1. Introduction
Commercialization of algal-based processes for biofuels and co-products is constrained upstream during cultivation by low productivity, contamination by invasive species, unsustainable and inefficient supply of nutrients (e.g., nitrogen, sulfur, and phosphorous), inorganic carbon, and water, exposure to environmental factors, and limited available land acreage for inoculum and subsequent large-scale cultures. It is also constrained downstream by costly and inefficient harvesting, cell disruption, product extraction, thermochemical conversion, hydrotreatment, and upgrading. A novel two-stage cultivation process was previously proposed to address various challenges of mass microalgal cultivation for biofuels and co-products and demonstrated using an oleaginous, haloalkaline-tolerant, and multi-trophic green
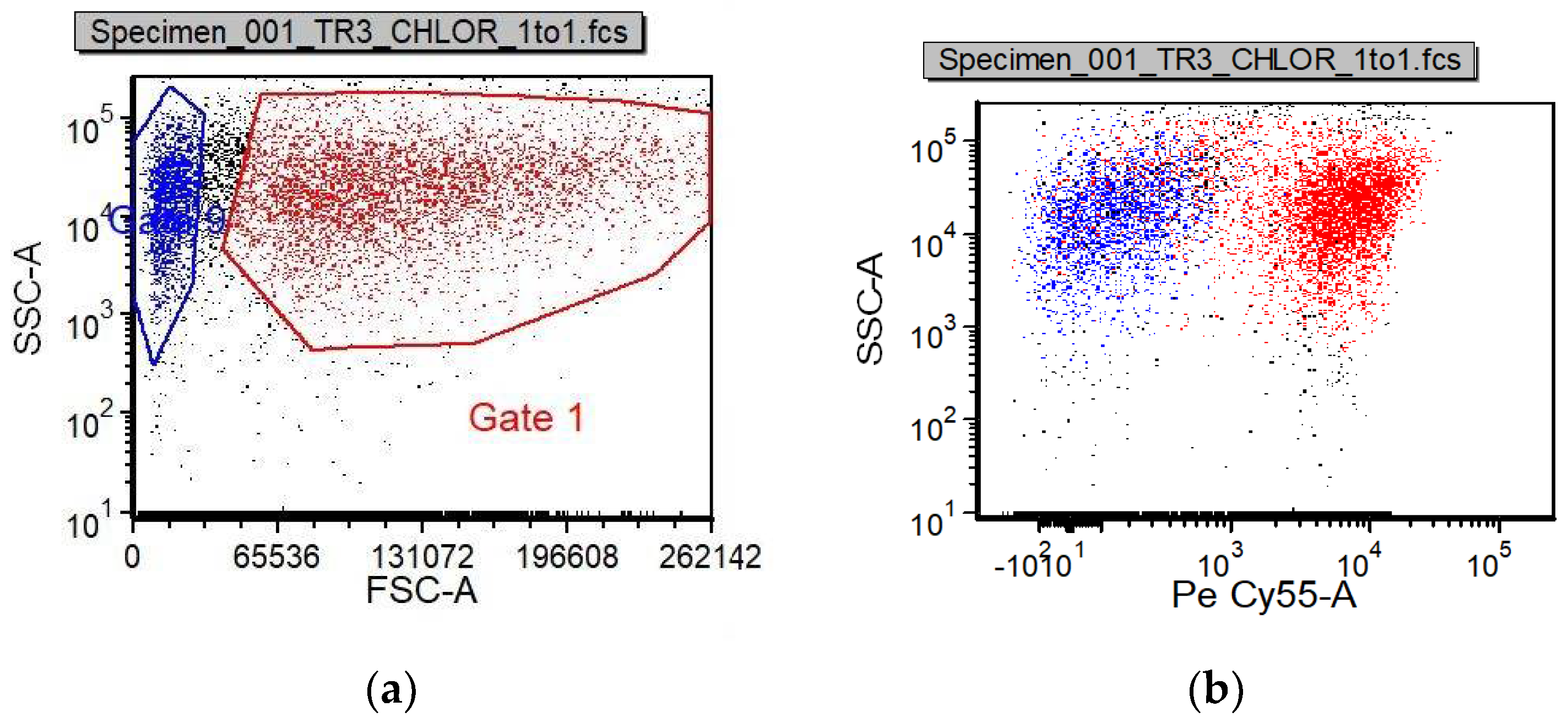
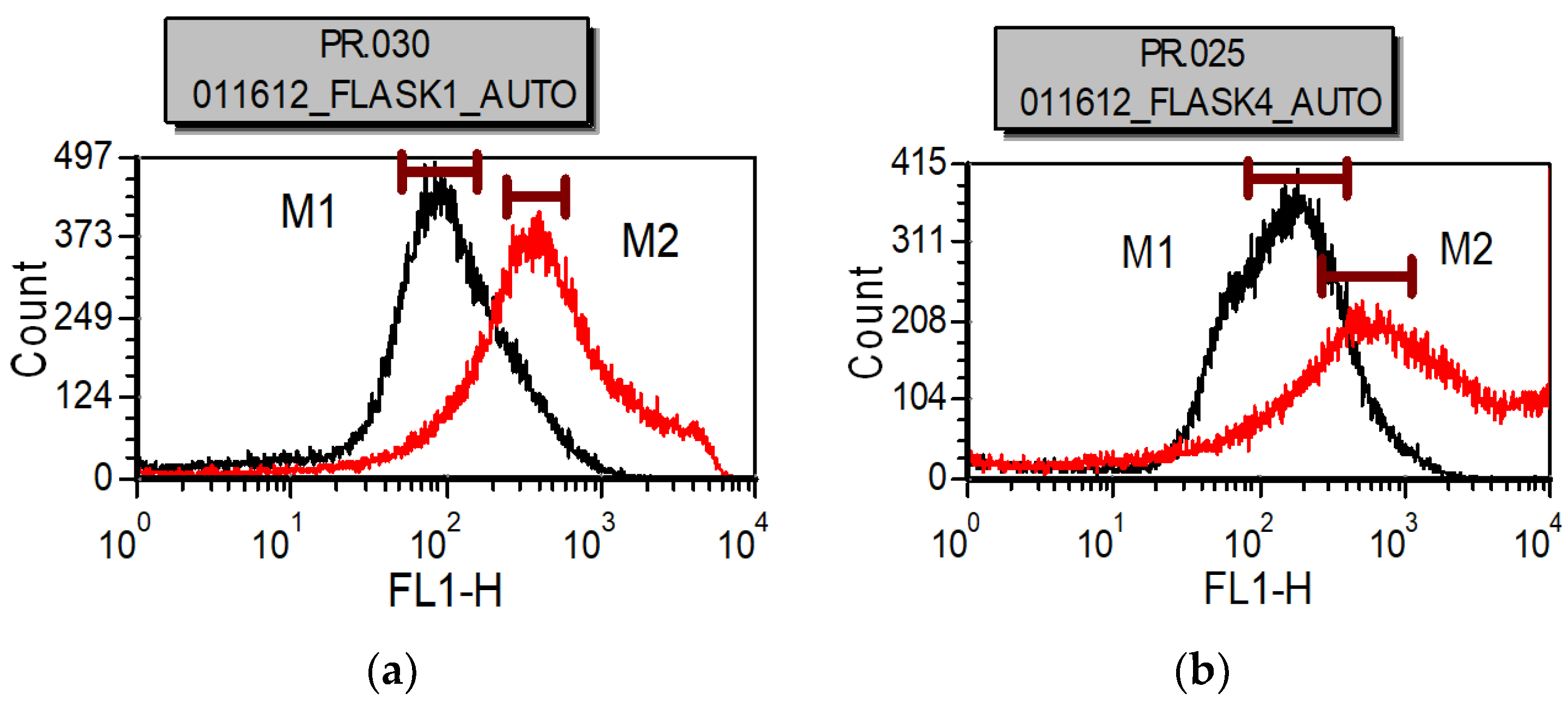
Chlorella sp. ALP2 isolated from a soda lake [
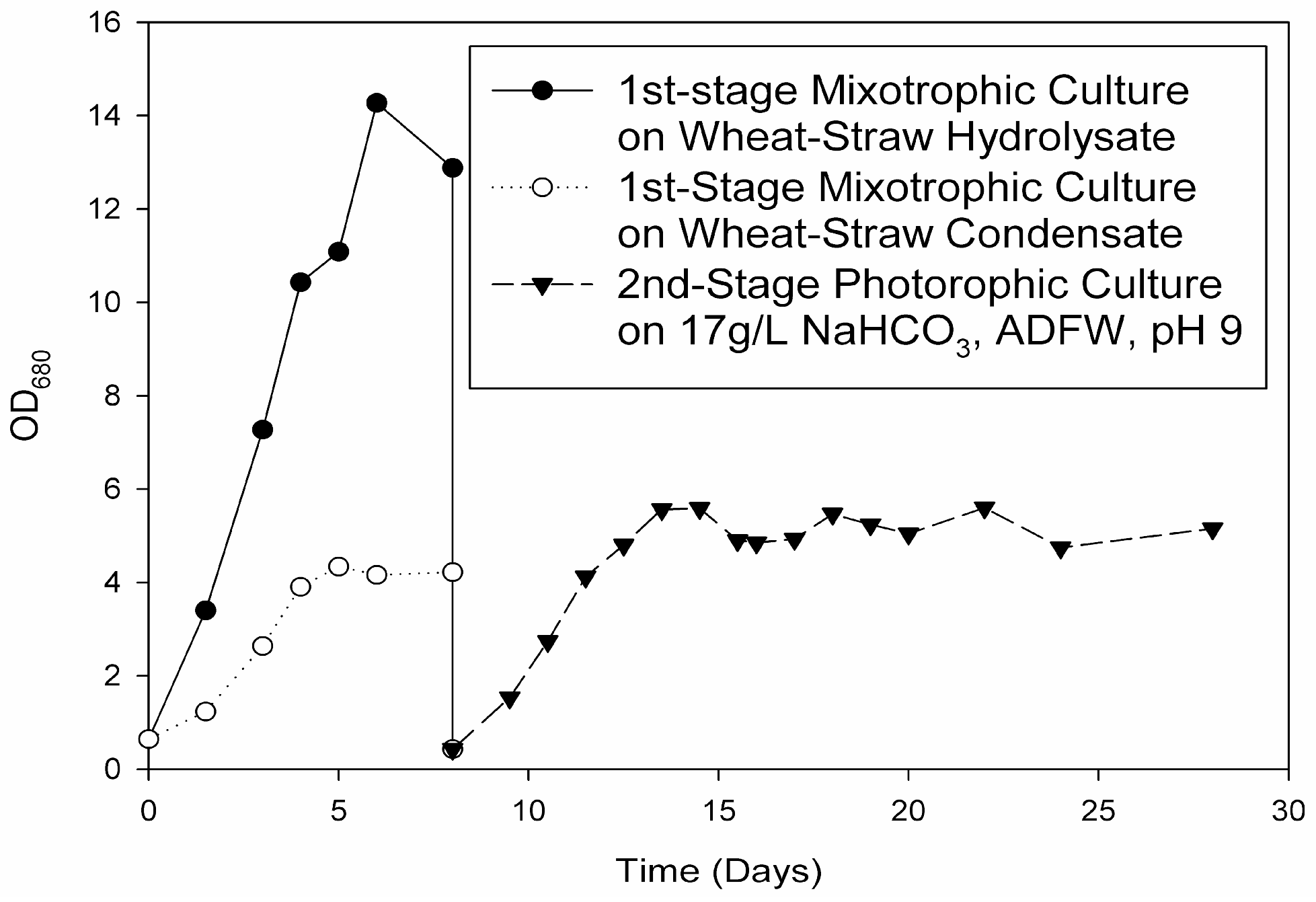
1]. This involved cultivating the microalgae in a fermenter heterotrophically or photobioreactor mixotrophically on organic carbon (first-stage) to rapidly obtain high cell densities and inoculate an open-pond phototrophic culture (second-stage) featuring high levels of NaHCO
3, pH, and salinity. The combination of high cell densities and extreme haloalkaline conditions in the second phototrophic stage could then minimize residence time and limit contamination [
2], facilitate auto-flocculation harvesting [
3], efficiently supply inorganic carbon [
4], and enhance neutral lipid accumulation [
5]. This two-stage process can be further improved upon with sustainable supplies of organic carbon for the first stage and nitrogen and phosphorous nutrients for the second stage.
Organic carbon for the first-stage enclosed microalgal cultures can be derived from hydrolysates and/or condensates from processed ligno-cellulosic agricultural and/or forestry wastes. For instance, a glucose-containing enzymatic hydrolysate from high-temperature ethanol-treated rice-straw and an acetic acid-containing condensate from fast-pyrolyzed softwood were previously used to cultivate the microalgae
Chlorella pyrenoidosa [
6] and
Chlamydomonas reinhardtii, respectively [
7]. Hydrolysates from treated ligno-cellulosic biomass are advantageous over those resulting from starch and juice of agricultural crops such as Jerusalem artichoke, cassava, and sugarcane [
6]. Namely, they contain both C
6 and C
5 sugars, avoid the “food vs. fuel” controversy, and are more cost-effective and higher-yielding than purchased glucose, which, in turn, was previously shown to be the highest-yielding carbon substrate for the
Chlorella vulgaris ALP2 microalgae, particularly under mixotrophic conditions [
1]. Alternatively, condensates from thermochemically processing lignocellulosic biomass, i.e., fast-pyrolysis, represent a way of removing the carboxylic acids and levoglucosan used for microalgal [
7] and yeast cultivation [
8] while simultaneously stabilizing pH, reducing corrosivity, and increasing heating-value of an intended bio-oil intermediary product that is later refined to fuel (i.e., green gasoline) or other chemicals.
Despite these improvements, the conventional dilute sulfuric acid approach for hydrolysates and the fast-pyrolysis approach for condensates both have limitations. First, they require relatively expensive capital equipment for high-temperature and/or high-pressure operating conditions [
9]. Second, such extreme conditions result in high levels of compounds in the hydrolysate and/or condensate that may inhibit microalgal growth [
10]. Third, they fail to adequately address major logistical challenges and high costs (i.e., 30% of feedstock costs) associated with initially harvesting, transporting, storing, and grinding waste ligno-cellulosic biomass feedstock to uniform particle size for greater process efficiency prior to pre-treatment at a biorefinery [
11]. Fourth, the selected lignocellulosic biomass may not be the most suitable. For instance, pyrolyzed forestry residues (i.e., softwood and hardwood) will yield less acetic acid and be less available, particularly during dark and cold winters when either indoor microalgal hetero-/mixotrophic cultivation or firewood combustion are implemented, compared to the very abundant, grassy agricultural crop residues (i.e., wheat-straw) after fall harvests [
12,
13]. Such by-products comprised up to 50% by weight of the world’s 2 billion t year
−1 cereal crop production in 2011 [
6].
Rather than incorporating these aforementioned lignocellulosic biomass treatment methods to supply organic carbon for the first of a two-stage microalgal cultivation process, a novel, more cost-effective, hybrid, thermo-biochemical pre-treatment method involving torrefaction of wheat-straw and subsequent steps was therefore devised. Compared to fast-pyrolysis, torrefaction is a lower-temperature (200–300 °C) and self-sustaining thermochemical process occurring in an inert, O
2-less atmosphere [
11] that can be conducted in relatively simpler reactors like those previously used by Tawainese farmers [
14]. It results not only in a drier, more brittle, and hydrophobic solid biomass to alleviate feedstock logistical issues [
15], but also in high-heating value gases (CO, CH
4) combusted to run it [
16], and a liquid condensate containing a mixture of water and carboxylic acids [
11]. These torrefaction-derived acids arise mostly from the thermochemical degradation of hemi-cellulose as a by-product, while the pyrolysis-derived acids and phenolic growth inhibitors arise mostly from the lignin depolymerization as an intended bio-oil product [
11]. The liquid condensate remaining after torrefaction, requiring less extensive purification due to the lower temperatures, can potentially supply organic carbon (acetate) to a first-stage microalgal culture. In this way, a microalgae that is unable to uptake and/or metabolize directly C
5 sugars (i.e., xylose, mannose, ribose) in hydrolysates can use instead the acetic acid in the condensate obtained from the same hemicellulose fraction. The solid biomass remaining after torrefaction can also potentially be further pre-treated with ozonolysis (O
3) and soaking in recyclable ammonia, enzymatically hydrolyzed, and purified to supply organic carbon (C
6 sugar glucose) to another first-stage microalgal culture. Compared to the dilute sulfuric acid method, these novel pre-treatment steps involving O
3/NH
4 to generate hydrolysate were previously shown to require less operation time and milder operating conditions, yielding higher levels of C
6 sugars and lower levels of phenolic inhibitors [
10].
Nitrogen and phosphorous nutrients for second-stage, large-scale outdoor open-pond microalgal cultures can be sustainably supplied by effluents from the integrated, bio-gas generating, anaerobic-digestion (AD) of waste. AD using mesophilic or thermophilic bacteria has been widely used instead of energy-intensive aeration-based activated sludge processes to partially treat swine, poultry, and dairy manure waste from farm concentrated animal feeding operations by reducing organic matter and waste volume via fermentative degradation of organic constituents and prevent eutrophication upon its discharge into water bodies [
17]. Effluents’ unmetabolized inorganic nitrogen (i.e., ammonia) can be used by the microalgae [
18], and biogas (i.e., typically a mixture of CH
4, CO
2, H
2S, and H
2) can be purified and used for on-site electrical energy to power compressors, pumps, and harvesting centrifuges. Microalgal photosynthesis during cultivation on such waste effluents evolves dissolved O
2 to kill contaminating anaerobes. It is also more metabolically efficient [
19] and cost-effective [
20] than that using NaNO
3, which represents the most expensive (750–950 USD t
−1) and concentrated (1.500 g L
−1) component of the traditional BG-11 growth medium devoid of vitamins. For instance, based on flux balance analysis, nitrates are more metabolically expensive than ammonia because phototrophs, such as
Synechocystis PCC6803, divert and expend 9 moles of NADPH and ATP to convert 1 mole of NaNO
3 into 1 mole of preferred ammonia [
19,
21].
However, despite these advances, batch addition of effluents derived from anaerobically digesting animal waste manure to open-ponds also has limitations: first, manure effluents and even their dilutions tend to be turpid and can block transmission of sunlight to microalgae cultivated in second-stage outdoor open-ponds [
18], which has been shown to significantly influence productivity [
22]. Second, economic losses and pollution due to effluent ammonia volatilization [
23] during hot summers would likely be higher in the large-scale second-stage open-ponds proposed here with high surface areas, temperatures, pH, and wind-speeds. Third, the combination of the UV wavelength of polychromatic light (i.e., outdoor sunlight) and high concentrations of protonated ammonia in batch-added AD effluent are also reportedly more toxic to the PSII photosynthetic apparatus of phototrophs at the proposed elevated pH, where uncontrollable, diffusive, non-active transport across the cell boundary occurs [
24].
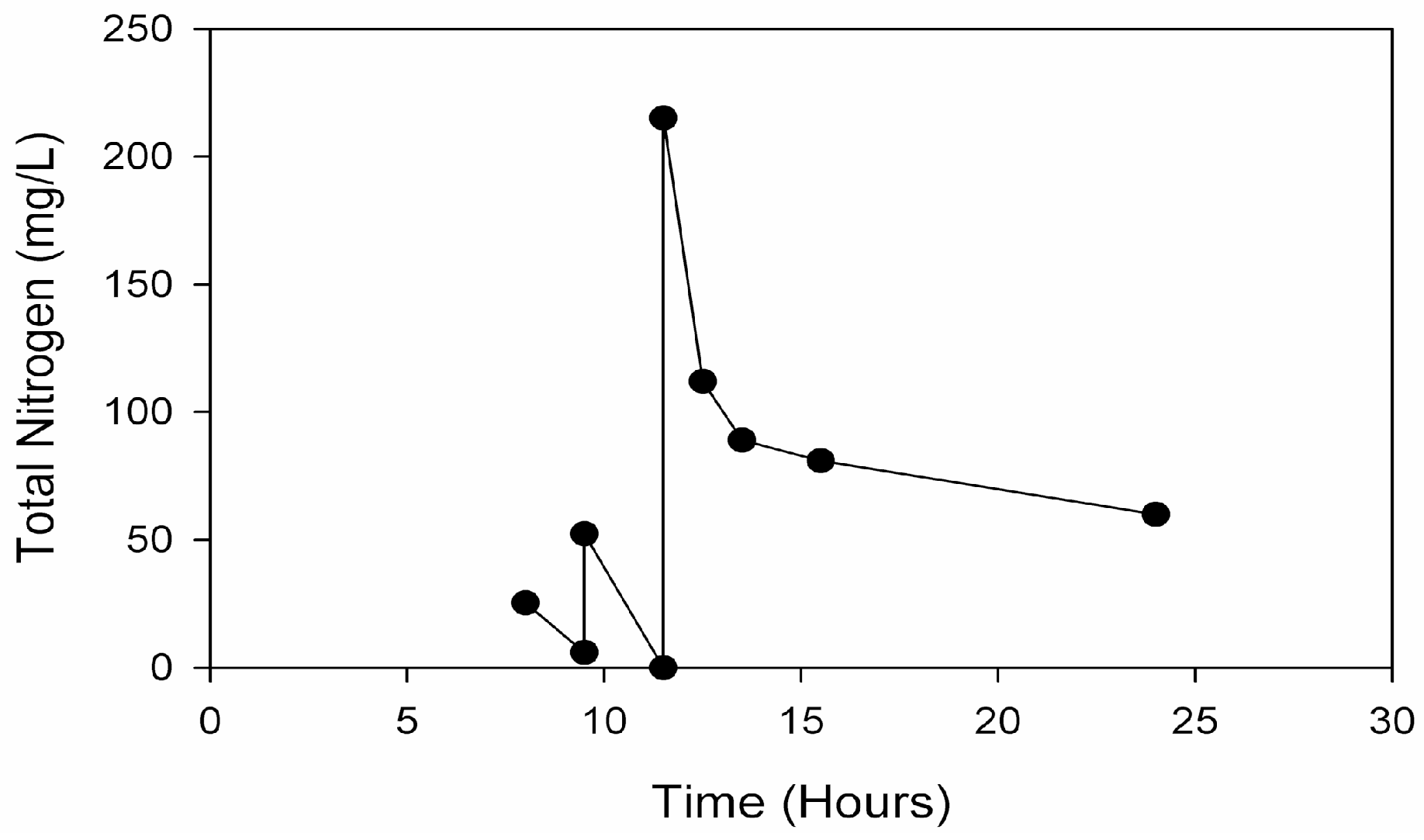
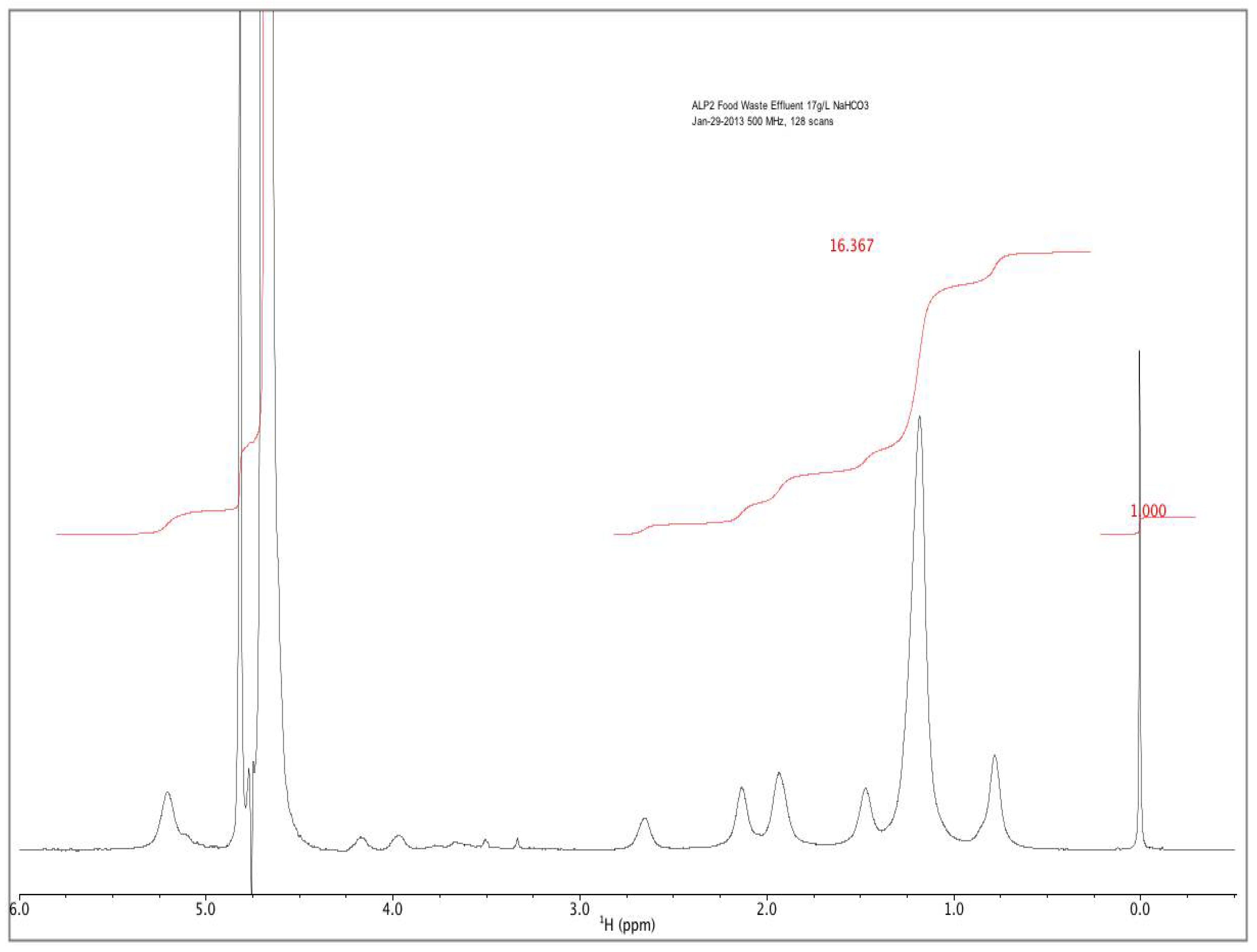
Rather than incorporating the aforementioned batch addition of AD manure effluents to supply nitrogen and phosphorous for the second of a two-stage microalgal cultivation process, a novel method involving fed-batch addition of AD effluent derived from food waste was developed. Food waste from municipalities and food processing facilities represents another alternative source of AD effluents. Food waste diversion from landfills is considered as a way to increase landfill capacity and reduce methane emissions, with a large potential energy content amounting to 2% of total U.S. energy consumption [
25]. Compared to the turbid dairy manure effluents, light yellow–brown effluent from starchy food-waste additionally represents greater microalgal open-pond light transmission. An effluent fed-batch strategy at high pH in the proposed second stage culture should employ an exponentially increasing mass flow [
26]. This would need to balance (1) the rate of effluent supplementation required for both sufficient growth and terminal nitrogen starvation-induced lipid accumulation, with (2) the rate of microalgal ammonia consumption and (3) the rate of high pH-induced volatilization.
In this study, proof of concept was established by cultivating for the first time an oleaginous, multi-trophic, haloalkaline-tolerant microalgae in an improved two-stage process using integrated, sustainable supplies of organic carbon from wheat-straw for mixotrophic first-stage and nitrogen and phosphorous from food waste for the phototrophic second stage. Carbon and nutrient sources were additionally analyzed in various conditions.