Abstract
Bone regeneration is a central focus of maxillofacial research, especially when dealing with dental implants or critical sized wound sites. While bone has great regeneration potential, exogenous delivery of growth factors can greatly enhance the speed, duration, and quality of osseointegration, making a difference in a patient’s quality of life. Bone morphogenic protein 2 (BMP-2) is a highly potent growth factor that acts as a recruiting molecule for mesenchymal stromal cells, induces a rapid differentiation of them into osteoblasts, while also maintaining their viability. Currently, the literature data shows that the liposomal direct delivery or transfection of plasmids containing BMP-2 at the bone wound site often results in the overexpression of osteogenic markers and result in enhanced mineralization with formation of new bone matrix. We reviewed the literature on the scientific data regarding BMP-2 delivery with the help of liposomes. This may provide the ground for a future new bone regeneration strategy with real chances of reaching clinical practice.
Keywords:
BMP-2; liposomes; drug delivery; growth factors; osseointegration; implantology; transfection 1. Introduction
In maxillofacial and orthopedic research, bone regeneration represents one of the main focuses. Critical size bone tissue loss resulted after trauma, infection, tumors, systemic diseases, osteoporosis, or surgical resection and often need consolidation or replacement using different biomaterials, autografts, allografts, or xenografts [1,2,3]. Nonunion fractures can account for up to 12 percent of all fractures and carry the risk of complications such as severe pain or loss of function, as well as prolonged hospitalization which results in higher costs [4].
Archeological findings attest to the replacement of missing teeth starting with the ancient Egyptians and the Mayan civilization [5]. Since 1949, when Goldberg and Gershkoff published the first scientific article describing the use of metals as dental implants [6], novel biomaterials and surgical approaches have made dental implantation an everyday procedure and, today, millions are performed every year with success [5]. Underlying periodontal disease [7], insufficient bone and a long, difficult recuperation period before the patient can fully regain use of function, are the main challenges in current implantology. During this time, the patient must comply with a series of recommendations which drastically impact the quality of their life. If periodontal disease is present, the underlying bone often has a poor quality, and implants fail to integrate. A good osteoinductive material has a role in support, as well as the capability to recruit mesenchymal stromal cells (MSCs), deliver the growth factors which are necessary in the differentiation process, and rendering a faster, enhanced bone formation [8,9].
In bone remodeling, the main growth factors are the members of the TGFβ superfamily, mainly bone morphogenetic proteins (BMPs) [10,11,12,13]. The delivery of these factors is a problem not yet solved. In the literature, numerous drug delivery systems are described but concerns are that none of these are ideal. Adding growth factors to metals increases the price of the implants significantly. Delivery by calcium-phosphate ceramics increases the necessity of growth factors and there is a risk for factor degradation [14]. Liposomes are bioactive vesicles which can encapsulate many types of molecules and specific genetic sequences that transfected into the cells can increase the secretion of specific proteins. They are excellent carriers, highly biocompatible, but overlooked in many areas, such as bone regeneration. Including growth factors in liposomes, reduces the amount used, and subsequently the costs and the risks of side effects [12].
In this review article, the aim is to compile the evidence regarding the use of liposomes as growth factor delivery systems in bone regeneration, most specifically, in the osseointegration process of dental implantation.
2. Components of Bone Regeneration
For either the insertion of a simple screw or the most complicated implant, it is imperative to find better and faster bone regeneration techniques [15]. Stem cells, biomaterials, and bioactive molecules are the major factors in current bone regeneration research.
2.1. Cells
Osteoblasts and osteoclasts are the two major cellular elements in bone remodeling. Osteoclasts attach to the old bone and reabsorb the damaged tissue, while osteoblasts migrate to the lesion site attracted by the secreted cytokines and fill up the gap created by the bone resorption [16]. In regenerative research, MSCs are used to obtain osteoblast, as they can differentiate into diverse cell types depending on the external stimuli. They have been defined as a population of plastic adherent, stem-like cells which can be isolated from various tissues [17]. These cells have the potential to differentiate into osteoblasts [18], chondroblasts [19], pancreatic cells [20], adipocytes [21] or myocytes [22] depending on the molecules of the micro medium in which they are seeded. They were first isolated from bone marrow, but lately were retrieved from numerous tissues, such as umbilical cord [23], adipose tissue, as well as healthy and diseased oral structures [8,24,25]. BMPs induce differentiation of MSCs into pre-osteoblasts and mature osteoblasts [26].
Osteoblastic differentiation requires the expression of two main transcription factors: Runx2 and osterix (Osx) [27]. Stem cells have the ability to migrate to the site of bone regeneration and secrete different biomolecules. Expression of Runx2 is low in MSCs, but rises when the cells differentiate and start to secrete BMP-2 [28,29,30]. Runx2 also encodes for other cellular markers involved in osteochondral calcification, such as alkaline phosphatase (ALP) [31], osteocalcin (OC), and osteopontin (OP) [32]. Transgenic mice which lack Runx2 have completely cartilaginous skeletons [33], because they do not have osteoblasts and mineralization does not occur [34,35]. Similarly, mice which lack Osx, have perfectly structured skeletons, but without ossification. The Osx gene acts downstream from Runx2. Mice lacking Runx2 do not express Osx, but those missing Osx can have Runx2 intact, therefore, many consider Runx2 as the main gene in osteoblastic differentiation [36,37].
Osteoinduction is the cellular process during which an undifferentiated osteoprogenitor cell transforms in bone tissue under the influence of the local environment.
2.2. Biomaterials
There are many materials used in dental medicine, from the simplest suture thread to the most complicated implants. Biocompatibility is a paramount aspect of any material which comes in contact with tissues. Dental implants are made from various materials such as titanium, bioceramics, composites, natural and synthetic polymers, carbons [38,39,40,41,42], and their combination. Every one of these materials has advantages, but also disadvantages, and the perfect implant is yet to be invented [43]. From all the biomaterials, the closest to ideal is titanium, a versatile metal used both in pure form and as an alloy. Its superior biocompatibility is due to the fact that it is a highly reactive metal which has a high affinity to oxygen and spontaneously forms a very stable oxide layer at its surface in less than a millisecond after exposure to atmosphere [44]. Studies have shown a seven year survival rate of titanium implants between 94.6–95.7% [45,46,47]. The main disadvantage of titanium is represented by its high modulus of elasticity, which is five to ten times greater than the underlying human cortical bone in which it is implanted [48]. Because of the difference between the two, bone is resorbed, the implant loosens, and revision surgery is needed [49].
With the purpose of enhancing the success rate and reducing the osseointegration time, research has focused on improving the interface between the organism and the inorganic substrate by functionalization of the implant with different biolayers and biomolecules. The surface of the implant is the part which interacts with the recipient tissue and for better results we need to fully comprehend all the mechanical, physical, and chemical interactions that take place here [50,51].
2.3. Growth Factors
The most important class of biomolecules in bone regeneration are the bone morphogenetic proteins (BMPs), members of the TGFβ superfamily [26,52]. They play a fundamental part in differentiation, embryonic development [53], and even tumorigenesis and cancer progression [54,55]. BMPs were first identified by Urist, in 1965 [56,57], and purified by Wang et al. two decades later [58]. After the identification and cloning of the BMP genes the manufacturing of recombinant human BMPs followed [59]. There are 20 different types of BMPs described in the literature as markers of the osteogenic cell differentiation process. BMP-2 and BMP-7 are currently approved by the FDA for human use [52,60]. BMP-2 is the most potent molecule in osteoinduction, essential for new bone formation. During fracture healing, the molecule is released during the degradation of the bone by osteoclasts and acts like a beacon for MSCs to find the lesion sites. During in vitro experiments, it is added to the culture media in the process of differentiation of stem cells into osteoblastic lineages for a better and faster osteogenic transformation. Attempts to replicate this in vivo frequently fail, because growth factors have a short half-life, and they are rapidly removed from the lesion site by the circulatory system. Human recombinant BMP-2 (rhBMP-2) is expensive, and its stability and biological activity in vivo is limited [61]. Off label use results, sometimes, in significant complications such as dysphagia [62], airway swelling [63,64], ectopic and heterotopic bone formation [65,66], immune response, or tumorigenesis [67]. For this reason, we have to optimize its intake by the cells by adding them to slow-release delivery systems. Pre-differentiation of MSCs, produces BMP-2 and attracts the organisms own stem cells to the injury site [68,69]. There are no standards of dosage for in vivo animal studies, the use of BMP-2 for cartilage and bone regeneration ranges between 0.015 and 150 µg/implant and even the FDA-controlled formulations are used in supra-physiological doses [70].
2.4. Growth Factor Delivery Systems
The major problem related to the in vivo use of growth factors in bone regeneration is represented by the lack of a reliable administration method [71]. In order to modulate osteogenic differentiation, different strategies can be applied. Systemic delivery methods often fail because of the accumulation of the growth factors in the kidneys and subsequent elimination. Injection to the site of the injury is most often used, but the lack of a good vessel leads to absorption into the systemic circulation and elimination. Specificity for a certain organ or even cell type is very important, and the lack of it is the biggest drawback of systemic drug delivery. Different delivery systems have been used to obtain a slow, controlled release of active molecules. Protection of the active molecule can be done by inclusion in carriers [72] or fixating them to the surface of the implant [73]. Biocompatibility is one of the most important features of the carriers. Natural compounds, i.e., collagen [74], gelatin [75], fibrin and fibronectin [76], chitosan [77], hyaluronic acid [78]; synthetic polymers, i.e., poly(lactic acid) [79], poly(glycolic acid) [80], poly(lactic-co-glycolic acid) [81]; or inorganic materials, i.e., hydroxyapatite (HA) [82,83], tricalcium phosphate (TCP) [84], and combinations of them are used for slow delivery.
3. Liposomes
Liposomes are biocompatible, self-assembled, spheric vesicles composed of concentric phospholipid bilayers wrapped around an aqueous compartment. Phospholipids are composed of a hydrophilic polar head and a hydrophobic non-polar tail. They were first described in the 1960s [85] and have been used as delivery systems since the 1970s [86]. As their structure is similar to cell membranes, they can easily merge with cells, penetrating them, and discarding their cargo. They can incorporate many types of molecules depending on the structure of the liposome and the hydrophilicity of the entrapped molecule. The hydrophilic drugs are entrapped into their inner compartment and the hydrophobic ones are linked to them directly or indirectly, either on the surface or between the two lipid layers (Figure 1). They are extremely versatile. Although there is little researched on their role in bone regeneration, their role in other areas is well documented. Liposomes are used as a vector in more than 20% of approved clinical trials in controlled drug delivery [87]. There are numerous FDA-approved clinical applications of liposomes in vaccine development, as well as antibiotic and analgetic delivery [88]. In cancer therapy, antibody conjugated liposomes (immunoliposomes) have been used in clinical trials to eliminate circulating cancer cells, preventing metastasis [89]. In the COVID-19 pandemic, the first approved vaccines were using liposomal transfection of messenger RNA [90]. By conjugating with magnetic nanoparticles and imaging agents, in vivo traceability of liposomes has been achieved. Targeted release has been obtained by exposure to ultrasound [91] or magnetic actuation [92,93]. Using a combination of radioactive molecules bonded to liposomes, diagnostics, targeting, and treatment have been obtained, which has been called theranostics [94].
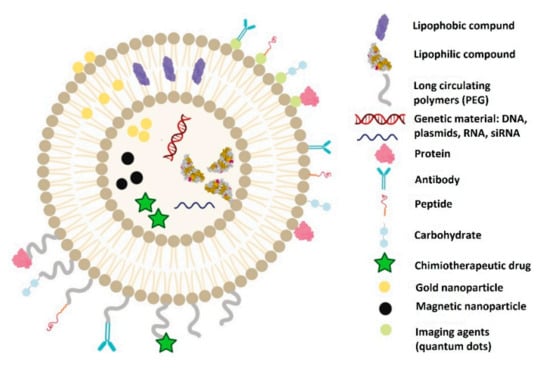
Figure 1.
Structure of liposomes and the molecules that can be delivered through them.
Attempts were made to immobilize liposomal BMP-2 onto different biocompatible scaffolds for a sustained, long-term release. Electrospun poly(L-lactic acid) fibers, functionalized with hydroxyapatite (HA) can be a good carrier for BMP-2 loaded liposomes. Adipose tissue-derived MSCs were seeded onto the scaffolds. The levels of ALP and calcium ions were significantly higher in the liposome and HA containing scaffolds than in the control group (HA-coated scaffolds with free BMP-2). The expression level of the genes related to osteogenesis were three-fold as compared with the control group. The osteoconductivity of the constructs was tested in vivo by subcutaneous implantation into rats. At the site of the implants, MSCs aggregated and primary ossification centers appeared [95].
Magnetic liposomes have also been used to carry BMP-2 and in combination with magnets and have had good results in maintaining the proteins at the injury site for a prolonged period of time. Entrapment efficiency was approximated to be the same with fluorescein isothiocyanate-conjugated dextran (FD-40) which has the same molecular weight as BMP-2. The magnetic liposomes had entrapped a lower quantity of FD-40 than the conventional ones. A critical size bone defect was created in the animal’s femur and a magnet was inserted. Different magnetic and non-magnetic liposomes were injected at the injury site, at different timepoints. Only the animals injected with magnetic liposomes immediately after the surgery, presented complete bone bridge formation [96].
In situ gels are a good alternative for drug delivery systems. They are liquid ex vivo and turn into gels in the organism depending on several factors. Growth factors included in liposomes can be entrapped in gels for a prolonged and controlled release, resulting in longer and more stable plasma levels of the protein and significantly more bone formation when injected into critical size bone defects [97].
Hydroxyapatite is found only in bone tissue and designing systems that can link to it is an important goal in osteogenetic research. Bisphosphonates (BPs) are ligands with a high affinity to osseous tissue, which prevent bone resorption by impairing the function of osteoclasts [98,99]. They can be conjugated with active molecules such as BMPs. Produced by two methods, Wang et al. found that the BP micelles and BP liposomes had a strong affinity to HA vs. the PEGilated ones, while the in vitro and in vivo bone-inducing capacity of BMP-2 was maintained [100]. The studies from the literature are detailed in Table 1.

Table 1.
Direct liposomal administration of BMP-2.
4. Transfection
Gene therapy is used to transfer genetic material into specific cells to obtain the secretion of a certain protein. DNA, plasmids, siRNAs, miRNAs can be delivered into target cells using vectors which protect them and facilitate their transport through the cell wall [101,102]. This method may be more effective than the exogenous utilization of the molecule, because it restricts the migration of the molecule and the accumulation in other organs. Transfection of DNA into cells is the ideal way to study some functions of proteins. The cells will become, by this method, protein producing factories at the site of the injury. Viruses represent a good vector, as they developed natural ways to enter the host cell and integrate in their genetic material. Adenoviruses, retroviruses, adeno-associated viruses, and lentiviruses are used as vectors the most efficiently [103,104,105], but they carry significant side effects and limitations, such as inflammatory and immune reactions, limit of included DNA size, or certain tumorigenic mutations [15,106,107,108].
Non-viral vectors are mostly cationic polymers or cationic liposomes, which interact with the negatively charged genetic material and can be transported into the cell. Liposomal transfection is not as efficient as the viral ones [109], but it yields a series of advantages such as lack of immune response, toxic byproducts, ectopic bone formation, and accumulation in organs [67]. Liposomes are a viable solution for transfection of large genetic structures, as their capacity to carry genetic material is not limited by size [110].
The genetic sequence is first loaded into the liposome. After the construct enters the cell, the gene is released, it enters the nucleus, where it integrates into the cell’s DNA. Thus, the cell produces BMP-2 (Figure 2).
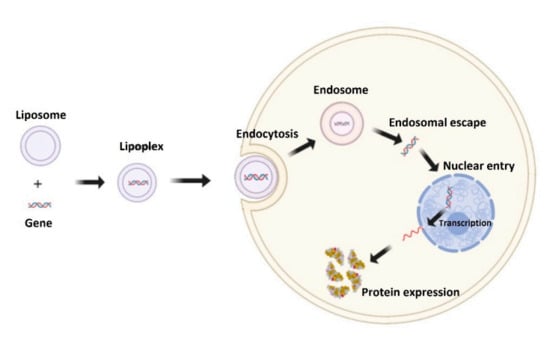
Figure 2.
Mechanism of liposomal transfection.
There are very few studies in the literature in which direct loading of BMP-2 is used; however, more studies concentrate on transfection of BMP-2 genes into cells.
Park et al. conducted a study on pigs where they created calvarial bone defects in which they inserted implants with or without BMP-2 transfected liposomes. Previously, they had assessed the transfection efficiency of the liposomal vector by introducing green fluorescent protein. The osteogenic capacity was measured at 7 and 28 days and at three different regions of interest. The bone regeneration was significantly enhanced in the group with the liposomal vector applied to the surface of the implant. The direct application of the vector was sufficient for complete bone healing at the margins of the bone defect, but not in the center [111]. Using collagen for the liposome/BMP-2 carrier allows migrating cells to express the protein even after 28 days [13].
Neo-angiogenesis is one of the main components of bone regeneration and, at the same time, one of the biggest challenges. For the formation of new bone, it is important for nutrients to be provided, the acid-base balance to be maintained, and the metabolic by-products to be eliminated. The combined release of BMP-2 and VEGF could solve this issue [112,113,114]. Xiao-bin et al. evaluated the efficacy of transfection of mouse bone marrow stromal cells (mBMSC) with BMP-2 and VEGF165 in order to assess the neoangiogenic and ectopic bone tissue forming capability of these molecules. They found co-expression of BMP-2 and VEGF165 mRNA in vitro by immunohistochemistry and RT-PCR. In mice, they obtained ectopic trabecular-like bone formation at 4 weeks after injection [115]. Guo-ping et al. researched, in vitro, the transfection efficiency of a vector which co-expressed hBMP-2 and hVEGF165 and the resulted protein levels. They found that the transcription of hVEGF may be upregulated by hBMP-2 by RT-PCR analysis of the proteins. Western blot did not show this cooperativity. Osteocalcin mRNA and collagen I were high in the groups transfected with BMP-2, but negligible in the groups with VEGF alone [116].
When comparing liposomes with polyethylene glycol (PEG) as gene carriers, BMP-2 levels in cells and mRNA levels of BMP-2 were double in PEG group than in liposomal group. The liposomal group was only used as control in vitro, but not in vivo [117].
Liposome-loaded DNA have been introduced in multilayer HA coatings deposited on titanium disks using the layer-by-layer technique. The amount of DNA was increased by each additional layer, the plasmids were released, and cells were transfected, with an increased expression of Runx2, Osx, ALP, and OC, but without calcified nodule formation at 14 days [118]. At implantation in rabbits, the uncoated implants yielded new woven bone, showing a statistically significant difference at 4 weeks (but not at 2 or 8 weeks) in favor of the BMP-2 gene coated implants. However, the bone-to-implant contact was consistently lower than in the control group, which the authors explained by the short persistence of the protein at the site [119].
PEG membranes are biodegradable materials often used in bone tissue engineering. In adult pig experimental model, PEG membranes containing liposomal BMP-2 transfected osteoblasts facilitated a significantly higher new bone regeneration, cell survival, and protein synthesis at 1, 2, 4, and 8 weeks after implantation. The combination of PEG matrixes and osteoblasts transfected with BMP-2 allowed for a good spatial fixation of the implanted cells in the defect [120,121].
Kroczek et al. compared the effect of BMPs to other members of the TGFβ superfamily such as TGFβ and IGF1. They transfected the genes into BMSCs, implanted the cells into mini-pigs, and evaluated the bone formation. Cells transfected with TGFβ and IGF1 did not enhance bone formation as compared with the negative control, while those with BMP-2/7 yielded good quality bone tissue with enhanced mineralization and organized architecture [122].
The extracellular matrix of bone consists of 70–90% of hydroxyapatite (HA) and 10–30% organic material, mainly collagen [123]. HA ceramics have been used as a substitute for autologous bone or as a carrier for bioactive molecules. Adding liposomal BMP-2 cDNA to HA scaffolds leads to better bone formation than HA alone or liposomal BMP-2 alone. The BMP-2 expression was present at 3 and 6 weeks after which it decreased gradually [124]. Human amnion mesenchymal stem cells (hAMSC) transfected with BMP-2 in a liposomal formulation seeded on nano HA/collagen/poly(1-lactide) had a similar proliferation and differentiation capability as those cultured in osteogenic culture media. The cells transfected had higher expression of OC and Runx2 [125].
Recently, stem cells of buco-maxillar origin have been identified and isolated [8]. Dental follicle, alveolar bones, and ligaments have proven to be excellent sources of stem cells. They are readily available from discarded medical waste and have proven to be superior in osteogenesis as compared with stem cells of other origins. The periodontal ligament plays an important role in stability, nutrition, and regeneration of the teeth. Stem cells isolated from it have been successfully used for differentiation into osteoblasts when transfected with BMP-2 plasmids using a liposomal vector [126].
The studies conducted on liposomal delivery of BMP-2 through transfection are detailed in Table 2.

Table 2.
Transfection of BMP-2 genes.
Comparative studies of efficiency of different vectors of BMP transfection.
Blum et al. compared the activity of luciferase one day after performing adenoviral, retroviral, and lipiosome mediated BMP-2 transfection into rat MSCs. The reporter gene was delivered efficiently by all three vectors. They obtained the best results using adenoviruses [104].
Park et al. compared liposome- and adenovirus- mediated gene transfer of BMP-2 cDNA in rat BMSCs and transplanted these cells into periosteal tissue. Gene expression lasted more than 14 days using either method, but adenoviral transfer resulted in double the amount of positive cells. In vivo healing of critical size bone defects by liposome-mediated gene transfer was slower, but the new bone had a normal configuration and physiologic orientation as compared with the adenoviral group in which the bone was significantly thicker [67]. In cartilaginous regeneration, liposomal transfection is also less efficient, forming only low rigidity fibro-cartilaginous tissue. Cells transfected by adenovirus formed tissue similar to hyaline cartilage [130].
5. Conclusions
In this review article, we detailed the existing BMP-2 delivery systems by liposomes. In the literature we found two methods described: direct addition of the growth factor and transfection through gene carrying. There are hardly any studies in which BMP-2 was added directly into liposomes, but the existing studies report good results both in vitro and in vivo, on animal studies. More papers are written on transfection of BMP-2 gene carrying liposomes. In vitro experiments show an excellent transfection efficiency by liposomes [111]. Combination therapy with VEGF yields an improved osteogenic differentiation [115,116], while the combination with TGFβ and IGF1 do not enhanced bone formation [122]. Animal studies are also promising, showing enhanced mineralization [119,122] and spatial fixation [120,121]. Due to these encouraging results, we anticipate that delivery of BMP-2 by liposomes will gain terrain in bone regeneration research. There is much need of better bone regeneration techniques, but we are still far away from the point where clinical translation is to be achieved before application of the method to humans. Future research has to establish the right dosage of BMP-2 delivery. In addition, in this review, we did not find studies comparing the two methods of BMP-2 delivery by liposomes.
Liposomes seem to be a good carrier for BMP-2. They enhance the osseointegration quality and shorten the required time. However, further investigation is needed in this area to properly translate it to clinical settings.
Author Contributions
This article is part of Dirzu Noemi’s doctoral studies: “Optimization of the bio integration of implantable materials used in dental medicine”, coordinator R.S.C.; Conceptualization, N.D., O.L. and D.S.D.; writing, N.D., D.C., D.S.D., B.C., L.T. and O.S.; supervision, R.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants awarded by the Romanian Ministry of Research and Innovation: Grants for Young Research Teams 2020–2022, grant no. PN-III-P1-1.1-TE-2019-0271 (grant director Tomuleasa Ciprian Ionut); PN-III-P4-ID-PCE-2020-1118 within PNCDI IV, Projects for Exploratory Medicine (grant director Gabriel Ghiaur).
Institutional Review Board Statement
The manuscript was approved by the institutional review board of the Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca.
Informed Consent Statement
No informed consent was required as it did not present any identifiable data.
Data Availability Statement
No informed consent was required as it did not present any identifiable data.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TGFβ | Transforming Growth Factor β |
| MSC | Mesenchymal Stromal Cell |
| BMP-2 | Bone morphogenetic protein 2 |
| rhBMP-2 | recombinant human BMP-2 |
| Runx2 | Runt-related transcription factor 2 |
| Osx | Osterix transcription factor |
| ALP | Alkaline Phosphatase |
| OC | osteocalcin |
| OP | osteopontin |
| FDA | Food and Drug Administration |
| HA | hydroxyapatite |
| TCP | tricalcium phosphate |
| DNA | deoxyribonucleic acid |
| siRNA | small interfering ribonucleic acid |
| miRNA | micro ribonucleic acid |
| VEGF | Vascular Endothelial Growth Factor |
| hVEGF | human Vascular Endothelial Growth Factor |
| hBMP-2 | human Bone morphogenetic protein 2 |
| BMSC | Bone Marrow Stromal Cell |
| mRNA | messenger ribonucleic acid |
| RT-PCR | Revers Transcription Polymerase Chain Reaction |
| Peg | polyethylene glycol |
| IGF1 | Insulin growth factor 1 |
| cDNA | complementary deoxyribonucleic acid |
| Q-PCR | quantitative PCR |
| BP | bisphosphonates |
| GADPH | glyceraldehyde 3-phosphate dehydrogenase |
References
- Peng, X.-Y.; Hu, M.; Liao, F.; Yang, F.; Ke, Q.-F.; Guo, Y.-P.; Zhu, Z.-H. La-Doped Mesoporous Calcium Silicate/Chitosan ScafFolds for Bone Tissue Engineering. Biomater. Sci. 2019, 7, 1565–1573. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, M.K. Bone Loss in the Oral Cavity. J. Bone Miner. Res. 2009, 8, S467–S473. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Templeman, D.; Weinlein, J.C. Nonunion of the Femur and Tibia. Orthop. Clin. N. Am. 2016, 47, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current Trends in Dental Implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Goldberg, N.I.; Gershkoff, A. The Implant Lower Denture. Dent. Dig. 1949, 55, 490–494. [Google Scholar]
- Ionel, A.; Lucaciu, O.; Bondor, C.; Moga, M.; Ilea, A.; Feurdean, C.; Buhățel, D.; Hurubeanu, L.; Câmpian, R.S. Assessment of the Relationship between Periodontal Disease and Cardiovascular Disorders: A Questionnaire-Based Study. Clujul Med. 2016, 89, 534–541. [Google Scholar] [CrossRef]
- Lucaciu, O.; Soritau, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Campian, R.; Băciuţ, G.; Popa, C.; et al. Dental Follicle Stem Cells in Bone Regeneration on Titanium Implants. BMC Biotechnol. 2015, 15, 1–18. [Google Scholar] [CrossRef]
- Lucaciu, O.; Crisan, B.; Hedesiu, M.; Soritau, O.; Dirzu, N.; Crisan, L.; Campian, R.; Baciut, G.; Baciut, M.; Onisor, F.; et al. The Role of BMP-2, Low-Level Laser Therapy and Low X-Ray Doses in Dental Follicle Stem Cell Migration. Part. Sci. Technol. 2017, 36, 981–988. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Wang, W.; Yang, A. BMP2 Induces hMSC Osteogenesis and Matrix Remodeling. Mol. Med. Rep. 2020, 23, 125. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, X.; Gao, L.; Mao, M.; Gao, D.; Huang, Z. Osteomodulin Positively Regulates Osteogenesis through Interaction with BMP2. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Wang, P.; Perche, F.; Midoux, P.; Cabral, C.S.; Malard, V.; Correia, I.J.; EI-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo Bone Tissue Induction by Freeze-Dried Collagen-Nanohydroxyapatite Matrix Loaded with BMP2/NS1 MRNAs Lipopolyplexes. J. Control. Release 2021, 334, 188–200. [Google Scholar] [CrossRef]
- Lutz, R.; Park, J.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Schlegel, K.A. Bone Regeneration after Topical BMP-2-Gene Delivery in Circumferential Peri-Implant Bone Defects. Clin. Oral Implant. Res. 2008, 19, 590–599. [Google Scholar] [CrossRef]
- Agrawal, V.; Sinha, M. A Review on Carrier Systems for Bone Morphogenetic Protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 4, 904–925. [Google Scholar] [CrossRef]
- Oliveira, A.; Ferraz, M.; Monteiro, F.; Simões, S. Cationic liposome–DNA Complexes as Gene Delivery Vectors: Development and Behaviour towards Bone-Like Cells. Acta Biomater. 2009, 5, 2142–2151. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis*. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Huang, A.; Zhang, X.; Wang, Y.; Geng, W.; Xu, R.; Li, S.; He, H.; Zheng, B.; et al. INTS7–ABCD3 Interaction Stimulates the Proliferation and Osteoblastic Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells by Suppressing Oxidative Stress. Front. Physiol. 2021, 12, 758607. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Kamali, A.; Hosseini, S.; Eslaminejad, M.B. Higher Chondrogenic Potential of Extracellular Vesicles Derived from Mesenchymal Stem Cells Compared to Chondrocytes-EVs in vitro. BioMed Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Goodrich, A.D.; Ersek, A.; Varain, N.M.; Groza, D.; Cenariu, M.C.; Thain, D.S.; Almeida-Porada, G.; Porada, C.D.; Zanjani, E.D. In Vivo Generation of β-Cell–Like Cells from CD34+ Cells Differentiated from Human Embryonic Stem Cells. Exp. Hematol. 2010, 38, 516–525.e4. [Google Scholar] [CrossRef]
- Wen, Q.; Xie, X.; Ren, Q.; Du, Y. Polybrominated Diphenyl Ether Congener 99 (PBDE 99) Promotes Adipocyte Lineage Commitment of C3H10T1/2 Mesenchymal Stem Cells. Chemosphere 2021, 290, 133312. [Google Scholar] [CrossRef] [PubMed]
- Miksiunas, R.; Aldonyte, R.; Vailionyte, A.; Jelinskas, T.; Eimont, R.; Stankeviciene, G.; Cepla, V.; Valiokas, R.; Rucinskas, K.; Janusauskas, V.; et al. Cardiomyogenic Differentiation Potential of Human Dilated Myocardium-Derived Mesenchymal Stem/Stromal Cells: The Impact of HDAC Inhibitor SAHA and Biomimetic Matrices. Int. J. Mol. Sci. 2021, 22, 12702. [Google Scholar] [CrossRef] [PubMed]
- Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Manochantr, S. Bone Morphogenetic Protein-2 Enhances the Osteogenic Differentiation Capacity of Mesenchymal Stromal Cells Derived from Human Bone Marrow and Umbilical Cord. Int. J. Mol. Med. 2017, 39, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Soancă, A.; Lupse, M.; Moldovan, M.; Pall, E.; Cenariu, M.C.; Roman, A.; Tudoran, O.; Surlin, P.; Șorițău, O. Applications of Inflammation-Derived Gingival Stem Cells for Testing the Biocompatibility of Dental Restorative Biomaterials. Ann. Anat. 2018, 218, 28–39. [Google Scholar] [CrossRef]
- Páll, E.; Florea, A.; Soriţău, O.; Cenariu, M.; Petruţiu, A.S.; Roman, A. Comparative Assessment of Oral Mesenchymal Stem Cells Isolated from Healthy and Diseased Tissues. Microsc. Microanal. 2015, 21, 1249–1263. [Google Scholar] [CrossRef]
- Senta, H.; Park, H.; Bergeron, E.; Drevelle, O.; Fong, D.; Leblanc, E.; Cabana, F.; Roux, S.; Grenier, G.; Faucheux, N. Cell responses to bone morphogenetic proteins and peptides derived from them: Biomedical applications and limitations. Cytokine Growth Factor Rev. 2009, 20, 213–222. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A Review of Old Concepts with New Standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Mishra, S.; Vaughn, A.D.; Devore, D.I.; Roth, C.M. Delivery of siRNA Silencing Runx2 Using a Multifunctional Polymer-Lipid Nanoparticle Inhibits Osteogenesis in a Cell Culture Model of Heterotopic Ossification. Integr. Biol. 2012, 4, 1498–1507. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and Characterization of CD133+CD44+ Cancer Stem Cells from Human Laryngeal Squamous Cell Carcinoma Cell Lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef]
- Ge, Q.; Green, D.W.; Lee, D.-J.; Kim, H.-Y.; Piao, Z.; Lee, J.-M.; Jung, H.-S. Mineralized Polysaccharide Transplantation Modules Supporting Human MSC Conversion into Osteogenic Cells and Osteoid Tissue in a Non-Union Defect. Mol. Cells 2018, 41, 1016–1023. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Xia, Q.; Meng, M.; Ye, Z.; Tang, Z.; Feng, H.; Chen, X.; Chen, H.; Zeng, X.; et al. Enamel Matrix Derivative (EMD) Enhances the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells (BMSCs). Bioengineered 2021, 12, 7033–7045. [Google Scholar] [CrossRef]
- Nie, D.; Zhou, Y.; Wang, W.; Zhang, J.; Wang, J.H.-C. Mechanical Overloading Induced-Activation of mTOR Signaling in Tendon Stem/Progenitor Cells Contributes to Tendinopathy Development. Front. Cell Dev. Biol. 2021, 9, 687856. [Google Scholar] [CrossRef]
- Ding, M.; Lu, Y.; Abbassi, S.; Li, F.; Li, X.; Song, Y.; Geoffroy, V.; Im, H.-J.; Zheng, Q. Targeting Runx2 Expression in Hypertrophic Chondrocytes Impairs Endochondral Ossification during Early Skeletal Development. J. Cell. Physiol. 2011, 227, 3446–3456. [Google Scholar] [CrossRef]
- Catheline, S.E.; Hoak, D.; Chang, M.; Ketz, J.P.; Hilton, M.J.; Zuscik, M.J.; Jonason, J.H. Chondrocyte-Specific RUNX2 Overexpression Accelerates Post-Traumatic Osteoarthritis Progression in Adult Mice. J. Bone Miner. Res. 2019, 34, 1676–1689. [Google Scholar] [CrossRef]
- Rashid, H.; Chen, H.; Javed, A. Runx2 is Required for Hypertrophic Chondrocyte Mediated Degradation of Cartilage Matrix during Endochondral Ossification. Matrix Biol. Plus 2021, 12, 100088. [Google Scholar] [CrossRef]
- Lai, K.; Xi, Y.; Du, X.; Jiang, Z.; Li, Y.; Huang, T.; Miao, X.; Wang, H.; Wang, Y.; Yang, G. Activation of Nell-1 in BMSC Sheet Promotes Implant Osseointegration Through Regulating Runx2/Osterix Axis. Front. Cell Dev. Biol. 2020, 8, 868. [Google Scholar] [CrossRef]
- Artigas, N.; Ureña, C.; Rodríguez-Carballo, E.; Rosa, J.L.; Ventura, F. Mitogen-Activated Protein Kinase (MAPK)-Regulated Interactions between Osterix and Runx2 Are Critical for the Transcriptional Osteogenic Program. J. Biol. Chem. 2014, 289, 27105–27117. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and Release Kinetics of Growth Factors on Barrier Membranes for Guided Tissue/Bone Regeneration: A Systematic Review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef]
- Edelhoff, D.; Schweiger, J.; Prandtner, O.; Stimmelmayr, M.; Güth, J.F. Metal-free Implant-Supported Single-Tooth Restorations. Part I: Abutments and Cemented Crowns. Quintessence Int. 2019, 50, 176–184. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant Materials, Design, and Surface Topographies: Their Influence on Osseointegration of Dental Implants. J. Long-Term Eff. Med. Implant. 2003, 13, 18–501. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized Nanofiber Segments Coupled with Calcium-Binding BMP-2 Peptides for Alveolar Bone Regeneration. Acta Biomater. 2018, 85, 282–293. [Google Scholar] [CrossRef]
- Morton, D.; Gallucci, G.; Lin, W.; Pjetursson, B.; Polido, W.; Roehling, S.; Sailer, I.; Aghaloo, T.; Albera, H.; Bohner, L.; et al. Group 2 ITI Consensus Report: Prosthodontics and Implant Dentistry. Clin. Oral Implant. Res. 2018, 29, 215–223. [Google Scholar] [CrossRef]
- Kasemo, B. Biocompatibility of Titanium Implants: Surface Science Aspects. J. Prosthet. Dent. 1983, 49, 832–837. [Google Scholar] [CrossRef]
- Becker, W.; Hujoel, P.; Becker, B.E.; Wohrle, P. Dental Implants in an Aged Population: Evaluation of Periodontal Health, Bone Loss, Implant Survival, and Quality of Life. Clin. Implant Dent. Relat. Res. 2015, 18, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Meijndert, C.M.; Raghoebar, G.M.; Stellingsma, K.; Vissink, A.; Meijer, H.J.A. Single Implants in the Aesthetic Region Preceded by Local Ridge Augmentation; a 10-year Randomized Controlled Trial. Clin. Oral Implant. Res. 2016, 28, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Marconcini, S.; Giammarinaro, E.; Mijiritsky, E.; Gelpi, F.; Covani, U. Clinical Outcomes of Implants Placed in Extraction Sockets and Immediately Restored: A 7-Year Single-Cohort Prospective Study. Clin. Implant Dent. Relat. Res. 2016, 18, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.-W.; Jung, H.-D.; Park, C.-H.; Shin, K.-H.; Koh, Y.-H.; Estrin, Y.; Kim, H.-E. Reverse Freeze Casting: A New Method for Fabricating Highly Porous Titanium Scaffolds with Aligned Large Pores. Acta Biomater. 2012, 8, 2401–2410. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Jing, Z.; Liu, X.; Zheng, Y.; Cai, H.; Wei, F.; Jiang, L.; Yu, M.; et al. Three-Dimensional-Printed Individualized Porous Implants: A New “Implant-Bone” Interface Fusion Concept for Large Bone Defect Treatment. Bioact. Mater. 2021, 6, 3659–3670. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.; Mikos, A.G. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Poynton, A.R.; Lane, J.M. Safety Profile for the Clinical Use of Bone Morphogenetic Proteins in the Spine. Spine 2002, 27, S40–S48. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic Activity of the Fourteen Types of Human Bone Morphogenetic Proteins (BMPs). JBJS 2003, 85, 1544–1552. [Google Scholar] [CrossRef]
- Davis, H.; Raja, E.; Miyazono, K.; Tsubakihara, Y.; Moustakas, A. Mechanisms of Action of Bone Morphogenetic Proteins in Cancer. Cytokine Growth Factor Rev. 2015, 27, 81–92. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and Characterization of Other Distinct Bone-Inducing Factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel Regulators of Bone Formation: Molecular Clones and Activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in Bone Regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Gerhart, T.N.; Kirker-Head, C.A.; Kriz, M.J.; Holtrop, M.E.; Hennig, G.E.; Hipp, J.; Schelling, S.H.; Wang, E. Healing Segmental Femoral Defects in Sheep Using Recombinant Human Bone Morphogenetic Protein. Clin. Orthop. Relat. Res. 1993, 293, 317–326. [Google Scholar] [CrossRef]
- Vaidya, R.; Carp, J.; Sethi, A.; Bartol, S.; Craig, J.; Les, C.M. Complications of Anterior Cervical Discectomy and Fusion Using Recombinant Human Bone Morphogenetic Protein-2. Eur. Spine J. 2007, 16, 1257–1265. [Google Scholar] [CrossRef]
- Tumialán, L.M.; Rodts, G.E. To the Editor. Spine J. 2007, 7, 509–510. [Google Scholar] [CrossRef]
- Smucker, J.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased Swelling Complications Associated with Off-Label Usage of rhBMP-2 in the Anterior Cervical Spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef]
- Joseph, V.; Rampersaud, Y.R. Heterotopic Bone Formation with the Use of rhBMP2 in Posterior Minimal Access Interbody Fusion. Spine 2007, 32, 2885–2890. [Google Scholar] [CrossRef]
- Chen, N.-F.; Smith, Z.A.; Stiner, E.; Armin, S.; Sheikh, H.; Khoo, L.T. Symptomatic Ectopic Bone Formation after Off-Label Use of Recombinant Human Bone Morphogenetic Protein-2 in Transforaminal Lumbar Interbody Fusion. J. Neurosurg. Spine 2010, 12, 40–46. [Google Scholar] [CrossRef]
- Park, J.; Rieß, J.; Gelse, K.; Kloss, F.; Von Der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone Regeneration in Critical Size Defects by Cell-Mediated BMP-2 Gene Transfer: A Comparison of Adenoviral Vectors and Liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Wu, Y.; Ye, D.; Wang, S.; Zou, D.; Zhang, X.; Kaplan, D.; Jiang, X. VEGF and BMP-2 Promote Bone Regeneration by Facilitating Bone Marrow Stem Cell Homing and Differentiation. Eur. Cells Mater. 2014, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chim, H.; Miller, E.; Gliniak, C.; Alsberg, E. Stromal-Cell-Derived Factor (SDF) 1-alpha in Combination with BMP-2 and TGF-β1 Induces Site-Directed Cell Homing and Osteogenic and Chondrogenic Differentiation for Tissue Engineering without the Requirement for Cell Seeding. Cell Tissue Res. 2012, 350, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled Release Strategies for Bone, Cartilage, and Osteochondral Engineering—Part II: Challenges on the Evolution from Single to Multiple Bioactive Factor Delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of Different Growth Factors and Carriers on Bone Regeneration: A Systematic Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423. [Google Scholar] [CrossRef]
- Pranskunas, M.; Galindo-Moreno, P.; Padial-Molina, M. Extraction Socket Preservation Using Growth Factors and Stem Cells: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e7. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Cassari, L.; D’Auria, G.; Falcigno, L.; Franchi, S.; Contini, G.; Marsotto, M.; Battocchio, C.; Iucci, G.; et al. Chitosan Covalently Functionalized with Peptides Mapped on Vitronectin and BMP-2 for Bone Tissue Engineering. Nanomaterials 2021, 11, 2784. [Google Scholar] [CrossRef]
- Cheng, A.; Krishnan, L.; Tran, L.; Stevens, H.Y.; Xia, B.; Lee, N.; Williams, J.K.; Gibson, G.; Guldberg, R.E. The Effects of Age and Dose on Gene Expression and Segmental Bone Defect Repair after BMP-2 Delivery. JBMR Plus 2018, 3, e10068. [Google Scholar] [CrossRef]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic Crosslinked Gelatin 3D Scaffolds for Bone Tissue Engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef]
- Chen, P.; Ouyang, J.; Xiao, J.; Han, Z.; Yu, Q.; Tian, J.; Zhang, L. Co-Injection of Human Adipose Stromal Cells and RhBMP-2/Fibrin Gel Enhances Tendon Graft Osteointegration in a Rabbit Anterior Cruciate Ligament-Reconstruction Model. Am. J. Transl. Res. 2018, 10, 535–544. [Google Scholar]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, K.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, M. Dental Pulp Stem Cells in Chitosan/Gelatin Scaffolds for Enhanced Orofacial Bone Regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable Hydrogels from Enzyme-Catalyzed Crosslinking as BMSCs-laden Scaffold for Bone Repair and Regeneration. Mater. Sci. Eng. C 2018, 96, 841–849. [Google Scholar] [CrossRef]
- Czerwinski, M.J.; Desiderio, V.; Shkeir, O.; Papagerakis, P.; Lapadatescu, M.C.; Owen, J.H.; Athanassiou-Papaefthymiou, M.; Zheng, L.; Papaccio, G.; Prince, M.E.; et al. In Vitro Evaluation of Sialyl Lewis X Relationship with Head and Neck Cancer Stem Cells. Otolaryngol. Neck Surg. 2013, 149, 97–104. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lohmann, C.H.; Somers, A.; Niederauer, G.G.; Wozney, J.M.; Dean, D.D.; Carnes, D.L.; Schwartz, Z. Potential of Porous Poly-D, L-Lactide-Co-Glycolide Particles as a Carrier for Recombinant Human Bone Morphogenetic Protein-2 during Osteoinduction in Vivo. J. Biomed. Mater. Res. 1999, 46, 51–59. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Reyes, A.B.J.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. BioMed Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef]
- DeConde, A.S.; Sidell, D.; Lee, M.; Bezouglaia, O.; Low, K.; Elashoff, D.; Grogan, T.; Tetradis, S.; Aghaloo, T.; John, M.S. Bone Morphogenetic Protein-2-Impregnated Biomimetic Scaffolds Successfully Induce Bone Healing in a Marginal Mandibular Defect. Laryngoscope 2013, 123, 1149–1155. [Google Scholar] [CrossRef]
- Zaffarin, A.S.M.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Nano-Hydroxyapatite as a Delivery System for Promoting Bone Regeneration In Vivo: A Systematic Review. Nanomaterials 2021, 11, 2569. [Google Scholar] [CrossRef]
- Overman, J.R.; Farré-Guasch, E.; Helder, M.N.; Bruggenkate, C.M.T.; Schulten, E.A.; Klein-Nulend, J. Short (15 Minutes) Bone Morphogenetic Protein-2 Treatment Stimulates Osteogenic Differentiation of Human Adipose Stem Cells Seeded on Calcium Phosphate Scaffolds In Vitro. Tissue Eng. Part A 2013, 19, 571–581. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Gregoriadis, G. Enzyme Entrapment in Liposomes. Methods Enzymol. 1976, 44, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-C.; Huang, L. Liposome-Based Gene Therapy. Pharm. Sci. Technol. Today 1998, 1, 206–213. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ishihara, H. Difference in the Lipid Nanoparticle Technology Employed in Three Approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) Drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Evjen, T.J.; Nilssen, E.A.; Fowler, R.A.; Røgnvaldsson, S.; Brandl, M.; Fossheim, S.L. Lipid Membrane Composition Influences Drug Release from Dioleoylphosphatidylethanolamine-Based Liposomes on Exposure to Ultrasound. Int. J. Pharm. 2011, 406, 114–116. [Google Scholar] [CrossRef]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Podaru, G.; Ogden, S.; Baxter, A.; Shrestha, T.; Ren, S.; Thapa, P.; Dani, R.K.; Wang, H.; Basel, M.T.; Prakash, P.; et al. Pulsed Magnetic Field Induced Fast Drug Release from Magneto Liposomes via Ultrasound Generation. J. Phys. Chem. B 2014, 118, 11715–11722. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of Hybrid Scaffold Based on Hydroxyapatite-Biodegradable Nanofibers Incorporated with Liposomal Formulation of BMP-2 Peptide for Bone Tissue Engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef]
- Matsuo, T.; Sugita, T.; Kubo, T.; Yasunaga, Y.; Ochi, M.; Murakami, T. Injectable Magnetic Liposomes as a Novel Carrier of Recombinant Human BMP-2 for Bone Formation in a Rat Bone-Defect Model. J. Biomed. Mater. Res. 2003, 66A, 747–754. [Google Scholar] [CrossRef]
- Hassan, A.H.; Hosny, K.M.; Murshid, Z.A.; Alhadlaq, A.; Yamani, A.; Naguib, G.; Alkhalidi, H.M.; Afify, A.R. Controlled Release of Injectable Liposomal in Situ Gel Loaded with Recombinant Human Bone Morphogenetic Protein-2 for the Repair of Alveolar Bone Clefts in Rabbits. J. Liposome Res. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Okada, E.; Nakata, H.; Yamamoto, M.; Kasugai, S.; Kuroda, S. Indirect Osteoblast Differentiation by Liposomal Clodronate. J. Cell. Mol. Med. 2017, 22, 1127–1137. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.O.; Crisan, B.; Crisan, L.; Baciut, M.; Onisor, F.; Baciut, G.; Câmpian, R.S.; Bran, S. Systemic Drugs that Influence Titanium Implant Osseointegration. Drug Metab. Rev. 2016, 49, 92–104. [Google Scholar] [CrossRef]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludağ, H. Bisphosphonate-Decorated Lipid Nanoparticles Designed as Drug Carriers for Bone Diseases. J. Biomed. Mater. Res. Part A 2011, 100A, 684–693. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Zhao, Y.; Cao, C.; Wu, K.; Zhao, L.; Zhang, Y. Non-Viral Oligonucleotide antimiR-138 Delivery to Mesenchymal Stem Cell Sheets and the Effect on Osteogenesis. Biomaterials 2014, 35, 7734–7749. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Nazarali, A.; Foldvari, M. Non-Viral Nucleic Acid Delivery: Key Challenges and Future Directions. Curr. Drug Deliv. 2011, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Parrott, M.B.; Mikos, A.G.; Barry, M.A. Early Osteoblastic Differentiation Induced by Dexamethasone Enhances Adenoviral Gene Delivery to Marrow Stromal Cells. J. Orthop. Res. 2004, 22, 411–416. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Choi, J.Y.; Lieberman, J.R.; Huang, J.; Zuk, P.A.; Zhang, J.; Hedrick, M.H.; Benhaim, P. Bone Induction byBMP-2 Transduced Stem Cells Derived from Human Fat. J. Orthop. Res. 2003, 21, 622–629. [Google Scholar] [CrossRef]
- Laitinen, M.; Jortikka, L.; Halttunen, T.; Nevalainen, J.; Aho, A.J.; Marttinen, A.; Lindholm, T.S. Measurement of Total and Local Bone Morphogenetic Protein Concentration in Bone Tumours. Int. Orthop. 1997, 21, 188–193. [Google Scholar] [CrossRef]
- Jo, J.-I.; Tabata, Y. Non-Viral Gene Transfection Technologies for Genetic Engineering of Stem Cells. Eur. J. Pharm. Biopharm. 2008, 68, 90–104. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Rettig, W.J.; Lane, J.M.; Takaoka, K.; Alderman, E.; Rup, B.; Rosen, V.; Healey, J.; Huvos, A.G.; Garin-Chesa, P. Immunohistochemical Detection of Bone Morphogenetic Proteins in Bone and Soft-Tissue Sarcomas. Cancer 1994, 74, 842–847. [Google Scholar] [CrossRef]
- Luo, J.; Sun, M.; Kang, Q.; Peng, Y.; Jiang, W.; Luu, H.; Luo, Q.; Park, J.; Li, Y.; Haydon, R. Gene Therapy for Bone Regeneration. Curr. Gene Ther. 2005, 5, 167–179. [Google Scholar] [CrossRef]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of Nanomaterials for Bone-Targeted Drug Delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef]
- Park, J.; Lutz, R.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Neukam, F.W.; Schlegel, K.A. The Effect on Bone Regeneration of a Liposomal Vector to Deliver BMP-2 Gene to Bone Grafts in Peri-Implant Bone Defects. Biomaterials 2007, 28, 2772–2782. [Google Scholar] [CrossRef]
- Patel, Z.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual Delivery of an Angiogenic and an Osteogenic Growth Factor for Bone Regeneration in a Critical Size Defect Model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of Local Sequential VEGF and BMP-2 Delivery on Ectopic and Orthotopic Bone Regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable Dual Growth Factor Delivery from Polyelectrolyte Multilayer Films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef]
- Tian, X.-B.; Sun, L.; Yang, S.-H.; Fu, R.-Y.; Wang, L.; Lu, T.-S.; Zhang, Y.-K.; Fu, D.-H. Ectopic Osteogenesis of Mouse Bone Marrow Stromal Cells Transfected with BMP 2/VEGF165genesin Vivo. Orthop. Surg. 2009, 1, 322–325. [Google Scholar] [CrossRef]
- Guo-Ping, W.; Xiao-Chuan, H.; Zhi-Hui, Y.; Li, G. Influence on the Osteogenic Activity of the Human Bone Marrow Mesenchymal Stem Cells Transfected by Liposome-Mediated Recombinant Plasmid pIRES-hBMP2-hVEGF165 In Vitro. Ann. Plast. Surg. 2010, 65, 80–84. [Google Scholar] [CrossRef]
- Xu, X.; Yang, J.; Ding, L.; Li, J. Bone Morphogenetic Protein-2-Encapsulated Grafted-Poly-Lactic Acid–Polycaprolactone Nanoparticles Promote Bone Repair. Cell Biophys. 2014, 71, 215–225. [Google Scholar] [CrossRef]
- Jiang, Q.-H.; Liu, L.; Shen, J.-W.; Peel, S.; Yang, G.-L.; Zhao, S.-F.; He, F.-M. Influence of Multilayer rhBMP-2 DNA Coating on the Proliferation and Differentiation of MC3T3-E1 Cells Seeded on Roughed Titanium Surface. J. Biomed. Mater. Res. Part A 2012, 100A, 2766–2774. [Google Scholar] [CrossRef]
- Jiang, Q.-H.; Liu, L.; Peel, S.; Yang, G.-L.; Zhao, S.-F.; He, F.-M. Bone Response to the Multilayer BMP-2 Gene Coated Porous Titanium Implant Surface. Clin. Oral Implant. Res. 2011, 24, 853–861. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Molenberg, A.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. PEG Matrix Enables Cell-Mediated Local BMP-2 Gene Delivery and Increased Bone Formation in a Porcine Critical Size Defect Model of Craniofacial Bone Regeneration. Clin. Oral Implant. Res. 2011, 23, 805–813. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Molenberg, A.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Critical Size Defect Regeneration Using PEG-Mediated BMP-2 Gene Delivery and the Use of Cell Occlusive Barrier Membranes-the Osteopromotive Principle Revisited. Clin. Oral Implant. Res. 2012, 24, 910–920. [Google Scholar] [CrossRef]
- Kroczek, A.; Park, J.; Birkholz, T.; Neukam, F.; Wiltfang, J.; Kessler, P. Effects of Osteoinduction on Bone Regeneration in Distraction: Results of a Pilot Study. J. Cranio-Maxillofac. Surg. 2009, 38, 334–344. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Poundarik, A.A.; Cabral, J.M.S.; Da Silva, C.L.; Vashishth, D. Biomimetic Matrices for Rapidly Forming Mineralized Bone Tissue Based on Stem Cell-Mediated Osteogenesis. Sci. Rep. 2018, 8, 14388. [Google Scholar] [CrossRef]
- Ono, I.; Yamashita, T.; Jin, H.-Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Nakasu, M.; Ogawa, T.; Jimbow, K. Combination of Porous Hydroxyapatite and Cationic Liposomes as a Vector for BMP-2 Gene Therapy. Biomaterials 2004, 25, 4709–4718. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Z.; Song, J.; Li, M.; Li, W. Evaluation of BMP-2 Enhances the Osteoblast Differentiation of Human Amnion Mesenchymal Stem Cells Seeded on Nano-Hydroxyapatite/Collagen/Poly(l-Lactide). Int. J. Mol. Sci. 2018, 19, 2171. [Google Scholar] [CrossRef]
- Jian, C.-X.; Fan, Q.-S.; Hu, Y.-H.; He, Y.; Li, M.-Z.; Zheng, W.-Y.; Ren, Y.; Li, C.-J. Effects of rhBMP-2 Gene Transfection to Periodontal Ligament Cells on Osteogenesis. Biosci. Rep. 2017, 37, BSR20160585. [Google Scholar] [CrossRef]
- Wu, G.; He, X.; Yang, Z.; Guo, L. Influence of Liposome-Mediated Recombinant Plasmid PIRES-HBMP-2-HVEGF165 on Osteogenic Activity of HBMSCs in Vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi Chin. J. Reparative Reconstr. Surg. 2009, 23, 1124–1128. [Google Scholar]
- Wang, Z.; Xu, X.; Yang, J.; Ding, L.; Li, J. Bone Morphogenetic Protein-2-Encupsulated PEG-Grafted-Poly-Lactic Acid-PolyCaprolactone Nanoparticles Promote Bone Repair. Zhonghua Yi Xue Za Zhi 2015, 95, 865–869. [Google Scholar] [PubMed]
- Thorwarth, M.; Schlegel, K.A.; Wiltfang, J.; Rupprecht, S.; Park, J.H. Experimentelle Untersuchung zur Oberflächenaktivierung von Implantaten Durch Liposomale Vektoren—eine Pilotstudie. Mund- Kiefer-und Gesichtschirurgie 2004, 8, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Mühle, C.; Franke, O.; Park, J.; Jehle, M.; Durst, K.; Göken, M.; Hennig, F.; Von Der Mark, K.; Schneider, H. Cell-Based Resurfacing of Large Cartilage Defects: Long-Term Evaluation of Grafts from Autologous Transgene-Activated Periosteal Cells in a Porcine Model of Osteoarthritis. Arthritis Care Res. 2008, 58, 475–488. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).