Cardioprotective Mechanisms of Interrupted Anesthetic Preconditioning with Sevoflurane in the Setting of Ischemia/Reperfusion Injury in Rats
Abstract
1. Introduction
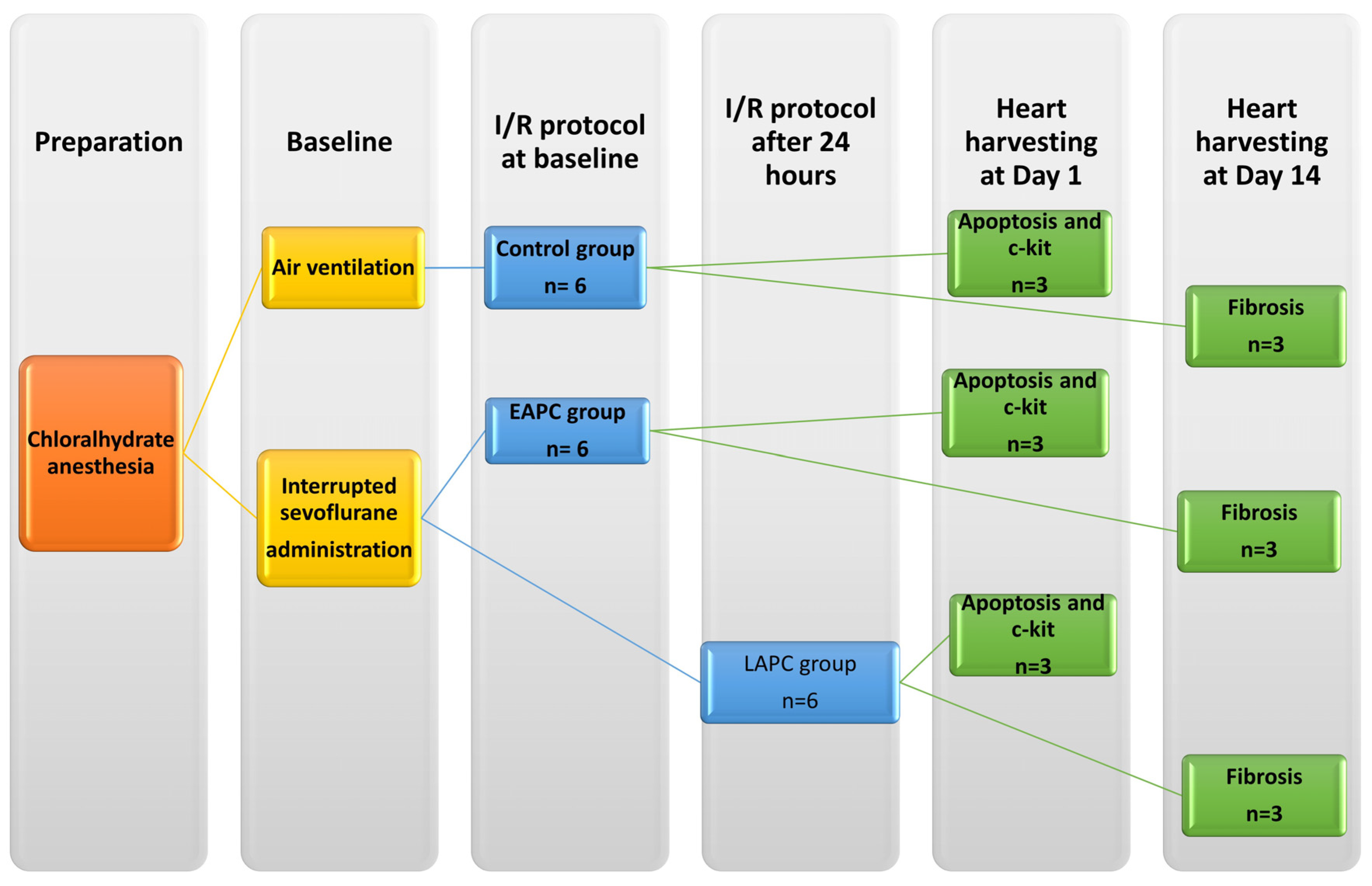
2. Materials and Methods
2.1. Cytokine Study
2.2. Ischemia/Reperfusion Study
2.2.1. Evaluation of Myocardial Apoptosis and C-kit+ Cell Infiltration
2.2.2. Fibrosis Study
2.3. Statistical Analysis
3. Results
3.1. Sevoflurane AP Increases Blood Levels of Plasma Cytokines Implicated in Chemotaxis of MSC
3.2. Sevoflurane Preconditioning Protects the Heart against Reperfusion Ischemia Injury by Decreasing the Degree of Apoptosis, Increasing the Number of C-kit+ Cells in the Damaged Myocardium, and Decreasing the Degree of Fibrosis
3.2.1. Estimation of Myocardial Apoptosis
3.2.2. Late AP Increases the Number of MSC in the Myocardium Subjected to Ischemia/Reperfusion Injury
3.2.3. AP Is Associated with a Decreased Area of Myocardial Fibrosis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kersten, J.R.; Schmeling, T.J.; Pagel, P.S.; Gross, G.J.; Warltier, D.C. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology 1997, 87, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, M.; Lucchinetti, E.; Spahn, D.R.; Pasch, T.; Schaub, M.C. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology 2002, 97, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Stadnicka, A.; Marinovic, J.; Ljubkovic, M.; Bienengraeber, M.W.; Bosnjak, Z.J. Volatile anesthetic-induced cardiac preconditioning. J. Anesth. 2007, 21, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, E.; Zeisberger, S.M.; Baruscotti, I.; Wacker, J.; Feng, J.; Zaugg, K.; Dubey, R.; Zisch, A.H.; Zaugg, M. Stem cell-like human endothelial progenitors show enhanced colony-forming capacity after brief sevoflurane exposure: Preconditioning of angiogenic cells by volatile anesthetics. Anesth. Analg. 2009, 109, 1117–1126. [Google Scholar] [CrossRef]
- Kamota, T.; Li, T.S.; Morikage, N.; Murakami, M.; Ohshima, M.; Kubo, M.; Kobayashi, T.; Mikamo, A.; Ikeda, Y.; Matsuzaki, M.; et al. Ischemic Pre-Conditioning Enhances the Mobilization and Recruitment of Bone Marrow Stem Cells to Protect Against Ischemia/Reperfusion Injury in the Late Phase. J. Am. Coll. Cardiol. 2009, 53, 1814–1822. [Google Scholar] [CrossRef]
- Takahashi, M.; Li, T.-S.; Suzuki, R.; Kobayashi, T.; Ito, H.; Ikeda, Y.; Matsuzaki, M.; Hamano, K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H886–H893. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Watson, T.; Lip, G.Y.H. Endothelial Progenitor Cells in Cardiovascular Disorders. J. Am. Coll. Cardiol. 2007, 49, 741–752. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, C.; Zhang, G.; Tao, J. Endothelial progenitor cells in cardiovascular diseases. Aging Med. 2018, 1, 204–208. [Google Scholar] [CrossRef]
- Kalenka, A.; Maurer, M.H.; Feldmann, R.E.; Kuschinsky, W.; Waschke, K.F. Volatile anesthetics evoke prolonged changes in the proteome of the left ventricule myocardium: Defining a molecular basis of cardioprotection? Acta Anaesthesiol. Scand. 2006, 50, 414–427. [Google Scholar] [CrossRef]
- Popescu, M.; Munteanu, A.; Isvoranu, G.; Suciu, L.; Pavel, B.; Marinescu, B.; Zagrean, L. Dynamics of endothelial progenitor cells following sevoflurane preconditioning. Roum. Arch. Microbiol. Immunol. 2011, 70, 109–113. [Google Scholar] [PubMed]
- Vlad, A.; Niculescu, L.; Stancu, C.; Popescu, M.; Stanca, I.; Corneci, D.; Ceafalan, L.; Gilca, M.; Surcel, M.; Popescu, A.; et al. Augmented Survival, Angiogenic Properties and Mobilization of Human Endothelial Progenitor Cells Following Exposure to Sevoflurane. FASEB J. 2019, 33, 820–833. [Google Scholar] [CrossRef]
- Lucchinetti, E.; Aguirre, J.; Feng, J.; Zhu, M.; Suter, M.; Spahn, D.R.; Härter, L.; Zaugg, M. Molecular evidence of late preconditioning after sevoflurane inhalation in healthy volunteers. Anesth. Analg. 2007, 105, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, E.; Hofer, C.; Bestmann, L.; Hersberger, M.; Feng, J.; Zhu, M.; Furrer, L.; Schaub, M.C.; Tavakoli, R.; Genoni, M.; et al. Gene regulatory control of myocardial energy metabolism predicts postoperative cardiac function in patients undergoing off-pump coronary artery bypass graft surgery: Inhalational versus intravenous anesthetics. Anesthesiology 2007, 106, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Weihrauch, D.; Schwabe, D.A.; Bienengraeber, M.; Warltier, D.C.; Kersten, J.R.; Pratt, P.F.; Pagel, P.S. Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1α and vascular endothelial growth factor expression in rats. Anesth. Analg. 2006, 103, 281–288. [Google Scholar] [CrossRef]
- Liu, Y.; Paterson, M.; Baumgardt, S.L.; Irwin, M.G.; Xia, Z.; Bosnjak, Z.J.; Ge, Z.D. Vascular endothelial growth factor regulation of endothelial nitric oxide synthase phosphorylation is involved in isoflurane cardiac preconditioning. Cardiovasc. Res. 2019, 115, 168–178. [Google Scholar] [CrossRef]
- Nogae, C.; Makino, N.; Hata, T.; Nogae, I.; Takahashi, S.; Suzuki, K.I.; Taniguchi, N.; Yanaga, T. Interleukin 1α-induced expression of manganous superoxide dismutase reduces myocardial reperfusion injury in the rat. J. Mol. Cell. Cardiol. 1995, 27, 2091–2099. [Google Scholar] [CrossRef]
- Billah, M.; Ridiandries, A.; Allahwala, U.; Mudaliar, H.; Dona, A.; Hunyor, S.; Khachigian, L.M.; Bhindi, R. Circulating mediators of remote ischemic preconditioning: Search for the missing link between non-lethal ischemia and cardioprotection. Oncotarget 2019, 10, 216–244. [Google Scholar] [CrossRef]
- Dawn, B.; Xuan, Y.T.; Guo, Y.; Rezazadeh, A.; Stein, A.B.; Hunt, G.; Wu, W.J.; Tan, W.; Bolli, R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc. Res. 2004, 64, 61–71. [Google Scholar] [CrossRef]
- Wu, J.W.; Hu, H.; Li, D.; Ma, L.K. Hypoxia-inducible factor 2-alpha-dependent induction of IL-6 protects the heart from ischemia/reperfusion injury. Aging 2021, 13, 3443–3458. [Google Scholar] [CrossRef]
- Rozier, R.; Paul, R.; Madji Hounoum, B.; Villa, E.; Mhaidly, R.; Chiche, J.; Verhoeyen, E.; Marchetti, S.; Vandenberghe, A.; Raucoules, M.; et al. Pharmacological preconditioning protects from ischemia/reperfusion-induced apoptosis by modulating Bcl-xL expression through a ROS-dependent mechanism. FEBS J. 2021, 288, 3547–3569. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Ma, H.; Wang, H.; Chen, C.; Ye, J.; Ahmed, A.M.; Zheng, H. Sevoflurane preconditioning attenuates hypoxia/reoxygenation injury of H9c2 cardiomyocytes by activation of the HIF-1/PDK-1 pathway. PeerJ 2020, 8, e10603. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tong, H. Precondition of sevoflurane upregulates TIMP3 expression to alleviate myocardial ischemia/reperfusion injury. Perfusion 2021, 36, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ren, Q.; Yu, S.; Gao, X. Sevoflurane protects cardiomyocytes against hypoxia/reperfusion injury via LINC01133/miR-30a-5p axis. Biosci. Rep. 2020, 40, BSR20200713. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, J.; Guo, R.; Jiao, L.; Zhang, Y.; Lau, W.B.; Lopez, B.; Christopher, T.; Gao, E.; Cao, J.; et al. Sevoflurane Pre-conditioning Ameliorates Diabetic Myocardial Ischemia/Reperfusion Injury Via Differential Regulation of p38 and ERK. Sci. Rep. 2020, 10, 23. [Google Scholar] [CrossRef]
- Sergeev, P.; Silva, R.D.; Lucchinetti, E.; Zaugg, K.; Pasch, T.; Schaub, M.C.; Zaugg, M. Trigger-dependent gene expression profiles in cardiac preconditioning: Evidence for distinct genetic programs in ischemic and anesthetic preconditioning. Anesthesiology 2004, 100, 474–488. [Google Scholar] [CrossRef]
- Pons, J.; Huang, Y.; Arakawa-Hoyt, J.; Washko, D.; Takagawa, J.; Ye, J.; Grossman, W.; Su, H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem. Biophys. Res. Commun. 2008, 376, 419–422. [Google Scholar] [CrossRef]
- Cébe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef]
- Kawata, H.; Yoshida, K.I.; Kawamoto, A.; Kurioka, H.; Takase, E.; Sasaki, Y.; Hatanaka, K.; Kobayashi, M.; Ueyama, T.; Hashimoto, T.; et al. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ. Res. 2001, 88, 696–704. [Google Scholar] [CrossRef]
- Leone, A.M.; Rutella, S.; Bonanno, G.; Contemi, A.M.; de Ritis, D.G.; Giannico, M.B.; Rebuzzi, A.G.; Leone, G.; Crea, F. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. Int. J. Cardiol. 2006, 111, 202–208. [Google Scholar] [CrossRef]
- Kelle, S.; Roes, S.D.; Klein, C.; Kokocinski, T.; de Roos, A.; Fleck, E.; Bax, J.J.; Nagel, E. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J. Am. Coll. Cardiol. 2009, 54, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.N.; Gunton, J.; Nucifora, G.; McGavigan, A.D.; Selvanayagam, J.B. Impact of Late Gadolinium Enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 254, 230–237. [Google Scholar] [CrossRef]
- Flamme, I.; Breier, G.; Risau, W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2(flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev. Biol. 1995, 169, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Kato, S.; Ito, Y.; Eshima, K.; Ogawa, F.; Takahashi, R.; Sekiguchi, K.; Tamaki, H.; Sakagami, H.; Shibuya, M.; et al. The Role of Vascular Endothelial Growth Factor Receptor-1 Signaling in the Recovery from Ischemia. PLoS ONE 2015, 10, e0131445. [Google Scholar] [CrossRef] [PubMed]
- Clahsen, T.; Schaper, F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008, 84, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Wang, I.F.; Chiang, P.M.; Wang, L.C.; Shen, C.K.J.; Tsai, K.J. G-CSF-mobilized Bone Marrow Mesenchymal Stem Cells Replenish Neural Lineages in Alzheimer’s Disease Mice via CXCR4/SDF-1 Chemotaxis. Mol. Neurobiol. 2017, 54, 6198–6212. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Kim, M.; Jan, M.; Emala, C.W. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am. J. Physiol.-Renal Physiol. 2006, 291, F67–F78. [Google Scholar] [CrossRef]
- Landoni, G.; Bignami, E.; Oliviero, F.; Zangrillo, A. Halogenated anaesthetics and cardiac protection in cardiac and non-cardiac anaesthesia. Ann. Card. Anaesth. 2009, 12, 4. [Google Scholar] [CrossRef]
- Siddique, A.; Shantsila, E.; Lip, G.Y.; Varma, C. Endothelial progenitor cells: What use for the cardiologist? J. Angiogenes. Res. 2010, 2, 6. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Adler, K.; Aicher, A.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001, 103, 2885–2890. [Google Scholar] [CrossRef]
- De Winter, R.J.; Klomp, M. Understanding the Role of Endothelial Progenitor Cells in Cardiovascular Disease, Coronary Artery Lesion Progression, and In-Stent Restenosis; Editorials published in JACC: Cardiovascular Interventions reflect the views of the authors and do not necessarily represent the views of JACC: Cardiovascular Interventions or the American College of Cardiology. JACC: Cardiovasc. Interv. 2010, 3, 87–89. [Google Scholar]
- Aoki, J.; Serruys, P.W.; Van Beusekom, H.; Ong, A.T.L.; McFadden, E.P.; Sianos, G.; Van Der Giessen, W.J.; Regar, E.; De Feyter, P.J.; Davis, H.R.; et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: The HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First in Man) registry. J. Am. Coll. Cardiol. 2005, 45, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Co, M.; Tay, E.; Lee, C.H.; Poh, K.K.; Low, A.; Lim, J.; Lim, I.H.; Lim, Y.T.; Tan, H.C. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: Intermediate- to long-term clinical follow-up. Am. Heart J. 2008, 155, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ovize, M.; Kloner, R.A.; Hale, S.L.; Przyklenk, K. Coronary cyclic flow variations "precondition" ischemic myocardium. Circulation 1992, 85, 779–789. [Google Scholar] [CrossRef][Green Version]
- Fräßdorf, J.; Borowski, A.; Ebel, D.; Feindt, P.; Hermes, M.; Meemann, T.; Weber, R.; Müllenheim, J.; Weber, N.C.; Preckel, B.; et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2009, 137, 1436–1442. [Google Scholar] [CrossRef]
- Bein, B.; Renner, J.; Caliebe, D.; Hanss, R.; Bauer, M.; Fraund, S.; Scholz, J. The effects of interrupted or continuous administration of sevoflurane on preconditioning before cardio-pulmonary bypass in coronary artery surgery: Comparison with continuous propofol. Anaesthesia 2008, 63, 1046–1055. [Google Scholar] [CrossRef]
- Lucchinetti, E.; Wacker, J.; Maurer, C.; Keel, M.; Harter, L.; Zaugg, K.; Zaugg, M. Helium breathing provides modest antiinflammatory, but no endothelial protection against ischemia-reperfusion injury in humans in vivo. Anesth. Analg. 2009, 109, 101–108. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, M.K.; Lee, H.Y.; Park, K.W.; Lee, W.; Cho, Y.S.; Koo, B.K.; Choi, D.J.; Park, Y.B.; Kim, H.S. Five-year results of intracoronary infusion of the mobilized peripheral blood stem cells by granulocyte colony-stimulating factor in patients with myocardial infarction. Eur. Heart J. 2012, 33, 3062–3069. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, M.K.; Kim, M.G.; Choi, D.J.; Yoon, J.H.; Park, Y.B.; Kim, H.S. A multicenter, prospective, randomized, controlled trial evaluating the safety and efficacy of intracoronary cell infusion mobilized with granulocyte colony-stimulating factor and darbepoetin after acute myocardial infarction: Study design and rationale of the ‘MAGIC cell-5-combination cytokine trial’. Trials 2011, 12, 33. [Google Scholar] [CrossRef]
- Achilli, F.; Pontone, G.; Bassetti, B.; Squadroni, L.; Campodonico, J.; Corrada, E.; Facchini, C.; Mircoli, L.; Esposito, G.; Scarpa, D.; et al. G-CSF for Extensive STEMI: Results from the STEM-AMI OUTCOME CMR Substudy. Circ. Res. 2019, 125, 295–306. [Google Scholar] [CrossRef]
- Achilli, F.; Malafronte, C.; Lenatti, L.; Gentile, F.; Dadone, V.; Gibelli, G.; Maggiolini, S.; Squadroni, L.; Di Leo, C.; Burba, I.; et al. Granulocyte colony-stimulating factor attenuates left ventricular remodelling after acute anterior STEMI: Results of the single-blind, randomized, placebo-controlled multicentre STem cEll Mobilization in Acute Myocardial Infarction (STEM-AMI) Trial. Eur. J. Heart Fail. 2010, 12, 1111–1121. [Google Scholar] [CrossRef]
- Pratt, P.F.; Wang, C.; Weihrauch, D.; Bienengraeber, M.W.; Kersten, J.R.; Pagel, P.S.; Warltier, D.C. Cardioprotection by volatile anesthetics: New applications for old drugs? Curr. Opin. Anaesthesiol. 2006, 19, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K. Stem Cells in the Infarcted Heart. J. Cardiovasc. Transl. Res. 2010, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pagel, P.S. Induction of heat shock protein 70 and preconditioning by sevoflurane: A potent protective interaction against myocardial ischemia-reperfusion injury. Anesth. Analg. 2008, 107, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, S.; Miyagawa, S.; Fukushima, S.; Tsuchimochi, H.; Sonobe, T.; Fujii, Y.; Pearson, J.T.; Saito, A.; Harada, A.; Toda, K.; et al. Influence of coronary architecture on the variability in myocardial infarction induced by coronary ligation in rats. PLoS ONE 2017, 12, e0183323. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.R.; Pavel, B.; Isvoranu, G.; Ceafalan, L.C.; Panaitescu, A.M.; Sava, R.I.; Vlad, A.; Zagrean, L. Cardioprotective Mechanisms of Interrupted Anesthetic Preconditioning with Sevoflurane in the Setting of Ischemia/Reperfusion Injury in Rats. Appl. Sci. 2022, 12, 1476. https://doi.org/10.3390/app12031476
Popescu MR, Pavel B, Isvoranu G, Ceafalan LC, Panaitescu AM, Sava RI, Vlad A, Zagrean L. Cardioprotective Mechanisms of Interrupted Anesthetic Preconditioning with Sevoflurane in the Setting of Ischemia/Reperfusion Injury in Rats. Applied Sciences. 2022; 12(3):1476. https://doi.org/10.3390/app12031476
Chicago/Turabian StylePopescu, Mihaela Roxana, Bogdan Pavel, Gheorghita Isvoranu, Laura Cristina Ceafalan, Anca Maria Panaitescu, Ruxandra Irina Sava, Adelina Vlad, and Leon Zagrean. 2022. "Cardioprotective Mechanisms of Interrupted Anesthetic Preconditioning with Sevoflurane in the Setting of Ischemia/Reperfusion Injury in Rats" Applied Sciences 12, no. 3: 1476. https://doi.org/10.3390/app12031476
APA StylePopescu, M. R., Pavel, B., Isvoranu, G., Ceafalan, L. C., Panaitescu, A. M., Sava, R. I., Vlad, A., & Zagrean, L. (2022). Cardioprotective Mechanisms of Interrupted Anesthetic Preconditioning with Sevoflurane in the Setting of Ischemia/Reperfusion Injury in Rats. Applied Sciences, 12(3), 1476. https://doi.org/10.3390/app12031476







