Experimental Investigation and Exergy Analysis of Dehumidification Performances for a Cascaded Phase Change Heat Storage Dehumidifier
Abstract
:1. Introduction
2. Exergy Analysis of Cascaded Phase Change Heat Storage
2.1. Analysis of Optimum Phase Change Temperature at all Stages
2.2. Analysis of Exergy Efficiency of Thermal Fluid
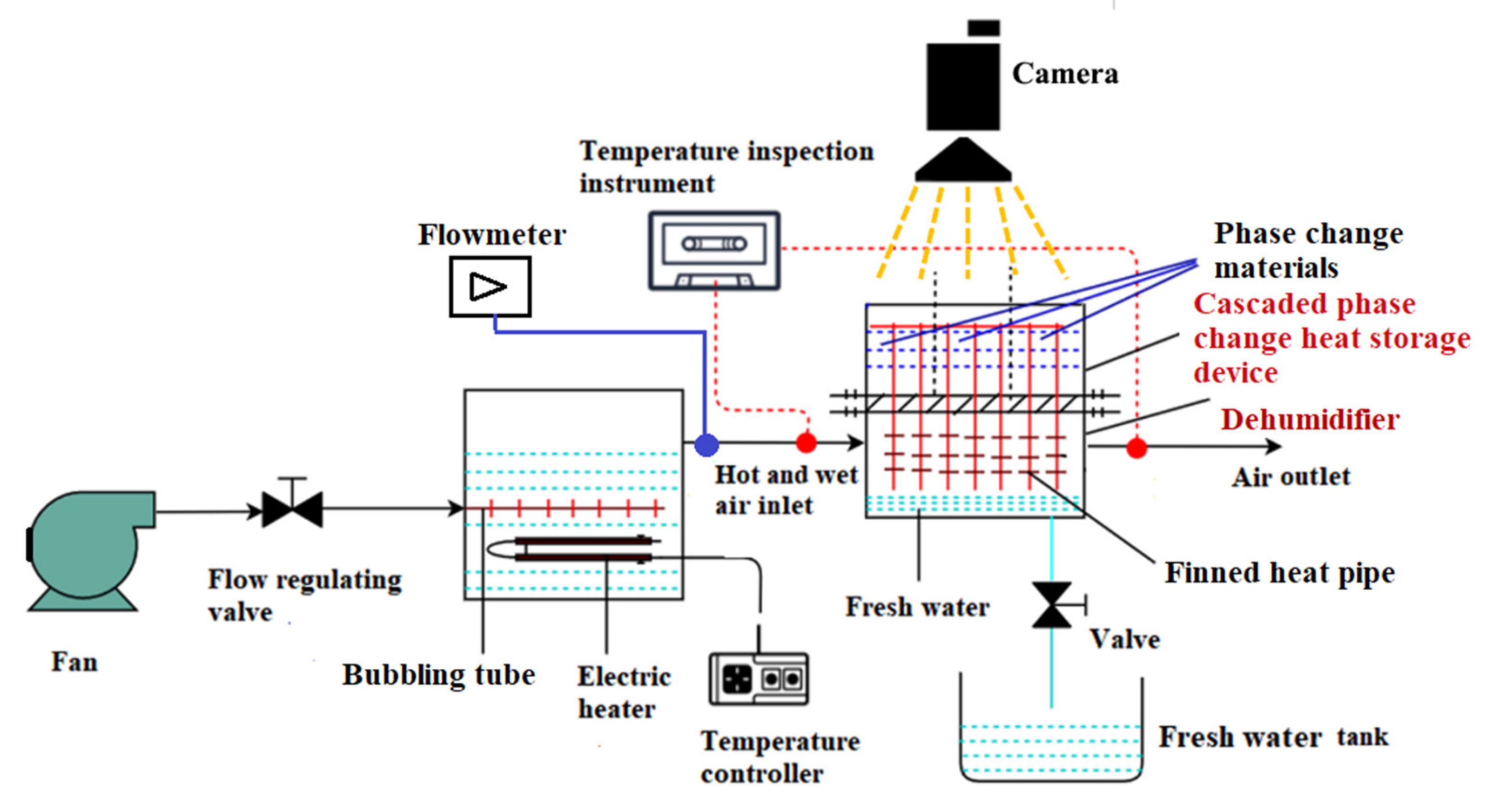
3. Cascaded Phase Change Heat Storage Dehumidifier and Its Experimental System
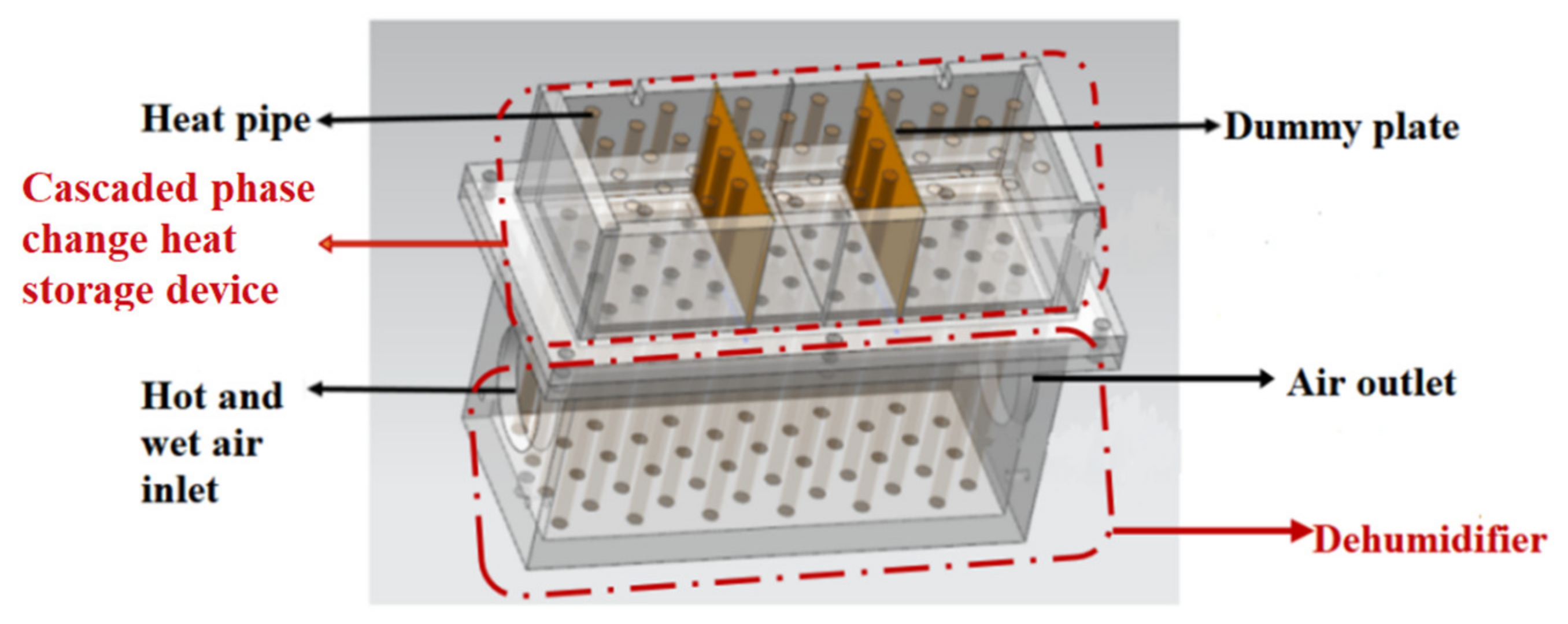
3.1. Structure of Cascaded Phase Change Heat Storage Dehumidifier
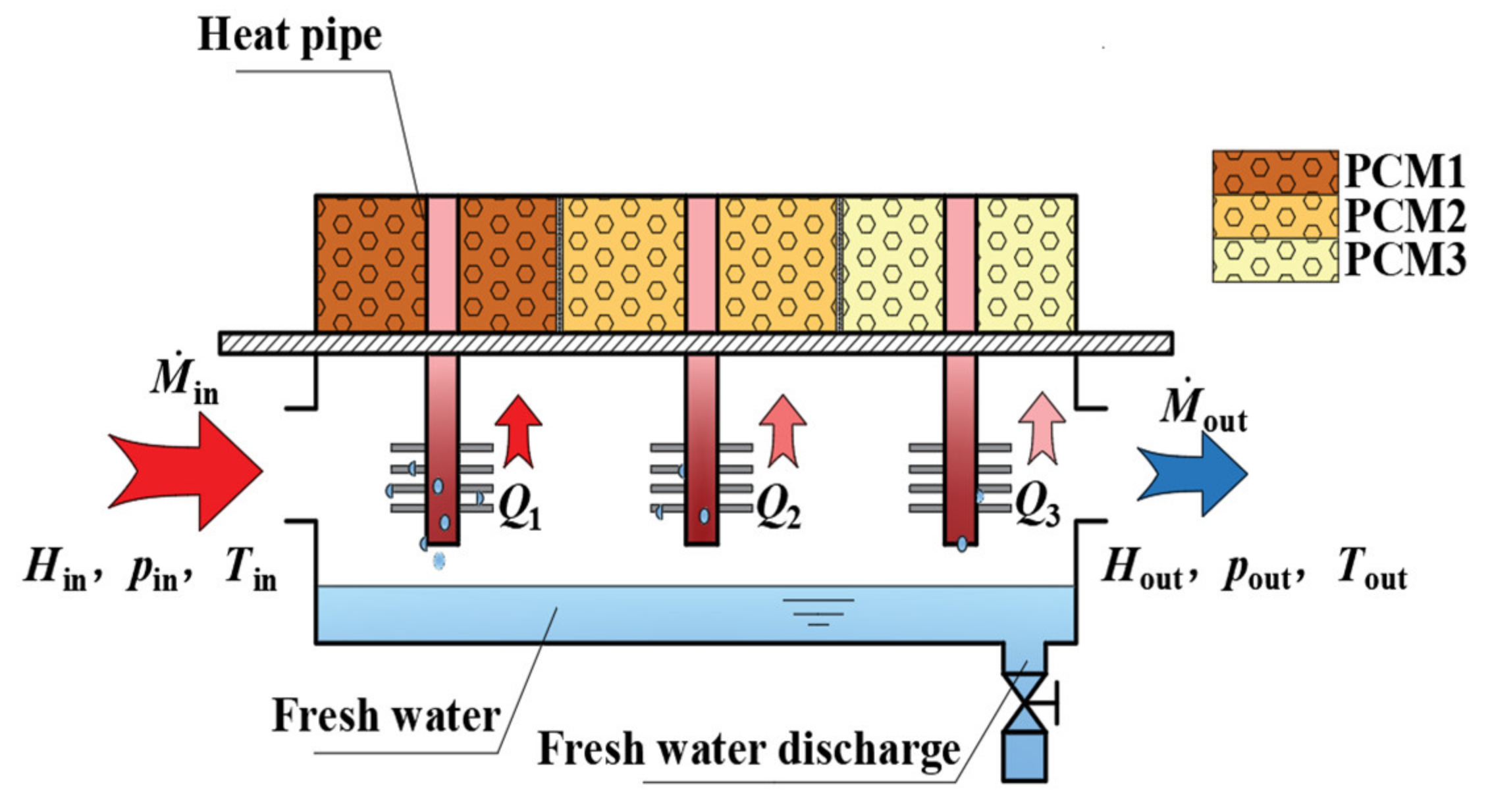
3.2. Working Principle of Cascaded Phase Change Heat Storage Dehumidifier
3.3. Experimental System
3.4. Definition of Experimental Performance Parameters
4. Experimental Results and Analysis
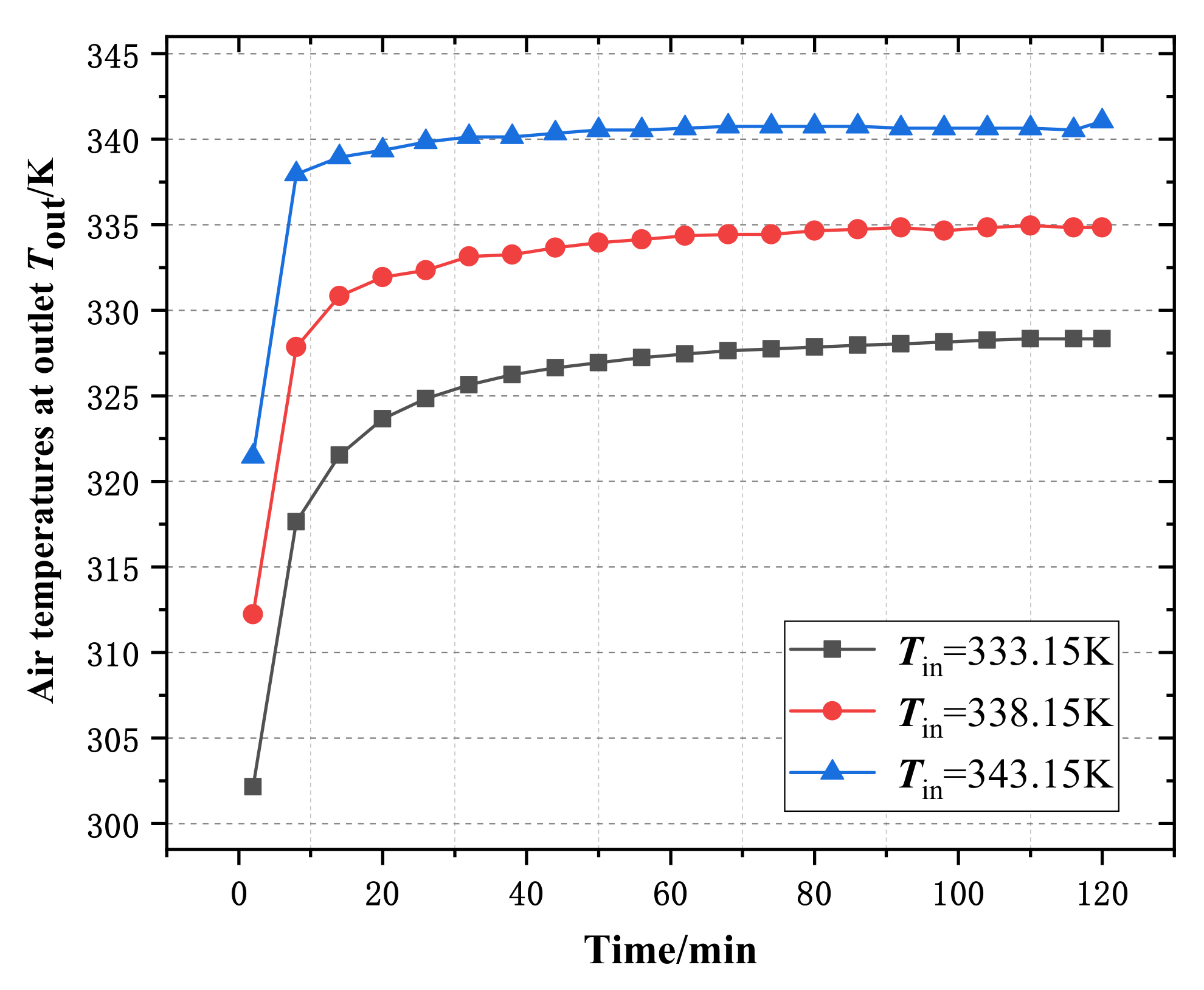
4.1. Influences of Wet Air Temperature at Dehumidifier Inlet on Experimental Results
4.1.1. Influence of Wet Air Temperatures at Inlet on Outlet of Dehumidifier
4.1.2. Effect of Wet Air Temperature at Dehumidifier Inlet on Temperature Field of Phase Change Heat Storage
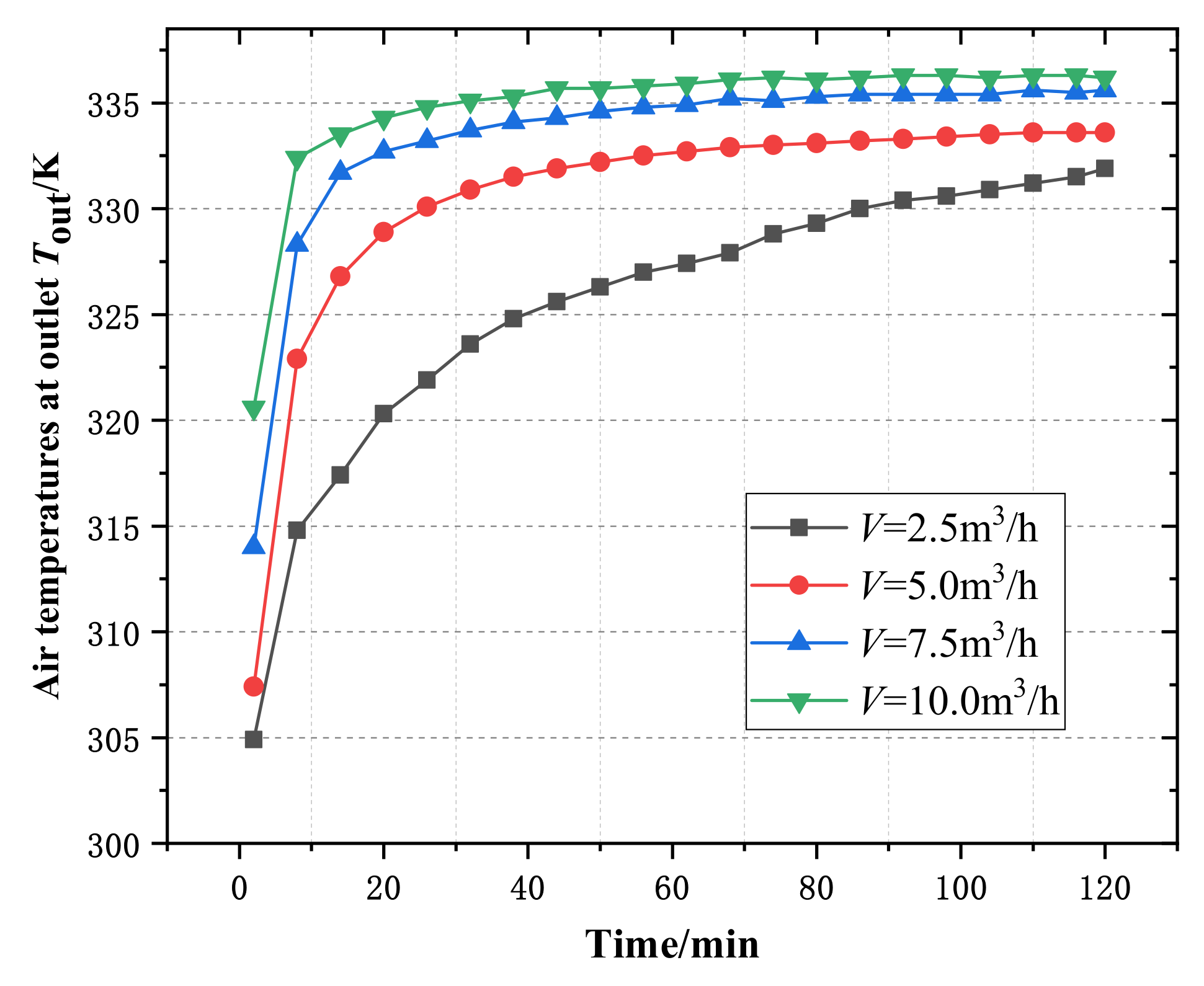
4.2. Influences of Wet Air Flow Rate at Dehumidifier Inlet on Experimental Results
4.2.1. Influence of Inlet Wet Air Flow Rate of Dehumidifier on Outlet Air Temperature
4.2.2. Effect of Inlet Wet Air Flow of Dehumidifier on Temperature Field of Phase Change Heat Storage
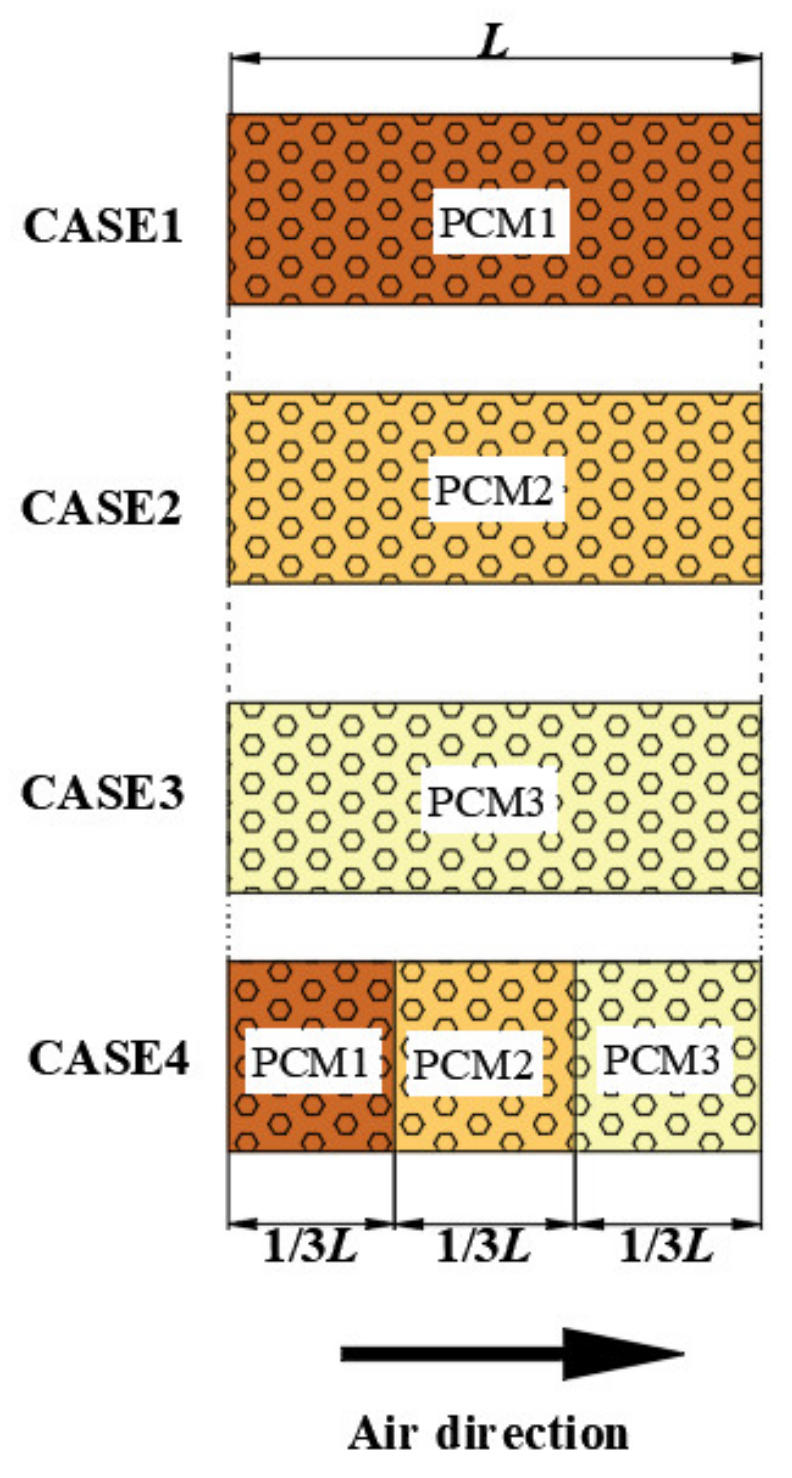
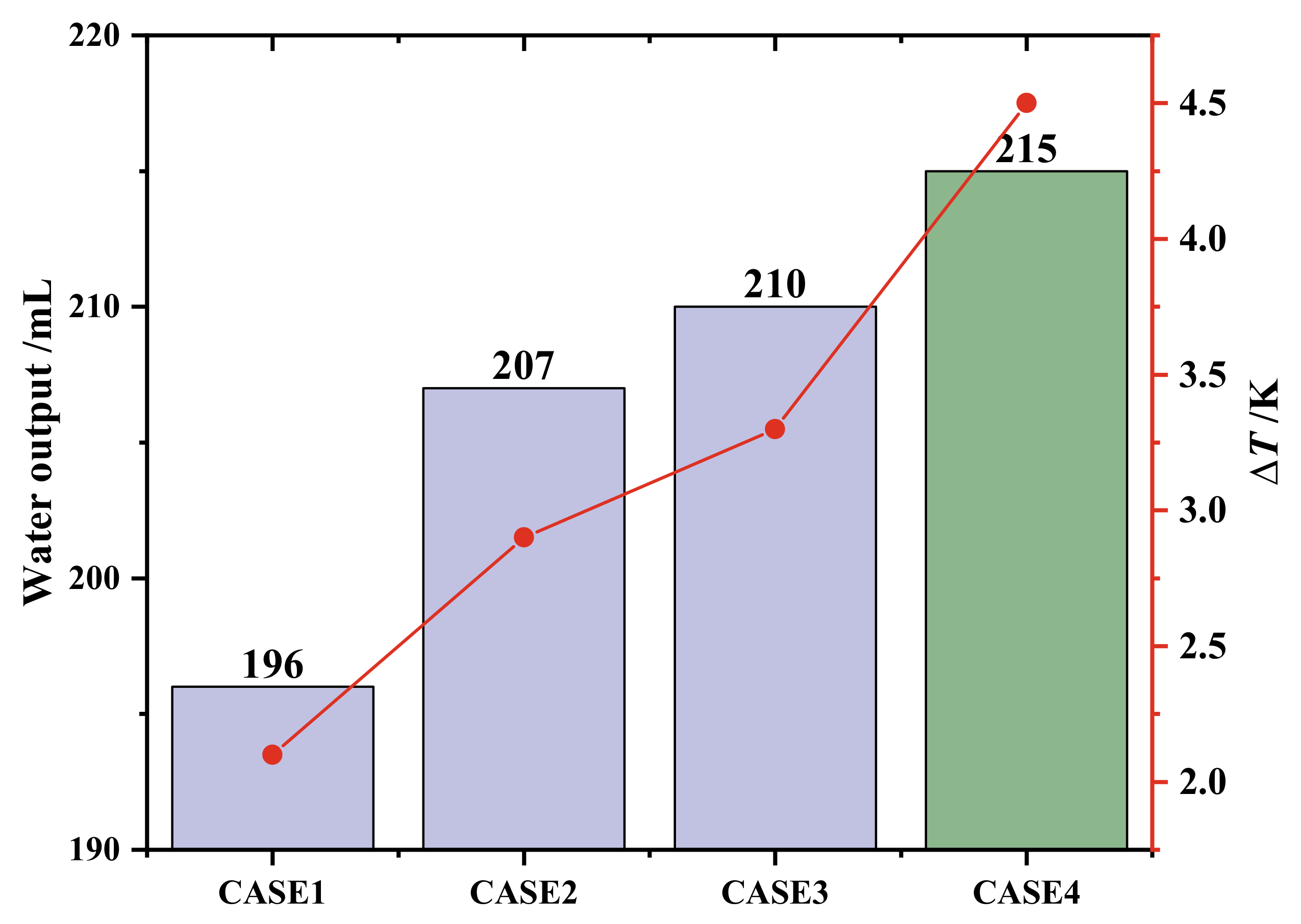
4.3. Comparison of Cascaded Number Layout of PCMs
5. Conclusions
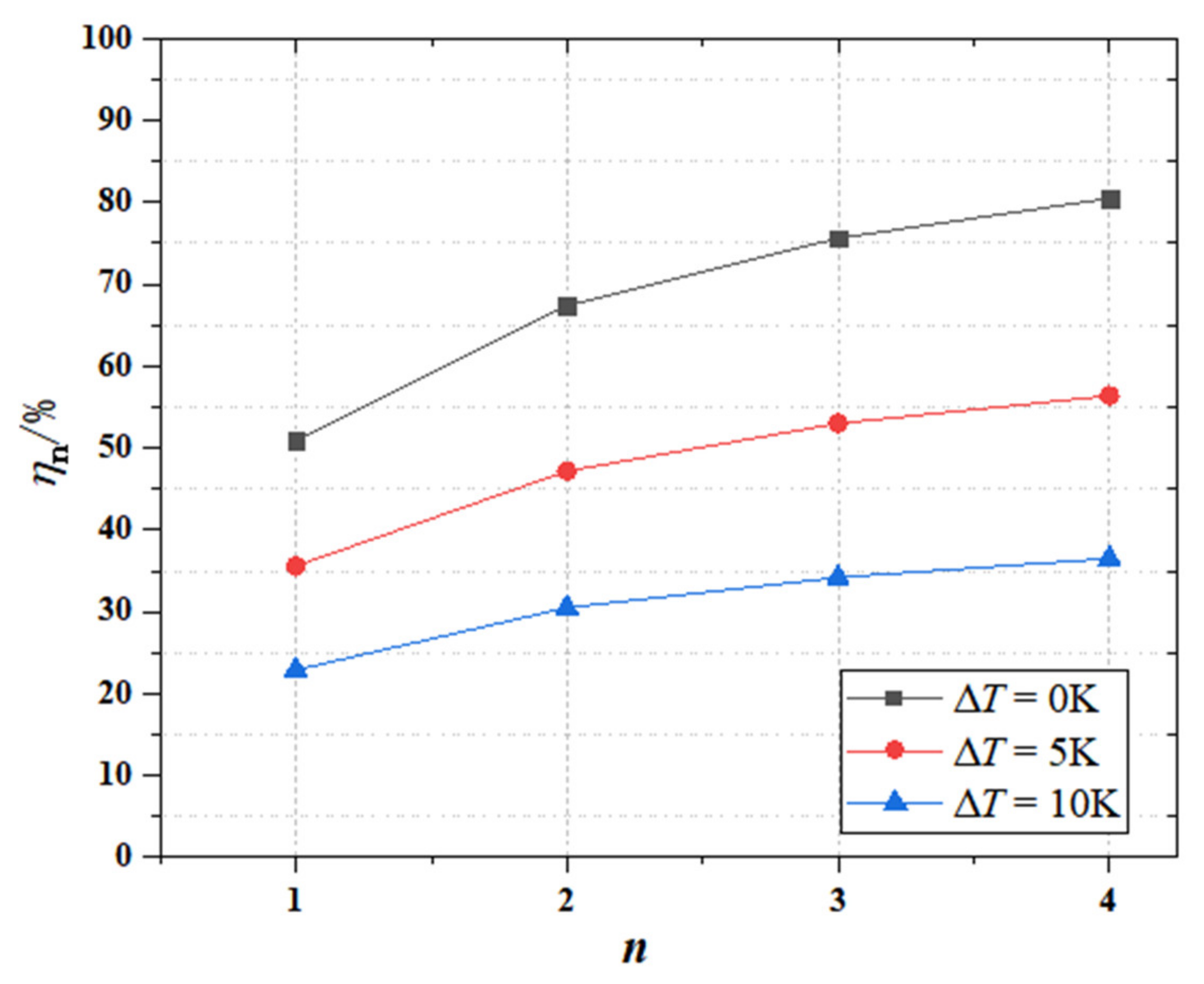
- The more stages of phase change materials exit in the cascaded phase change heat storage, the greater the exergy efficiency will be, but the relationship between them is not linear. The increase rate of the exergy efficiency decreases with the increase of the stage numbers. Considering the above factors, the complexity of a multi-stage phase change heat storage device and the difficulty of selecting phase change materials, it is recommended to select the number of phase change material stages as three. When the ∆T is 5 K, and the n is equal to 3, the can reach a maximum of 53.0%.
- With the increase of the temperature and volume flow of the wet air at the inlet of the dehumidifier, the time required for the air temperature at the outlet of the dehumidifier to reach stability is shortened, and the heat transfer capacity of the phase change heat storage dehumidifier is enhanced.
- The cascaded layout of PCMs can improve the water production performance of a dehumidifier. When the hot and wet air temperature and the volume flow rate at the inlet of the dehumidifier are 338.15 K and 5.0 m3/h, compared with dehumidifiers with three kinds of PCM single-stage layouts, the GOR of the dehumidifier with a PCM three-stage layout is increased by 9.69%, 3.86% and 2.40%, and the WPC is reduced by 8.82%, 3.69% and 2.31%, respectively. If the heat in the phase change heat storage device is used for the secondary water output, the water output and GOR will be increased by 25%, and the WPC will be reduced by 20%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahdi, J.M.; Lohrasbi, S.; Nsofor, E.C. Hybrid heat transfer enhancement for latent-heat thermal energy storage systems: A review. Int. J. Heat Mass Transf. 2019, 137, 630–649. [Google Scholar] [CrossRef]
- Al-Maghalseh, M.; Mahkamov, K. Methods of heat transfer intensification in PCM thermal storage systems: Review paper. Renew. Sustain. Energy Rev. 2018, 92, 62–94. [Google Scholar] [CrossRef]
- Christopher, S.; Parham, K.; Mosaffa, A.H.; Farid, M.M.; Ma, Z.; Thakur, A.K.; Xu, H.; Saidur, R. A critical review on phase change material energy storage systems with cascaded configurations. J. Clean. Prod. 2021, 283, 124653. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Elsheikh, A.H.; Hammad, F.A.; Ma, L.; Du, Y.; Kabeel, A.E.; Shalaby, S.M. Applications of cascaded phase change materials in solar water collector storage tanks: A review. Sol. Energy Mater. Sol. Cells 2019, 199, 24–49. [Google Scholar] [CrossRef]
- Farid, M.M.; Kanzawa, A. Thermal performance of a heat storage module using pcm’s with different melting temperatures: Mathematical modeling. J. Sol. Energy Eng. Trans. ASME 1989, 111, 152–157. [Google Scholar] [CrossRef]
- Xu, Y.; He, Y.-L.; Li, Y.-Q.; Song, H.-J. Exergy analysis and optimization of charging–discharging processes of latent heat thermal energy storage system with three phase change materials. Sol. Energy 2016, 123, 206–216. [Google Scholar] [CrossRef]
- Li, B.; Zhai, X.; Cheng, X. Thermal performance analysis and optimization of multiple stage latent heat storage unit based on entransy theory. Int. J. Heat Mass Transf. 2019, 135, 149–157. [Google Scholar] [CrossRef]
- Khor, J.O.; Sze, J.Y.; Li, Y.; Romagnoli, A. Overcharging of a cascaded packed bed thermal energy storage: Effects and solutions. Renew. Sustain. Energy Rev. 2020, 117, 109421. [Google Scholar] [CrossRef]
- Shamsi, H.; Boroushaki, M.; Geraei, H. Performance evaluation and optimization of encapsulated cascade PCM thermal storage. J. Energy Storage 2017, 11, 64–75. [Google Scholar] [CrossRef]
- Zhao, Y.; You, Y.; Liu, H.B.; Zhao, C.Y.; Xu, Z.G. Experimental study on the thermodynamic performance of cascaded latent heat storage in the heat charging process. Energy 2018, 157, 690–706. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, Y.; Lin, J.; Zhou, T.; Liu, Y.; Zhao, Q. Improvement of the efficiency of solar thermal energy storage systems by cascading a PCM unit with a water tank. J. Clean. Prod. 2020, 245, 118864. [Google Scholar] [CrossRef]
- Hassanpour, A.; Borji, M.; Ziapour, B.M.; Kazemi, A. Performance analysis of a cascade PCM heat exchanger and two-phase closed thermosiphon: A case study of geothermal district heating system. Sustain. Energy Technol. Assess. 2020, 40, 100755. [Google Scholar] [CrossRef]
- Cheng, X.; Zhai, X. Thermal performance analysis and optimization of a cascaded packed bed cool thermal energy storage unit using multiple phase change materials. Appl. Energy 2018, 215, 566–576. [Google Scholar] [CrossRef]
- Majumdar, R.; Saha, S.K. Computational study of performance of cascaded multi-layered packed-bed thermal energy storage for high temperature applications. J. Energy Storage 2020, 32, 101930. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Yao, Y.; Wu, H. Visualized experiment on solid-liquid phase change heat transfer enhancement with multiple PCMs. J. Chem. Ind. Eng. 2019, 70, 1263–1271. [Google Scholar]
- Zhu, Y.; Wang, D.; Li, P.; Yuan, Y.; Tan, H. Optimization of exergy efficiency of a cascaded packed bed containing variable diameter particles. Appl. Therm. Eng. 2021, 188, 116680. [Google Scholar] [CrossRef]
- Riahi, S.; Liu, M.; Jacob, R.; Belusko, M.; Bruno, F. Assessment of exergy delivery of thermal energy storage systems for CSP plants: Cascade PCMs, graphite-PCMs and two-tank sensible heat storage systems. Sustain. Energy Technol. Assess. 2020, 42, 100823. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Hashim, E.T.; Talebizadehsardari, P.; Nsofor, E.C. Solidification enhancement with multiple PCMs, cascaded metal foam and nanoparticles in the shell-and-tube energy storage system. Appl. Energy 2020, 257, 113993. [Google Scholar] [CrossRef]
- Mawire, A.; Ekwomadu, C.S.; Lefenya, T.M.; Shobo, A. Performance comparison of two metallic eutectic solder based medium-temperature domestic thermal energy storage systems. Energy 2020, 194, 116828. [Google Scholar] [CrossRef]
- Suresh, C.; Saini, R. Experimental study on combined sensible-latent heat storage system for different volume fractions of PCM. Sol. Energy 2020, 212, 282–296. [Google Scholar]
- Cheng, X.; Zhai, X.; Zheng, C. Performance Analysis and Optimization of a Cascade PCM Cold Storage Heat Exchanger. J. Shanghai Jiao Tong Univ. 2016, 50, 1500–1505, 1513. [Google Scholar]
- Zheng, Z.; Xu, Y.; He, Y. Study on the Performance of a Shell-and-Tube Latent-Heat Storage Unit Enhanced by Porous Medium with Graded Porosity. J. Eng. Thermophys. 2019, 40, 605–611. [Google Scholar]
- Ahmed, N.; Elfeky, K.; Lu, L.; Wang, Q. Thermal performance analysis of thermocline combined sensible-latent heat storage system using cascaded-layered PCM designs for medium temperature applications. Renew. Energy 2020, 152, 684–697. [Google Scholar] [CrossRef]
- Elfeky, K.E.; Li, X.; Ahmed, N.; Lu, L.; Wang, Q. Optimization of thermal performance in thermocline tank thermal energy storage system with the multilayered PCM(s) for CSP tower plants. Appl. Energy 2019, 243, 175–190. [Google Scholar] [CrossRef]
- Tehrani, S.S.M.; Shoraka, Y.; Nithyanandam, K.; Taylor, R.A. Cyclic performance of cascaded and multi-layered solid-PCM shell-and-tube thermal energy storage systems: A case study of the 19.9 MWe Gemasolar CSP plant. Appl. Energy 2018, 228, 240–253. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, Y. Thermal energy storage performance of a three-PCM cascade tank in a high-temperature packed bed system. Renew. Energy 2020, 152, 110–119. [Google Scholar] [CrossRef]
- Hu, F.; Lu, D.; Zhao, P.; Chen, Z. Thermodynamic analysis on optimum phase change temperature for multiple phase change materials. J. Chem. Ind. Eng. 2013, 64, 2322–2327. [Google Scholar]
- Hamed, M.H.; Omara, Z.M.; Sharshir, S.W.; Kabeel, A.E. Mathematical and experimental investigation of a solar humidification-dehumidification desalination unit. Desalination 2015, 358, 9–17. [Google Scholar] [CrossRef]
- Khayet, M. Solar desalination by membrane distillation: Dispersion in energy consumption analysis and water production costs (a review). Desalination 2013, 308, 89–101. [Google Scholar] [CrossRef]
- Zhang, H.F. Principle of Solar Thermal Utilization and Computer Simulation, 2nd ed.; Northwestern Polytechnical University Press: Xi’an, China, 2012; pp. 222–223. [Google Scholar]





| Stage Number i | |||||
|---|---|---|---|---|---|
| n = 1 | n = 2 | n = 3 | n = 4 | ||
| 0 | 1 | 312.79 | 317.83 | 320.38 | 321.92 |
| 2 | — | 307.80 | 312.79 | 315.80 | |
| 3 | — | — | 305.38 | 309.81 | |
| 4 | — | — | — | 303.90 | |
| 5 | 1 | 310.40 | 314.59 | 316.71 | 317.99 |
| 2 | — | 306.26 | 310.40 | 312.91 | |
| 3 | — | — | 304.21 | 307.91 | |
| 4 | — | — | — | 302.99 | |
| 10 | 1 | 307.99 | 311.34 | 313.03 | 314.05 |
| 2 | — | 304.67 | 307.99 | 309.99 | |
| 3 | — | — | 303.03 | 305.99 | |
| 4 | — | — | — | 302.05 | |
| PCM | Phase | Phase Change Temperature/K | Melting Latent Heat/(kJ/kg) |
|---|---|---|---|
| PCM1 | Solid | 325.15 | 210.3 |
| PCM2 | Solid | 323.15 | 179.0 |
| PCM3 | Solid | 321.15 | 160.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jia, Y.; Fan, Z.; Wang, K. Experimental Investigation and Exergy Analysis of Dehumidification Performances for a Cascaded Phase Change Heat Storage Dehumidifier. Appl. Sci. 2022, 12, 1303. https://doi.org/10.3390/app12031303
Zhang L, Jia Y, Fan Z, Wang K. Experimental Investigation and Exergy Analysis of Dehumidification Performances for a Cascaded Phase Change Heat Storage Dehumidifier. Applied Sciences. 2022; 12(3):1303. https://doi.org/10.3390/app12031303
Chicago/Turabian StyleZhang, Lixi, Yi Jia, Zhida Fan, and Kangbo Wang. 2022. "Experimental Investigation and Exergy Analysis of Dehumidification Performances for a Cascaded Phase Change Heat Storage Dehumidifier" Applied Sciences 12, no. 3: 1303. https://doi.org/10.3390/app12031303
APA StyleZhang, L., Jia, Y., Fan, Z., & Wang, K. (2022). Experimental Investigation and Exergy Analysis of Dehumidification Performances for a Cascaded Phase Change Heat Storage Dehumidifier. Applied Sciences, 12(3), 1303. https://doi.org/10.3390/app12031303





