google Scholar] [CrossRef]
- Ohshiro, T.; Matsubara, K.; Tsutsui, M.; Furuhashi, M.; Taniguchi, M.; Kawai, T. Single-Molecule Electrical Random Resequencing of DNA and RNA. Sci. Rep. 2012, 2, 501–507. [Google Scholar] [CrossRef]
- Ohshiro, T.; Tsutsui, M.; Yokota, K.; Taniguchi, M. Quantitative analysis of DNA with single-molecule sequencing. Sci. Rep. 2018, 8, 8517. [Google Scholar] [CrossRef] [Green Version]
- Restrepo-Perez, L.; Joo, C.; Dekker, C. Paving the way to single-molecule protein sequencing. Nat. Nanotechnol. 2018, 13, 786–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.L.; Huo, M.Z.; Ying, Y.L.; Long, Y.T. Biological Nanopore Approach for Single-Molecule Protein Sequencing. Angew. Chem. Int. Ed. 2020, 133, 14862–14873. [Google Scholar] [CrossRef]
- Norris, A.L.; Workman, R.E.; Fan, Y.; Eshleman, J.R.; Timp, W. Nanopore sequencing detects structural variants in cancer. Cancer Biol. Ther. 2016, 17, 246–253. [Google Scholar] [CrossRef]
- Yang, W.J.; Liu, Y.; Dong, R.Y.; Liu, J.; Lang, J.D.; Yang, J.L.; Wang, W.W.; Li, J.J.; Meng, B.; Tian, G. Accurate Detection of HPV Integration Sites in Cervical Cancer Samples Using the Nanopore MinION Sequencer Without Error Correction. Front. Genet. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Valle-Inclan, J.E.; Stangl, C.; de Jong, A.C.; van Dessel, L.F.; van Roosmalen, M.J.; Helmijr, J.C.; Renkens, I.; Janssen, R.; de Blank, S.; de Witte, C.J.; et al. Optimizing Nanopore sequencing-based detection of structural variants enables individualized circulating tumor DNA-based disease monitoring in cancer patients. Genome Med. 2021, 13, 86. [Google Scholar] [CrossRef]
- Quan, L.L.; Dong, R.Y.; Yang, W.J.; Chen, L.Y.; Lang, J.D.; Liu, J.; Song, Y.; Ma, S.Q.; Yang, J.L.; Wang, W.W.; et al. Simultaneous detection and comprehensive analysis of HPV and microbiome status of a cervical liquid-based cytology sample using Nanopore MinION sequencing. Sci. Rep. 2019, 9, 19337. [Google Scholar] [CrossRef]
- Minervini, C.F.; Cumbo, C.; Orsini, P.; Brunetti, C.; Anelli, L.; Zagaria, A.; Minervini, A.; Casieri, P.; Coccaro, N.; Tota, G.; et al. TP53 gene mutation analysis in chronic lymphocytic leukemia by nanopore MinION sequencing. Diagn. Pathol. 2016, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, D.L.; Tan, Q.L.; Wang, M.X.; Gu, L.Q. Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat. Nanotechnol. 2011, 6, 668–674. [Google Scholar] [CrossRef]
- Zhang, J.H.; Liu, X.L.; Hu, Z.L.; Ying, Y.L.; Long, Y.T. Intelligent identification of multi-level nanopore signatures for accurate detection of cancer biomarkers. Chem. Commun. 2017, 53, 10176–10179. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Y.; Fricke, B.L.; Gu, L.Q. Programming Nanopore Ion Flow for Encoded Multiplex MicroRNA Detection. ACS Nano 2014, 8, 3444–3450. [Google Scholar] [CrossRef]
- Tian, K.; He, Z.J.; Wang, Y.; Chen, S.J.; Gu, L.Q. Designing a Polycationic Probe for Simultaneous Enrichment and Detection of MicroRNAs in a Nanopore. ACS Nano 2013, 7, 3962–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, Y.L.; Li, D.W.; Li, Y.; Lee, J.S.; Long, Y.T. Enhanced translocation of poly(dt)45 through an α-hemolysin nanopore by binding with antibody. Chem. Commun. 2011, 47, 5690–5692. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Humphreys, G.I.; Venkatesan, B.M.; Munz, J.M.; Zou, X.Q.; Sathe, C.; Schulten, K.; Kosari, F.; Nardulli, A.M.; Vasmatzis, G.; et al. Detection and Quantification of Methylation in DNA using Solid-State Nanopores. Sci. Rep. 2013, 3, 1389. [Google Scholar] [CrossRef] [Green Version]
- Xi, D.M.; Li, Z.; Liu, L.P.; Ai, S.Y.; Zhang, S.S. Ultrasensitive Detection of Cancer Cells Combining Enzymatic Signal Amplification with an Aerolysin Nanopore. Anal. Chem. 2018, 90, 1029–1034. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Dou, L.; Wang, J.; Sun, K.; Zhang, X.; Song, G.; Zhao, C.; Li, K.; Bai, Y.; et al. Detection of Circulating Tumor Cells in Breast Cancer Patients by Nanopore Sensing with Aptamer-Mediated Amplification. ACS Sens. 2020, 5, 2359–2366. [Google Scholar] [CrossRef]
- Duan, L.; Yobas, L. Label-Free Multiplexed Electrical Detection of Cancer Markers on a Microchip Featuring an Integrated Fluidic Diode Nanopore Array. ACS Nano 2018, 12, 7892–7900. [Google Scholar] [CrossRef]
- Wang, S.Y.; Haque, F.; Rychahou, P.G.; Evers, B.M.; Guo, P.X. Engineered Nanopore of Phi29 DNA-Packaging Motor for Real-Time Detection of Single Colon Cancer Specific Antibody in Serum. ACS Nano 2013, 7, 9814–9822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.; Kim, Y.; Humphreys, G.I.; Nardulli, A.M.; Kosari, F.; Vasmatzis, G.; Taylor, W.R.; Ahlquist, D.A.; Myong, S.; Bashir, R. Nanopore-Based Assay for Detection of Methylation in Double-Stranded DNA Fragments. ACS Nano 2015, 9, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ying, Y.L.; Shi, X.; Liu, S.C.; Long, Y.T. Direct sensing of cancer biomarkers in clinical samples with a designed nanopore. Chem. Commun. 2017, 53, 11564–11567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.Y.; Song, P.; Zhou, K.; Liu, L.; Wu, H.C. Simultaneous Sensing of Multiple Cancer Biomarkers by a Single DNA Nanoprobe in a Nanopore. Anal. Chem. 2020, 92, 9405–9411. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.R.; Wang, H.; Yang, C.; Zhao, D.D.; Qian, Y.Y.; Li, Y.X. Nanopore-based Strategy for Selective Detection of Single Carcinoembryonic Antigen (CEA) Molecules. Anal. Chem. 2020, 92, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, T.; Zhang, S.W.; Song, P.; Guo, B.Y.; Zhao, Y.L.; Wu, H.C. Simultaneous Quantification of Multiple Cancer Biomarkers in Blood Samples through DNA-Assisted Nanopore Sensing. Angew. Chem. Int. Ed. 2018, 57, 11882–11887. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Wang, Y.; Reagan, C.; Fu, Y.M.; Wang, M.X.; Gu, L.Q. Designing DNA interstrand lock for locus-specific methylation detection in a nanopore. Sci. Rep. 2013, 3, 2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.H.; Zhang, Y.W.; Roozbahani, G.M.; Guan, X.Y. Salt-Mediated Nanopore Detection of ADAM-17. ACS Appl. Bio Mater. 2019, 2, 504–509. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, K.; Shi, R.C.; Gu, A.; Pennella, M.; Alberts, L.; Gates, K.S.; Li, G.F.; Fan, H.X.; Wang, M.X.; et al. Nanolock-Nanopore Facilitated Digital Diagnostics of Cancer Driver Mutation in Tumor Tissue. ACS Sens. 2017, 2, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, P.; Yan, S.; Yuan, W.; Wang, Y.; Li, X.; Dou, L.; Zhao, C.; Zhang, J.; Wang, Q.; et al. Ultrasensitive Nanopore Sensing of Mucin 1 and Circulating Tumor Cells in Whole Blood of Breast Cancer Patients by Analyte-Triggered Triplex-DNA Release. ACS Appl. Mater. Interfaces 2021, 13, 21030–21039. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Asghar, W.; Allen, P.B.; Duhon, H.; Ellington, A.D.; Iqbal, S.M. Electrical detection of cancer biomarker using aptamers with nanogap break-junctions. Nanotechnology 2012, 23, 275502. [Google Scholar] [CrossRef] [Green Version]
- Arima, A.; Tsutsui, M.; Yoshida, T.; Tatematsu, K.; Yamazaki, T.; Yokota, K.; Kuroda, S.; Washio, T.; Baba, Y.; Kawai, T. Digital Pathology Platform for Respiratory Tract Infection Diagnosis via Multiplex Single-Particle Detections. ACS Sens. 2020, 5, 3398–3403. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Lynch, S.E.; Rodoni, B.C.; Sawbridge, T.I.; Cogan, N.O.I. MinION nanopore sequencing of an influenza genome. Front. Microbiol. 2015, 6, 766. [Google Scholar]
- Batovska, J.; Lynch, S.E.; Rodoni, B.C.; Sawbridge, T.I.; Cogan, N.O. Metagenomic arbovirus detection using MinION nanopore sequencing. J. Virol. Methods 2017, 249, 79–84. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Federman, S.; Yu, G.; Mbala, P.; Bres, V.; Stryke, D.; Bouquet, J.; Somasekar, S.; Linnen, J.M.; et al. Rapid metagenomic identification of viral pathogens in clinical samples by real-time nanopore sequencing analysis. Genome Med. 2015, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzwiecki, D.J.; Iyer, R.; Borer, P.N.; Movileanu, L. Sampling a Biomarker of the Human Immunodeficiency Virus across a Synthetic Nanopore. ACS Nano 2013, 7, 3341–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanunu, M.; Dadosh, T.; Ray, V.; Jin, J.M.; McReynolds, L.; Drndic, M. Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat. Nanotechnol. 2010, 5, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.K.; Kim, J.S.; Lee, M.K.; Ryu, K.S.; Chi, S.W. Probing the Neuraminidase Activity of Influenza Virus Using a Cytolysin a Protein Nanopore. Anal. Chem. 2020, 92, 14303–14308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.T.; de Zoysa, R.S.S.; Wang, D.Q.; Jayawardhana, D.A.; Guan, X.Y. Real-Time Monitoring of Peptide Cleavage Using a Nanopore Probe. J. Am. Chem. Soc. 2009, 131, 6324–6325. [Google Scholar] [CrossRef]
- Ding, T.L.; Yang, J.; Pan, V.; Zhao, N.; Lu, Z.H.; Ke, Y.G.; Zhang, C. DNA nanotechnology assisted nanopore-based analysis. Nucleic Acids Res. 2020, 48, 2791–2806. [Google Scholar] [CrossRef] [Green Version]
- Wanunu, M.; Sutin, J.; Meller, A. DNA Profiling Using Solid-State Nanopores: Detection of DNA-Binding Molecules. Nano Lett. 2009, 9, 3498–3502. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Oh, S.; Lim, K.; Lee, B.; Yi, G.S.; Kim, Y.R.; Kim, K.B.; Lee, C.K.; Chi, S.W.; Lee, M.K. Tertiary RNA Folding-Targeted Drug Screening Strategy Using a Protein Nanopore. Anal. Chem. 2021, 93, 2811–2819. [Google Scholar] [CrossRef]
- Yao, F.J.; Duan, J.; Wang, Y.; Zhang, Y.; Guo, Y.L.; Guo, H.L.; Kang, X.F. Nanopore Single-Molecule Analysis of DNA–Doxorubicin Interactions. Anal. Chem. 2015, 87, 338–342. [Google Scholar] [CrossRef]
- Ohshiro, T.; Komoto, Y.; Konno, M.; Koseki, J.; Asai, A.; Ishii, H.; Taniguch, M. Direct Analysis of Incorporation of an Anticancer Drug into DNA at Single-Molecule Resolution. Sci. Rep. 2019, 9, 3886. [Google Scholar] [CrossRef] [Green Version]
- De Coster, W.; Van Broeckhoven, C. Newest methods for detecting structural variations. Trends Biotechnol. 2019, 37, 973–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.; Yang, H.; Yan, Q.; Qi, P.; Qing, Z.H.; Zheng, J.; Xu, X.; Zhang, L.H.; Feng, F.; Yang, R.H. Synchronous screening of multiplexed biomarkers of Alzheimer’s disease by a length-encoded aerolysin nanopore-integrated triple-helix molecular switch. Chem. Commun. 2019, 55, 6433. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, B.; Wei, X.J.; Watson, B.; Wang, X.Q.; Zhang, Z.H.; Li, C.Z.; Moss, M.; Liu, C. In Vitro Biosensing of β-Amyloid Peptide Aggregation Dynamics using a Biological Nanopore. Sens. Actuators B 2021, 338, 129863. [Google Scholar] [CrossRef] [PubMed]
- Houghtaling, J.; List, J.; Mayer, M. Nanopore-Based, Rapid Characterization of Individual Amyloid Particles in Solution: Concepts, Challenges, and Prospects. Small 2018, 14, 1802412. [Google Scholar] [CrossRef] [PubMed]
- Madampage, C.A.; Andrievskaia, O.; Lee, J.S. Nanopore detection of antibody prion interactions. Anal. Biochem. 2010, 396, 36–41. [Google Scholar] [CrossRef]
- Madampage, C.; Tavassoly, O.; Christensen, C.; Kumari, M.; Lee, J.S. Nanopore analysis: An emerging technique for studying the folding and misfolding of proteins. Prion 2012, 6, 116–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalampous, T.; Kay, G.L.; Richardson, H.; Aydin, A.; Baldan, R.; Jeanes, C.; Rae, D.; Grundy, S.; Turner, D.J.; Wain, J.; et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat. Biotechnol. 2019, 37, 783–792. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Frith, M.C.; Mizuguchi, T.; Miyatake, S.; Toyota, T.; Adachi, H.; Oma, Y.; Kino, Y.; Mitsuhashi, H.; Matsumoto, N. Tandem-genotypes: Robust detection of tandem repeat expansions from long DNA reads. Genome Biol. 2019, 20, 58. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Juan, A.; Stephens, J.; French, C.E.; Gleadall, N.; Mégy, K.; Penkett, C.; Shamardina, O.; Stirrups, K.; Delon, I.; Dewhurst, E.; et al. Complex structural variants in Mendelian disorders: Identification and breakpoint resolution using short- and long-read genome sequencing. Genome Med. 2018, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Kafetzopoulou, L.E.; Pullan, S.T.; Lemey, P.; Suchard, M.A.; Ehichioya, D.U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J.; Oestereich, L.; Wozniak, D.M.; et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science 2019, 363, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Minh, Q.N.; Tong, H.D.; Kuijk, A.; van de Bent, F.; Beekman, P.; van Rijn, C.J.M. Gas sensing performance at room temperature of nanogap interdigitated electrodes for detection of acetone at low concentration. RSC Adv. 2017, 7, 50279–50286. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.Y.; Gu, L.Q.; Cheley, S.; Braha, O.; Bayley, H. Stochastic sensing of TNT with a genetically engineered pore. ChemBioChem 2005, 6, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Ohshiro, T. Applications of Microfluidic Systems in Biology and Medicine: Nanopore Device for Single-Molecule Sensing Method and Its Application; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-981-13-6229-3. [Google Scholar] [CrossRef]
- Hou, X.; Guo, W.; Jiang, L. Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 2011, 40, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Kaji, N.; Okamoto, Y.; Tokeshi, M.; Baba, Y. Nanopillar, nanoball, and nanofibers for highly efficient analysis of biomolecules. Chem. Soc. Rev. 2010, 39, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Komoto, Y.; Taniguchi, M. Single-Molecule Counting of Nucleotide by Electrophoresis with Nanochannel-Integrated Nano-Gap Devices. Micromachines 2020, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Hoff, B.H.; Maier, S.A.; de Mello, J.C. Scalable Fabrication of Metallic Nanogaps at the Sub-10 nm Level. Adv. Sci. 2021, 8, 2102756. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Ying, Y.L.; Zhong, C.B.; Zhang, L.M.; Yan, F.; Long, Y.T. Instrumentational implementation for parallelized nanopore electrochemical measurements. Analyst 2021, 146, 4111. [Google Scholar] [CrossRef] [PubMed]
- Korlach, J.; Bjornson, K.P.; Chaudhuri, B.P.; Cicero, R.L.; Flusberg, B.A.; Gray, J.J.; Holden, D.; Saxena, R.; Wegener, J.; Turner, S.W. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. Trends Anal. Chem 2016, 82, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Dombi, P.; Papa, Z.; Vogelsang, J.; Yalunin, S.V.; Sivis, M.; Herink, G.; Schafer, S.; Gross, P.; Ropers, C.; Lienau, C. Strong-field nano-optics. Rev. Mod. Phys. 2020, 92, 025003. [Google Scholar] [CrossRef]
- Reisner, W.; Larsen, N.B.; Silahtaroglu, A.; Kristensen, A.; Tommerup, N.; Tegenfeldt, J.O.; Flyvbjerg, H. Single-molecule denaturation mapping of DNA in nanofluidic channels. Proc. Natl. Acad. Sci. USA 2010, 107, 13294–13299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Bosnick, K.; Maillard, M.; Brus, L. Single Molecule Raman Spectroscopy at the Junctions of Large Ag Nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Zrimsek, A.B.; Chiang, N.H.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Kiguchi, M.; Takase, M.; Nagasawa, F.; Nabika, H.; Ikeda, K.; Uosaki, K.; Ueno, K.; Misawa, H.; Murakoshi, K. Single Molecule Dynamics at a Mechanically Controllable Break Junction in Solution at Room Temperature. J. Am. Chem. Soc. 2013, 135, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Yasuraoka, K.; Kiguchi, M. Bias Voltage Induced Surface-Enhanced Raman Scattering Enhancement on the Single-Molecule Junction. J. Phys. Chem. C 2019, 123, 6502–6507. [Google Scholar] [CrossRef]
- Yang, W.; Lim, D.K. Recent Advances in the Synthesis of Intra-Nanogap Au Plasmonic Nanostructures for Bioanalytical Applications. Adv. Mater. 2020, 32, e2002219. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ohshiro, T.; Komoto, Y.; Takaai, T.; Yoshida, T.; Washio, T. High-Precision Single-Molecule Identification Based on Single-Molecule Information within a Noisy Matrix. J. Phys. Chem. C 2019, 123, 15867–15873. [Google Scholar] [CrossRef]
- Taniguchi, M. Combination of Single-Molecule Electrical Measurements and Machine Learning for the Identification of Single Biomolecules. ACS Omega 2020, 5, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Komoto, Y.; Ohshiro, T.; Taniguchi, M. Length discrimination of homo-oligomeric nucleic acids with single-molecule measurement. Anal. Sci. 2021, 37, 513–518. [Google Scholar] [CrossRef]
- Luh, F.; Yen, Y. FDA guidance for next generation sequencing-based testing: Balancing regulation and innovation in precision medicine. NPJ Genom. Med. 2018, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, M.; Fabi, A.; Buglioni, S.; Martayan, A.; Conti, L.; Pescarmona, E.; Ciliberto, G.; Giacomini, P. Tearing down the walls: FDA approves next generation sequencing (NGS) assays for actionable cancer genomic aberrations. J. Exp. Clin. Cancer Res. 2018, 37, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).