Abstract
American and European Foulbrood (AFB and EFB) are considered the most contagious infectious diseases affecting honeybees worldwide. New sustainable strategies need to be implemented for their prevention and control, and probiotics may represent one solution to investigate. In our study, we evaluated the efficacy of one strain of Lactobacillus plantarum (L. plantarum) isolated from northern Italy, orally administered to the bees for AFB and EFB prevention. From March to September 2014, a total of 979 honeybee colonies (9.6% of Viterbo province—Central Italy) were taken under observation from 22 apiaries. Overall prevalence of AFB was 5.3% in treated colonies and 5.1% in the untreated ones. On the contrary, EFB prevalence was lower in the treated colonies (2.5%) compared to the untreated ones (4.5%). L. plantarum showed a significant effect in reducing insurgence of cases of EFB up to 35 days after the end of the treatment (p-value: 0.034). Thanks to this study we could investigate the preventive efficacy of L. plantarum in controlling AFB and EFB, and obtain official data on their clinical prevalence in Central Italy.
1. Introduction
Recent losses of managed honeybee colonies and the need to reduce the use of veterinary medicines at the apiary level to reach sustainable beekeeping goals are leading to a growing interest in the development of new strategies for disease prevention and control, under the One Health approach [1].
American Foulbrood (AFB) and European Foulbrood (EFB) are the two major bacterial diseases affecting honeybee brood and present a considerable threat to beekeeping worldwide [2]. These diseases are classified within the OIE—World Organisation for Animal Health list, as having a socioeconomic impact in the international trade of bees and bee products [3,4].
AFB, considered the most serious and widespread brood infection, is caused by the spore forming, Gram-positive bacterium Paenibacillus larvae [5,6]. Young larvae are infected through ingestion of food contaminated by the extremely resilient endospores of the bacterium. The infection is transmitted through the resistant spores, which contaminate bees and hive equipment and remain viable for decades. AFB is highly contagious and destructive, often causing colony death if left untreated. As a result, in most countries AFB is a notifiable disease, must be reported to the relevant government authorities [6] and is included in the new EU Animal Health Law (Regulation (EU) 2016/429).
EFB is caused by the bacterium Melissococcus plutonius, although associated with secondary invaders like Paenibacillus alvei, Brevibacillus laterosporus, Enterococcus faecalis and Achromobacter euridice [7]. The causative agent does not form spores, and therefore the disease is considered less problematic than AFB. However, prevalence and severity of EFB are variable in different areas and appear to be influenced by environmental factors such as climatic conditions and food quality [7]. With regard to the distribution in Europe, EFB is the most widespread bacterial brood disease in Great Britain [8], and is of relevant interest in other countries, like Switzerland, where the incidence of the infection has risen consistently since the late 1990s, without a clear cause [9].
On a global scale, several antibiotics and chemotherapies are of interest in apiculture for the treatment of bacterial diseases, including: tetracyclines, streptomycin, sulfonamides, tylosin, erythromycin, lincomycin, chloramphenicol, nitrofurans, bacitracin, and penicillins [10]. Prophylactic treatment with oxytetracycline and tylosin is limited to some countries (e.g., USA, Canada, Argentina, Australia) and generally only in cases of initial stage disease [7,10]. In the European Union, antibiotics for honey bees are not registered [6], and their use is admitted through the ‘cascade’ system, as described in Article 11 of Directive, 2001/82/EC, as amended by Directive 2004/28/EC and Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC [10].
In many cases antibiotics have been used continuously and excessively or even illegally [11]. Several problems may be associated with the routine use of antibiotics: it has little impact on the epidemiology and control of bacterial diseases as they are not effective in destroying Paenibacillus larvae spores or in eliminate the reservoir of Melissococcus plutonius cells persisting in beehives. Moreover, their administration, especially for AFB, can only mitigate the disease by suppressing clinical signs and beekeepers may spread the infection between colonies [6,12]. Furthermore, it has been observed that the use of antibiotics may affect the longevity of the bees and the vitality of the brood [13] as well as cause disequilibria in the normal microbiota of the beehive [14]. Another relevant issue related to the use of antibiotics is the development of resistant strains of the pathogenic bacteria. In particular OTC and sulfathiazole-resistance in P. larvae has become widespread as a consequence of decade-long misuse [11,15,16,17,18]. Moreover, the transfer of resistance from animal pathogens to human ones is a possible threat that has to be considered [19]. Finally, residues of antibiotics contaminate beehive products, especially honey, affecting quality and safety for human consumption [11,20,21]. Due to lack of metabolism in the beehive, an elimination of residues within a certain period of time, as defined for other food producing animals, cannot be established in apiculture. Hence, the persistence of residues of antibiotics in honey leads to significant commercial problems [10].
Investigation of alternative, more sustainable methods to prevent and control honey bee bacterial diseases is needed. One of the most studied methods is the selection of honey bee colonies with hygienic behavior of adult bees toward infected larvae [22]. Other strategies are based on the use of natural substances, including: essential oils, plant extracts, propolis, royal jelly, non-conventional natural molecules, bacteriocins [23,24,25,26,27,28,29,30,31,32] and antagonistic bacteria [7,15,23,24,32,33,34]. The last may act as biocontrol agents representing a promising alternative to antibiotics. Frequently, to this scope, autochthonous strains isolated from honeybees are used [35,36,37].
Even if the microbiota associated with honeybees is not fully known, it is mainly composed of yeasts, Gram-positive bacteria (such as Lactobacillus spp., Bacillus spp., Brevibacillus spp., Streptococcus and Clostridium) and Gram-negative or Gram variable bacteria (Achromobacter, Citrobacter, Enterobacter, Erwinia, Escherichia coli, Flavobacterium, Klebsiella, Proteus and Pseudomonas) [33,38,39,40,41,42]. Gilliam [43] reported that these bacteria were likely to be endemic in the alimentary tract of adult bees and were dependent neither on seasonal factors nor on nutritional factors.
The ability of the spore-forming species to inhibit different fungi and bacteria, by secreting a broad range of bioactive compounds, that include peptides, lipopeptides, bacteriocins, and bacteriocin-like inhibitory substances, has been well documented [15]. More in detail, Bacillus subtilis, Bacillus pumilus, Bacillus licheniformis, Bacillus cereus, Bacillus megaterium and Brevibacillus laterosporus have been reported as normal microorganisms associated with honeybees and honey [40,44,45], characterized by high inhibitory capacities against P. larvae, as evidenced through in vitro studies by Alippi and Reynaldi [15]. More generally, antagonistic activity against P. larvae showed by Bacillus and Brevibacillus species has been confirmed by several studies [29,33,46].
The microbial group currently of major interest for honeybee probiotic purposes is represented by lactic acid bacteria (LAB) [47,48,49,50,51]. Lactobacillus and Bifidobacterium, the most important genera within LAB, are commonly beneficial commensals and are widely used as probiotics to modulate the composition of the microflora in order to protect the host from infections. LAB are in fact known to be good producers of antimicrobial substances such as organic acids, hydrogen peroxide and antimicrobial peptides [52]. Multiple factors are involved in the mechanisms associated with antibacterial activity in probiotic LAB and there is a clear variation in the production of antimicrobial agents including common organic acids, proteins, peptides, enzymes, and bacteriocins between the different LAB species [7,53].
A novel flora of LAB composed of Lactobacillus and Bifidobacterium has recently been identified in the honey stomach of honeybees [52,53]. A mutual dependence between honeybees and the novel LAB flora was hypothesized: the LAB obtaining nutrients, the honeybees and the honey in turn being protected by the LAB from harmful microorganisms. Previously, Evans and Lopez [54] observed a strong immune response in larvae fed with a mix of Lactobacillus and Bifidobacterium species, with the enhanced production of antibacterial peptides. Moreover, growth of both P. larvae and M. plutonius was inhibited by strain R4BT of the novel described species Lactobacillus apis [55].
The first findings above suggest that probiotic LAB linked to the honeybee gastrointestinal tract can have an important role for the tolerance of infective diseases. Forsgren [56] demonstrated a strong inhibitory effect of combined honeybee stomach LAB flora on the in vitro growth of P. larvae and, subsequently, proved that the administration of the LAB mixture to young larvae reared in vitro significantly reduced the proportion of larvae succumbing to AFB infection. Vásquez [57] administrated the LAB mixture isolated from the honey harvest to bee larvae reared in vitro exposed to M. plutonius and obtained a significant reduction of the number of individuals killed by EFB. Recently, Kačániová [58] isolated some Lactobacillus species and evaluated their antimicrobial activity against P. larvae.
The activity of L. plantarum in inhibiting honeybee pathogenic bacteria has so far been investigated through few studies, limited however to P. larvae. Mudroňová [34] isolated from the digestive tract of adult honeybees a L. plantarum strain and showed its strong inhibiting activity against P. larvae. Recently, Daisley [32] stated that administering Lactobacillus species (including L. plantarum ATCC 14917) improved honeybee survival and colony resilience against AFB.
Our study was designed to investigate the efficacy in reducing clinical symptomatic cases of AFB and EFB by the preventive administration of Lactobacillus plantarum into colonies at the beginning of the active season.
2. Materials and Methods
Field trials were carried out from March 2014 to September 2014 (220 days) in 22 apiaries distributed in the Viterbo province (Central Italy), in an area of approximately 2700 km2 (Figure 1).
Figure 1.
Apiaries distribution.
L. plantarum, stable stabulogen autochthonous strain (LMG P-21806), isolated from healthy bees and brood in northern Italy (Piacenza), was applied in water solution trickled on the bees. Then, 20 g containing 2 × 1011 CFU of L. plantarum were mixed in one litre of water to obtain a suspension of 2 × 108 CFU/mL. The amount of solution administered per colony (dosage) was of 5 mL for each intercomb space covered by bees. Trials involved 979 colonies, kept in 10 frame Dadant-Blatt hives, free of any other symptomatic disease at the beginning of the trial. These colonies were randomly divided into two groups (Table 1):

Table 1.
Number of colonies randomly assigned to the treatment and control groups in the 22 apiaries.
- L. plantarum group, consisted of 488 colonies treated once a week, for a total of 4 administrations.
- Control group, consisted of 491 colonies that received only water in the amount of 5 mL per hive, dropped onto the inter comb space covered by bees, for a total of 4 administrations.
All colonies were carefully checked at least once a month by visual inspection for the presence of clinical symptoms of disease. The on-field diagnosis was performed by the Public Veterinary Services and the confirmation was provided by the official laboratory Istituto Zooprofilattico Sperimentale del Lazio e della Toscana (IZSLT) following the methods reported in the OIE Manual of diagnostic tests and vaccines for terrestrial animals. Suspicious larval samples collected were cultured for the isolation and characterization of P. larvae or M. plutonius isolates, subsequently processed by RT-PCR [59,60].
All hives that resulted positive to EFB or AFB were recorded and immediately destroyed by the Public Veterinary Services.
Kaplan–Meier estimator of the survival function, followed by Log-Rank test was used to evaluate the efficacy of L. plantarum administration in preventing disease outbreaks in tested colonies. Data were processed by means of statistical software XLSTAT™ (Addinsoft, Paris, France, 2020).
3. Results
During the whole observation period, untreated colonies showed a peak of AFB cases in March (10 outbreaks) and April (9 outbreaks) while EFB showed the largest number of cases in the untreated group in April (Figure 2).
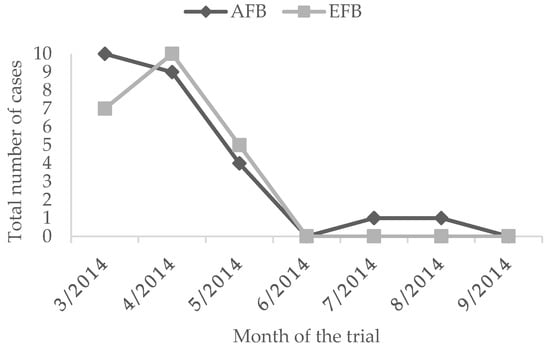
Figure 2.
Number of symptomatic cases of AFB and EFB in the untreated groups.
The overall prevalence of clinical cases in the untreated group was of 5.1% for AFB and 4.5% for EFB. The administration of L. plantarum for four weeks determined a reduced EFB prevalence of 2.0%. On the contrary, the prevalence of AFB in the treated group was higher than the untreated group of 0.2% (Figure 3 and Figure 4, Table 2). Moreover, during the whole observation period, the group treated with L. plantarum showed lower cases of EFB, except during June and July (Figure 3), after 4 months since the end of the treatment.
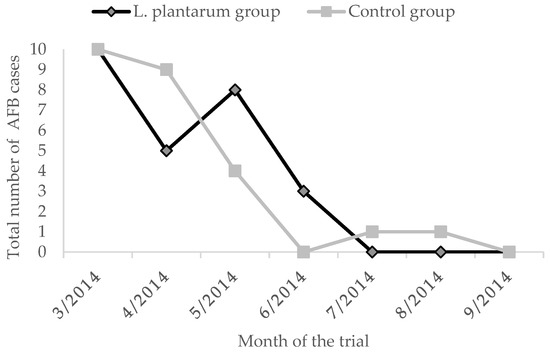
Figure 3.
AFB cases in the two groups.
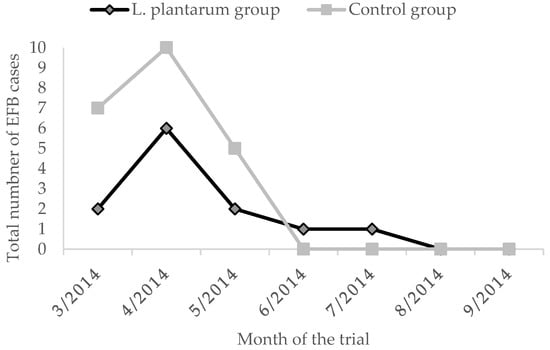
Figure 4.
EFB cases in the two groups.

Table 2.
Disease prevalence in L. plantarum and control groups.
Kaplan–Meier estimator of the survival function applied to the AFB cases is reported in Figure 5.
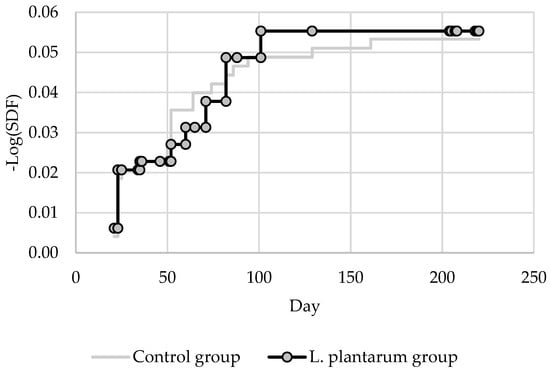
Figure 5.
Survival estimation curves—AFB cases.
p-value of Log-Rank test was 0.906 (obs. value 0.014) showing no statistical differences between the survival curve of the treated and untreated groups compared to the number of symptomatic AFB cases.
The Kaplan–Meier estimator of the survival function applied to the AFB cases is reported in Figure 6.
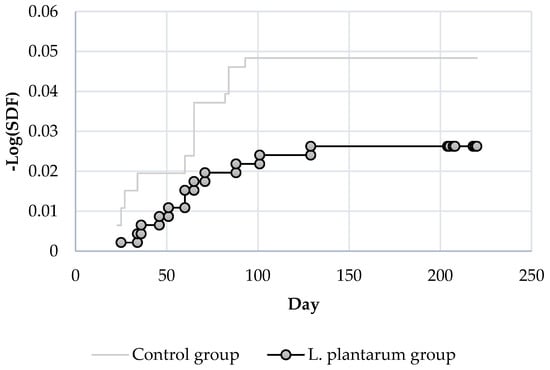
Figure 6.
Survival estimation curves—EFB cases.
p-value of Log-Rank test applied to EFB cases (Figure 6) resulted in 0.083 (obs. value 3.005) showing a higher difference compared to the survival curves of AFB.
In order to establish if the use of L. plantarum could have a preventive effect in reducing the number of cases of EFB based on the time elapsed from the end of the treatment period, the Log-Rank test has been applied to different time-frames, excluding from the analysis all the other cases that emerged during the trial. The p-values are reported in Table 3.

Table 3.
Log-Rank test values of EFB survival curves applied at different time-frames.
The p-value of Log-Rank test applied to survival curves of EFB cases in the treated and untreated groups showed a statistical difference at day 34.
4. Discussion
EFB is endemic in most parts of the world [61,62] except New Zealand [62]. For a long time the effects of the disease on colonies health have been underestimated and the prevalence has increased in the last decades in many countries such as Switzerland, UK, Netherlands and others [63,64,65,66,67,68]. AFB disease is present wherever honey bees are reared and its economic impact is still one of the most important across brood diseases [69]. According to Genersch [5] two subspecies exist, belonging to four ERIC genotypes (ERIC I–IV), but only P. l. larvae (genotypes ERIC I and ERIC II) can be frequently isolated from foulbrood diseased colonies in Europe and on the American continent.
The first EU-wide epidemiological dataset regarding foulbroods were obtained in 2012–2013, when 17 Member States took part to the first active epidemiological surveillance program on honeybee colony mortality in Europe (EPILOBEE) [66,67,68]. The overall clinical prevalence of AFB and EFB in 14 Member States was, respectively, lower than 12% for AFB and did not exceed 5% for EFB.
In Italy, 1682 colonies have been put under observation in the EPILOBEE project: in regard to AFB, during the experimental period, the prevalence ranged from 2.7% before wintertime; 0% after wintertime to 2.2% during the active season. Similarly, in regard to EFB, the prevalence observed in Italy was 0% before wintertime and during the season and 1.1% after wintertime [68].
The Viterbo province presents 10,201 honeybee colonies, distributed in 169 apiaries (Italian National Beekeeping Registry—December 2014). During our study, 979 colonies were monitored, the equivalent of 9.6% of the total amount of colonies in the whole province territory. The obtained prevalence of 5.1% for AFB and of 4.5% for EFB on 491 untreated colonies is the first data ever reported in Central Italy involving a substantial number of honey bee colonies.
The majority of outbreaks occurred during spring (March–May 2014) when the brood coverage in colonies is developing and an imbalance between the number of larvae and nurse bees as well as food shortages, particularly of protein, can play an important role [69,70].
Worldwide, the control of AFB and EFB relies on different strategies. In many countries, quarantine, incineration of symptomatic colonies or, alternatively, use of the shook swarm technique are considered priority measures regulated by health laws.
L. plantarum is widely used in the food industry. It is, in fact, a versatile bacterium living in several ecological niches, ranging from vegetable and plant fermentations to the human gastrointestinal tract. The EU Commission, by means of Regulation (EU) N. 93/2012, approves the use of Lactobacillus plantarum (strains DSM 8862 and DSM 8866) as a feed additive for all animal species, without maximum residue limit.
Several LAB species isolated from honey bees showed in laboratory conditions beneficial health effects at the individual bee level respect the AFB infection [52,56,71,72], but in literature no optimistic results have ever been obtained from trials on colonies [73]. The results obtained with the L. plantarum strain tested in our study underline that, at the moment, the best practice to avoid the spread of AFB and the development of antibiotic resistance is the incineration of symptomatic colonies [10].
In Italy, where both infections are almost endemic, quarantine measures and burning the symptomatic colonies are the usual legal requirements for AFB and EFB, limiting the chance of recovery to the colonies presenting an early stage of the disease; shook swarm is permitted and the best management practice for symptomatic EFB colonies is the shook swarm method into clean boxes with new frames. Supplemental feeding is highly suggested, and this technique should be adopted only on strong colonies able to draw combs and store sufficient honey for winter time [74,75].
The preventive administration at the beginning of the active season of the L. plantarum strain tested in our study reduced clinical symptomatic cases of EFB until 34 days after the application of the product. Only one reference [57] reported a preventive action on bee larvae reared in vitro exposed to M. plutonius. Considering that field data on the application of LAB are not available in the literature, our data suggest the importance of further investigations on LAB strains as biological preventive or control agents of EFB. Multiple treatment applications as a preventive technique or combining their use with the shook swarm method on symptomatic colonies could be tested, also verifying the absence of residues in hive products.
Author Contributions
Conceptualization, G.F., M.P. (Marco Pietropaoli) and M.P. (Massimo Palazzetti); methodology, G.F. and M.P. (Marco Pietropaoli); investigation, M.P. (Massimo Palazzetti), M.M., G.F. and M.P. (Marco Pietropaoli); data curation, M.P. (Marco Pietropaoli); writing—original draft preparation, M.P. (Marco Pietropaoli) and G.F.; writing—review and editing, E.C., G.F., M.P. (Marco Pietropaoli) and S.C.; visualization, M.P. (Marco Pietropaoli); supervision, G.F. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors would like to thank for his support Giuseppe Micarelli, Chief Veterinary Officer of Veterinary Services of Viterbo, Apituscia beekeepers association for their zeal and willingness in participating to the study and Chemicals Laif for the collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donkersley, P.; Elsner-Adams, E.; Maderson, S. A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Vet. Sci. 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Munn, P.A. The Worldwide Health Status of Honey Bees. Bee World 2005, 86, 88–101. [Google Scholar] [CrossRef]
- OIE Chapter 3.2.3. European Foulbrood of Honey Bees (Infection of Honey Bees with Melissococcus Plutonius). OIE Man. Diagn. Tests Vaccines Terr. Anim. Mamm. Birds Bees 2018, 1, 736–743.
- OIE Chapter 3.2.2. American Foulbrood of Honey Bees (Infection of Honey Bees with Paenibacillus Larvae). OIE Man. Diagn. Tests Vaccines Terr. Anim. Mamm. Birds Bees 2018, 1, 719–735.
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus Larvae Subsp. Pulvifaciens and Paenibacillus Larvae Subsp. Larvae as Paenibacillus Larvae without Subspecies Differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef]
- Genersch, E. American Foulbrood in Honeybees and Its Causative Agent, Paenibacillus Larvae. J. Invertebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef]
- Forsgren, E. European Foulbrood in Honey Bees. J. Invertebr. Pathol. 2010, 103, S5–S9. [Google Scholar] [CrossRef]
- Wilkins, S.; Brown, M.A.; Cuthbertson, A.G.S. The Incidence of Honey Bee Pests and Diseases in England and Wales. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 1062–1068. [Google Scholar] [CrossRef]
- Roetschi, A.; Berthoud, H.; Kuhn, R.; Imdorf, A. Infection Rate Based on Quantitative Real-Time PCR of Melissococcus Plutonius, the Causal Agent of European Foulbrood, in Honeybee Colonies before and after Apiary Sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Reybroeck, W.; Daeseleire, E.; De Brabander, H.F.; Herman, L. Antimicrobials in Beekeeping. Vet. Microbiol. 2012, 158, 1–11. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C. Limits of Chemotherapy in Beekeeping: Development of Resistance and the Problem of Residues. Bee World 2005, 86, 102–109. [Google Scholar] [CrossRef]
- Waite, R.J.; Brown, M.A.; Thompson, H.M.; Bew, M.H. Controlling European Foulbrood with the Shook Swarm Method and Oxytetracycline in the UK. Apidologie 2003, 34, 569–575. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.-S.C.; Mussen, E.; Fong, A.; Montague, M.A.; Tyler, T. Effects of Chlortetracycline of Honey Bee Worker Larvae Reared in Vitro. J. Invertebr. Pathol. 1992, 60, 127–133. [Google Scholar] [CrossRef]
- Anderson, K.E.; Sheehan, T.H.; Eckholm, B.J.; Mott, B.M.; DeGrandi-Hoffman, G. An Emerging Paradigm of Colony Health: Microbial Balance of the Honey Bee and Hive (Apis mellifera). Insectes Sociaux 2011, 58, 431. [Google Scholar] [CrossRef]
- Alippi, A.M.; Reynaldi, F.J. Inhibition of the Growth of Paenibacillus Larvae, the Causal Agent of American Foulbrood of Honeybees, by Selected Strains of Aerobic Spore-Forming Bacteria Isolated from Apiarian Sources. J. Invertebr. Pathol. 2006, 91, 141–146. [Google Scholar] [CrossRef]
- Miyagi, T.; Peng, C.Y.S.; Chuang, R.Y.; Mussen, E.C.; Spivak, M.S. Verification of Oxytetracycline-Resistant American Foulbrood Pathogen Paenibacillus Larvae in the United States. J. Invertebr. Pathol. 2000, 75, 95–96. [Google Scholar] [CrossRef]
- Evans, J.D. Diverse Origins of Tetracycline Resistance in the Honey Bee Bacterial Pathogen Paenibacillus Larvae. J. Invertebr. Pathol. 2003, 83, 46–50. [Google Scholar] [CrossRef]
- Piccini, C.; Zunino, P. American Foulbrood in Uruguay: Isolation of Paenibacillus Larvae Larvae from Larvae with Clinical Symptoms and Adult Honeybees and Susceptibility to Oxytetracycline. J. Invertebr. Pathol. 2001, 78, 176–177. [Google Scholar] [CrossRef]
- Alippi, A.M.; Leon, I.E.; López, A.C. Tetracycline-Resistance Encoding Plasmids from Paenibacillus Larvae, the Causal Agent of American Foulbrood Disease, Isolated from Commercial Honeys. Int. Microbiol. 2014, 17, 49–61. [Google Scholar]
- Bogdanov, S. Contaminants of Bee Products. Apidologie 2006, 37, 665–672. [Google Scholar] [CrossRef]
- Martel, A.-C.; Zeggane, S.; Drajnudel, P.; Faucon, J.-P.; Aubert, M. Tetracycline Residues in Honey after Hive Treatment. Food Addit. Contam. 2006, 23, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Reuter, G.S. Resistance to American Foulbrood Disease by Honey Bee Colonies Apis mellifera Bred for Hygienic Behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Janashia, I.; Choiset, Y.; Rabesona, H.; Hwanhlem, N.; Bakuradze, N.; Chanishvili, N.; Haertlé, T. Protection of Honeybee Apis Mellifera by Its Endogenous and Exogenous Lactic Flora against Bacterial Infections. Ann. Agrar. Sci. 2016, 14, 177–181. [Google Scholar] [CrossRef]
- Kuzyšinová, K.; Mudroňová, D.; Toporčák, J.; Molnár, L.; Javorský, P. The Use of Probiotics, Essential Oils and Fatty Acids in the Control of American Foulbrood and Other Bee Diseases. J. Apic. Res. 2016, 55, 386–395. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Cugnata, N.M.; Guaspari, E.; Pellegrini, M.C.; Aubone, I.; De Piano, F.G.; Antunez, K.; Fuselli, S.R. Natural Strategies for the Control of Paenibacillus Larvae, the Causative Agent of American Foulbrood in Honey Bees: A Review. Apidologie 2017, 48, 387–400. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Buczek, K.; Zambrowski, G.; Miastkowski, K.; Swiecicka, I. In Vitro Study of the Antimicrobial Activity of European Propolis against Paenibacillus Larvae. Apidologie 2017, 48, 411–422. [Google Scholar] [CrossRef][Green Version]
- Isidorov, V.A.; Buczek, K.; Segiet, A.; Zambrowski, G.; Swiecicka, I. Activity of Selected Plant Extracts against Honey Bee Pathogen Paenibacillus Larvae. Apidologie 2018, 49, 687–704. [Google Scholar] [CrossRef]
- Pellegrini, M.C.; Alonso-Salces, R.M.; Umpierrez, M.L.; Rossini, C.; Fuselli, S.R. Chemical Composition, Antimicrobial Activity, and Mode of Action of Essential Oils against Paenibacillus Larvae, Etiological Agent of American Foulbrood on Apis mellifera. Chem. Biodivers. 2017, 14, e1600382. [Google Scholar] [CrossRef]
- Bartel, L.C.; Abrahamovich, E.; Mori, C.; López, A.C.; Alippi, A.M. Bacillus and Brevibacillus Strains as Potential Antagonists of Paenibacillus Larvae and Ascosphaera Apis. J. Apic. Res. 2019, 58, 117–132. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The Terpenes of Leaves, Pollen, and Nectar of Thyme (Thymus Vulgaris) Inhibit Growth of Bee Disease-Associated Microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Ansari, M.J.; Khan, M.H.U.; Kamal, S.; Rahman, K.; Shoaib, M.; Man, S.; Khan, A.J.; Khan, S.U. Antimicrobial Potentials of Medicinal Plant’s Extract and Their Derived Silver Nanoparticles: A Focus on Honey Bee Pathogen. Saudi J. Biol. Sci. 2019, 26, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel Probiotic Approach to Counter Paenibacillus Larvae Infection in Honey Bees. ISME J. 2020, 14, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Armstrong, T.-N. Inhibition of the American Foulbrood Bacterium, Paenibacillus Larvae Larvae, by Bacteria Isolated from Honey Bees. J. Apic. Res. 2005, 44, 168–171. [Google Scholar] [CrossRef]
- Mudroňová, D.; Toporčák, J.; Nemcová, R.; Gancarčíková, S.; Hajdučková, V.; Rumanovská, K. Lactobacillus Sp. as a Potential Probiotic for the Prevention of Paenibacillus Larvae Infection in Honey Bees. J. Apic. Res. 2011, 50, 323–324. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Žiarovská, J.; Kowalczewski, P.Ł. In Vitro Antagonistic Effect of Gut Bacteriota Isolated from Indigenous Honey Bees and Essential Oils against Paenibacillus Larvae. Int. J. Mol. Sci. 2020, 21, 6736. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.; Tysset, C.; Durand, C. Presence of Streptococci of the Lancefield D Group in Healthy Working Bees (Alpis Mellifica L.). Interpretation of Their Presence in Alimentary Bacteriology. Bull. De L’academie Vet. Fr. 1969, 42, 173–186. [Google Scholar]
- Gilliam, M.; Morton, H.L. Bacteria Belonging to the Genus Bacillus Isolated from Honey Bees, Apis mellifera, Fed 2, 4-D and Antibiotics (1). Apidologie 1978, 9, 213–222. [Google Scholar] [CrossRef]
- Gilliam, M.; Prest, D.B. Microbiology of Feces of the Larval Honey Bee, Apis mellifera. J. Invertebr. Pathol. 1987, 49, 70–75. [Google Scholar] [CrossRef]
- Rada, V.; Machova, M.; Huk, J.; Marounek, M.; Dušková, D. Microflora in the Honeybee Digestive Tract: Counts, Characteristics and Sensitivity to Veterinary Drugs. Apidologie 1997, 28, 357–365. [Google Scholar] [CrossRef][Green Version]
- Mohr, K.I.; Tebbe, C.C. Diversity and Phylotype Consistency of Bacteria in the Guts of Three Bee Species (Apoidea) at an Oilseed Rape Field. Environ. Microbiol. 2006, 8, 258–272. [Google Scholar] [CrossRef]
- Gilliam, M. Identification and Roles of Non-Pathogenic Microflora Associated with Honey Bees. FEMS Microbiol. Lett. 1997, 155, 1–10. [Google Scholar] [CrossRef]
- Gilliam, M. Bacteria Belonging to the Genus Bacillus Isolated from Selected Organs of Queen Honey Bees, Apis mellifera. J. Invertebr. Pathol. 1978, 31, 389–391. [Google Scholar] [CrossRef]
- Gilliam, M. Microbiology of Pollen and Bee Bread: The Genus Bacillus. Apidologie 1979, 10, 269–274. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Carrillo, L.; Audisio, M.C. Inhibition of Paenibacillus Larvae and Ascosphaera Apis by Bacillus Subtilis Isolated from Honeybee Gut and Honey Samples. Res. Microbiol. 2009, 160, 193–199. [Google Scholar] [CrossRef]
- Lazzeri, A.M.; Mangia, N.P.; Mura, M.E.; Floris, I.; Satta, A.; Ruiu, L. Potential of Novel Food-Borne Lactobacillus Isolates against the Honeybee Pathogen Paenibacillus Larvae. Biocontrol Sci. Technol. 2020, 30, 897–908. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection—A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef]
- Kačániová, M.; Gasper, J.; Terentjeva, M. Antagonistic Effect of Gut Microbiota of Honeybee (Apis mellifera) against Causative Agent of American Foulbrood Paenibacillus Larvae. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 478–481. [Google Scholar] [CrossRef]
- Niode, N.J.; Salaki, C.L.; Rumokoy, L.J.M.; Tallei, T.E. Lactic Acid Bacteria from Honey Bees Digestive Tract and Their Potential as Probiotics. In Proceedings of the International Conference and the 10th Congress of the Entomological Society of Indonesia (ICCESI 2019), Bali, Indonesia, 6–9 October 2019; Atlantis Press: Dordrecht, The Netherlands, 2020; pp. 236–241. [Google Scholar]
- Lamei, S.; Stephan, J.G.; Nilson, B.; Sieuwerts, S.; Riesbeck, K.; de Miranda, J.R.; Forsgren, E. Feeding Honeybee Colonies with Honeybee-Specific Lactic Acid Bacteria (Hbs-LAB) Does Not Affect Colony-Level Hbs-LAB Composition or Paenibacillus Larvae Spore Levels, Although American Foulbrood Affected Colonies Harbor a More Diverse Hbs-LAB Community. Microbial ecology 2020, 79, 743–755. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vandamme, E.J. Bacteriocins of Lactic Acid Bacteria; Blackie Academic & Professional Inc.: New York, NY, USA, 1994. [Google Scholar]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An Overview of Beneficial Effects. Lact. Acid Bact. Genet. Metab. Appl. 2002, 82, 279–289. [Google Scholar]
- Olofsson, T.C.; Vásquez, A. Detection and Identification of a Novel Lactic Acid Bacterial Flora within the Honey Stomach of the Honeybee Apis mellifera. Curr. Microbiol. 2008, 57, 356–363. [Google Scholar] [CrossRef]
- Vásquez, A.; Olofsson, T.C.; Sammataro, D. A Scientific Note on the Lactic Acid Bacterial Flora in Honeybees in the USA–A Comparison with Bees from Sweden. Apidologie 2009, 40, 26–28. [Google Scholar] [CrossRef]
- Evans, J.D.; Lopez, D.L. Bacterial Probiotics Induce an Immune Response in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Killer, J.; Dubná, S.; Sedláček, I.; Švec, P. Lactobacillus Apis Sp. Nov., from the Stomach of Honeybees (Apis mellifera), Having an in Vitro Inhibitory Effect on the Causative Agents of American and European Foulbrood. Int. J. Syst. Evol. Microbiol. 2014, 64, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Olofsson, T.C.; Váasquez, A.; Fries, I. Novel Lactic Acid Bacteria Inhibiting Paenibacillus Larvae in Honey Bee Larvae. Apidologie 2010, 41, 99–108. [Google Scholar] [CrossRef]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as Major Modulators of Insect Health: Lactic Acid Bacteria and Honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Kačániová, M.; Gasper, J.; Terentjeva, M.; Kunová, S.; Kluz, M.; Puchalski, C. Antibacterial Activity of Bees Gut Lactobacilli against Paenibacillus Larvae in Vitro. Adv. Res. Life Sci. 2018, 2, 7–10. [Google Scholar] [CrossRef]
- Dainat, B.; Grossar, D.; Ecoffey, B.; Haldemann, C. Triplex Real-Time PCR Method for the Qualitative Detection of European and American Foulbrood in Honeybee. J. Microbiol. Methods 2018, 146, 61–63. [Google Scholar] [CrossRef]
- Bakonyi, T.; Derakhshifar, I.; Grabensteiner, E.; Nowotny, N. Development and Evaluation of PCR Assays for the Detection of Paenibacillus Larvae in Honey Samples: Comparison with Isolation and Biochemical Characterization. Appl. Environ. Microbiol. 2003, 69, 1504–1510. [Google Scholar] [CrossRef]
- Belloy, L.; Imdorf, A.; Fries, I.; Forsgren, E.; Berthoud, H.; Kuhn, R.; Charrière, J.-D. Spatial Distribution of Melissococcus Plutonius in Adult Honey Bees Collected from Apiaries and Colonies with and without Symptoms of European Foulbrood. Apidologie 2007, 38, 136–140. [Google Scholar] [CrossRef]
- Forsgren, E.; Lundhagen, A.C.; Imdorf, A.; Fries, I. Distribution of Melissococcus Plutonius in Honeybee Colonies with and without Symptoms of European Foulbrood. Microb. Ecol. 2005, 50, 369–374. [Google Scholar] [CrossRef]
- Forsgren, E.; Genersch, E. The Foulbroods of the Honeybee. Microbiol. Today 2011, 11, 238–241. [Google Scholar]
- Laurent, M.; Hendrikx, P.; Ribiere-Chabert, M.; Chauzat, M.-P. A Pan-European Epidemiological Study on Honeybee Colony Losses 2012–2014. EPILOBEE Rep. 2015. Available online: http://www.euroconsulting.be/upload/news/documents/20150505060346_bee-report_2012_2014_en.pdf (accessed on 9 December 2021).
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A Pan-European Epidemiological Study Reveals Honey Bee Colony Survival Depends on Beekeeper Education and Disease Control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Hendrikx, P.; Ribière-Chabert, M.; Chauzat, M.P. EPILOBEE: A European Epidemiological Program for the Surveillance of Honeybee Colony Losses. Épidémiologie St. Anim. 2014, 66, 43–50. [Google Scholar]
- Jacques, A.; Laurent, M.; Ribiere-Chabert, M.; Saussac, M.; Bougeard, S.; Hendrikx, P.; Chauzat, M. Statistical Analysis on the EPILOBEE Dataset: Explanatory Variables Related to Honeybee Colony Mortality in EU during a 2 Year Survey. EFSA Supporting Publ. 2016, 13, 883E. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Laurent, M.; Riviere, M.P.; Saugeon, C.; Hendrikx, P.; Ribiere-Chabert, M. Epilobee A Pan-European Epidemiological Study on Honeybee Colony Losses 2012–2013; European Union Reference Laboratory for Honeybee Health (EURL): Sophia Antipolis, France, 2014; Available online: https://ec.europa.eu/food/system/files/2017-04/la_bees_epilobee-report_2012-2013.pdf (accessed on 9 December 2021).
- Graham, J.M. The Hive and the Honey Bee; Dadant and Sons: Hamilton, IL, USA, 1992; ISBN 0915698099. [Google Scholar]
- McKee, B.A.; Djordjevic, S.P.; Goodman, R.D.; Hornitzky, M.A. The Detection of Melissococcus Pluton in Honey Bees (Apis mellifera) and Their Products Using a Hemi-Nested PCR. Apidologie 2003, 34, 19–27. [Google Scholar] [CrossRef]
- Lamei, S.; Stephan, J.G.; Riesbeck, K.; Vasquez, A.; Olofsson, T.; Nilson, B.; de Miranda, J.R.; Forsgren, E. The Secretome of Honey Bee-Specific Lactic Acid Bacteria Inhibits Paenibacillus Larvae Growth. J. Apic. Res. 2019, 58, 405–412. [Google Scholar] [CrossRef]
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative Correlation between Individual-Insect-Level Virulence and Colony-Level Virulence of Paenibacillus Larvae, the Etiological Agent of American Foulbrood of Honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347. [Google Scholar] [CrossRef]
- Stephan, J.G.; Lamei, S.; Pettis, J.S.; Riesbeck, K.; de Miranda, J.R.; Forsgren, E. Honeybee-Specific Lactic Acid Bacterium Supplements Have No Effect on American Foulbrood-Infected Honeybee Colonies. Appl. Environ. Microbiol. 2019, 85, e00606–e00619. [Google Scholar] [CrossRef]
- Shimanuki, H.; Knox, D.A. Summary of Control Methods. In Honey Bee Pests, Predators, & Disease, 3rd ed.; Morse, R.A., Flottum, K., Eds.; A.I. Root Company: Medina, OH, USA, 1997; pp. 505–507. [Google Scholar]
- Budge, G.E.; Barrett, B.; Jones, B.; Pietravalle, S.; Marris, G.; Chantawannakul, P.; Thwaites, R.; Hall, J.; Cuthbertson, A.G.S.; Brown, M.A. The Occurrence of Melissococcus Plutonius in Healthy Colonies of Apis Mellifera and the Efficacy of European Foulbrood Control Measures. J. Invertebr. Pathol. 2010, 105, 164–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).