A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation and Growth Conditions
2.2. Carotenogenesis
2.3. Techniques of Enumeration of H. pluvialis and Growth Cell Monitoring
2.4. HPLC Analysis
2.5. Data Analysis
3. Results
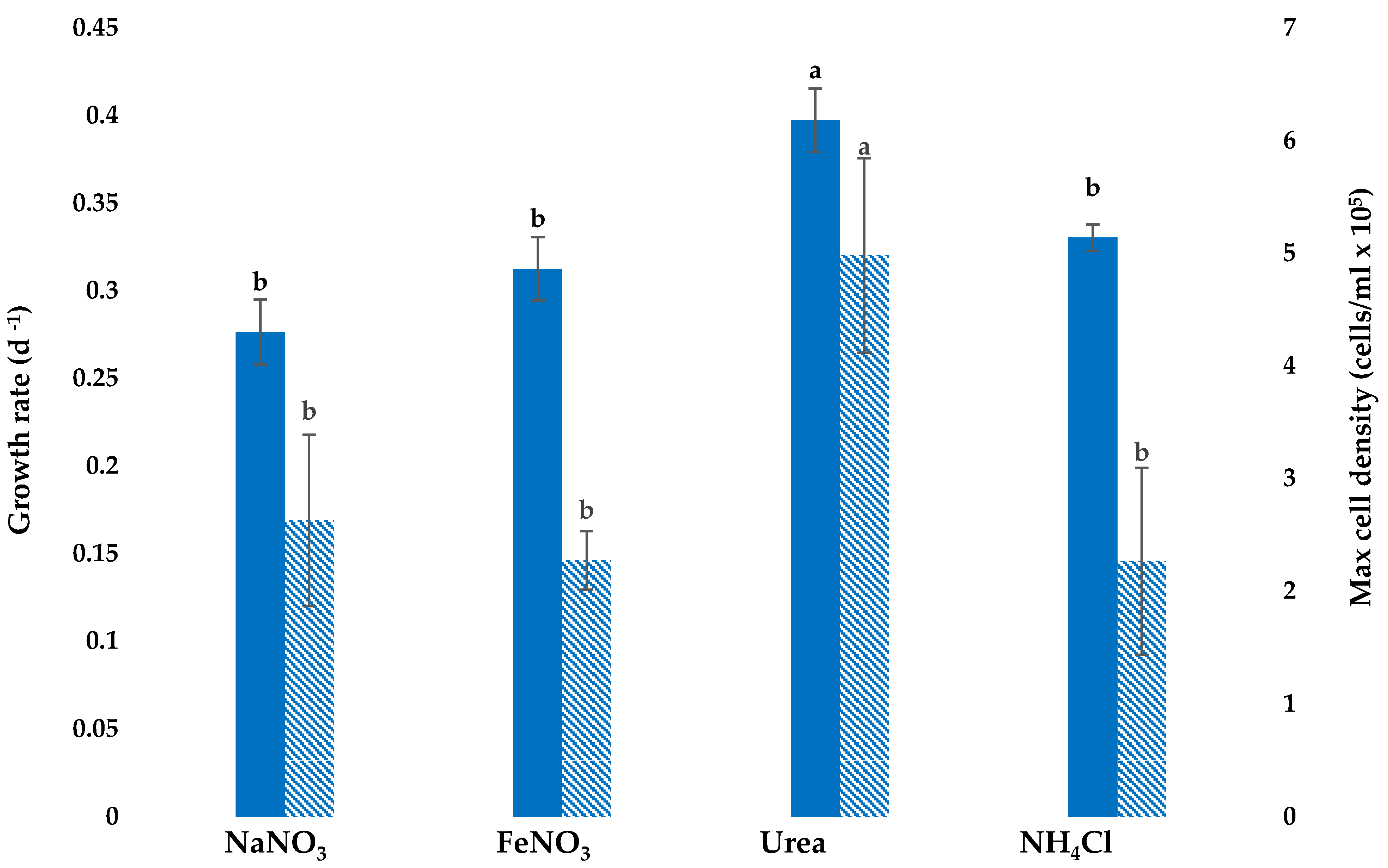
3.1. Growth with Different Nitrogen Sources
3.2. Assessment of Cell Density Following Application of Stress Conditions
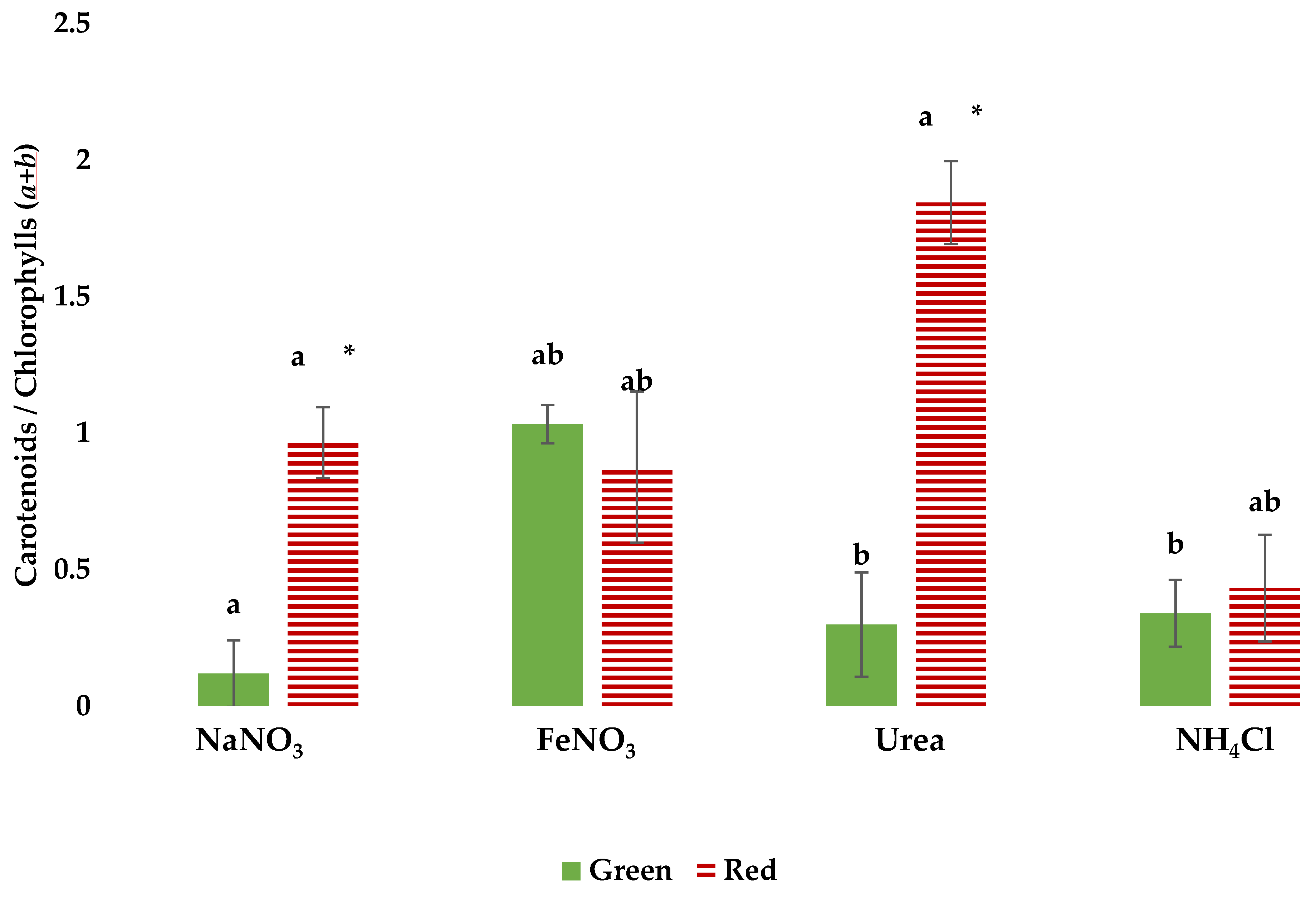
3.3. Pigment Profiles in Green and Red Growth Phases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colusse, G.A.; Duarte, M.E.R.; de Carvalho, J.C.; Noseda, M.D. Production of astaxanthin by Haematococcus pluvialis: Lab processes to scale up including the cost considerations. In Global Perspectives on Astaxanthin; Ravishankar, G.A., Rao, A.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 121–130. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs Natural Production. In Chemical and Biomolecular Engineering Publications and Other Works; The University of Tennessee Libraries: Knoxville, TN, USA, 2013. [Google Scholar]
- Li, F. Accumulation of Astaxanthin Was Improved by the Nonmotile Cells of Haematococcus Pluvialis. BioMed Res. Int. 2019, 2019, 8101762. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Sun, H.; Kong, Q.; Geng, Z.; Duan, L.; Yang, M.; Guan, B. Enhancement of Cell Biomass and Cell Activity of Astaxanthin-Rich Haematococcus Pluvialis. Bioresour. Technol. 2015, 186, 67–73. [Google Scholar] [CrossRef]
- Capelli, B.; Talbott, S.; Ding, L. Astaxanthin Sources: Suitability for Human Health and Nutrition. Funct. Foods Health Dis. 2019, 9, 430–445. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as Versatile Cellular Factories for Valued Products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus Pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Ahuja, K.; Rawat, A. Astaxanthin Market Size by Source (Synthetic, Natural), by Application (Dietary Supplement, Personal Care, Pharmaceuticals, Food & Beverages, Animal Feed Aquaculture, Livestock, Pets) Industry Outlook Report, Regional Analysis. Application Potential. Available online: https://www.marketresearch.com/One-Off-Global-Market-Insights-v4130/Astaxanthin-Size-Source-Synthetic-Natural-14839588 (accessed on 20 January 2022).
- Grand View Research. Functional Foods Market Size, Share & Trends Analysis Report by Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, and Segment Forecasts. 2019. Available online: https://www.researchandmarkets.com/reports/4764576/functional-foods-market-size-share-and-trends (accessed on 20 January 2022).
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A New Paradigm for Producing Astaxanthin from the Unicellular Green Alga Haematococcus Pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus Pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial Astaxanthin Production Derived by Green Alga Haematococcus Pluvialis: A Microalgae Process Model and a Techno-Economic Assessment All through Production Line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Geng, Y.H.; Li, Z.K.; Hu, H.J.; Li, Y.G. Production of Astaxanthin from Haematococcus in Open Pond by Two-Stage Growth One-Step Process. Aquaculture 2009, 295, 275–281. [Google Scholar] [CrossRef]
- Kiperstok, A.C.; Sebestyén, P.; Podola, B.; Melkonian, M. Biofilm cultivation of Haematococcus Pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res. 2017, 21, 213–222. [Google Scholar] [CrossRef]
- Suh, I.S.; Joo, H.-N.; Lee, C.-G. A Novel Double-Layered Photobioreactor for Simultaneous Haematococcus Pluvialis Cell Growth and Astaxanthin Accumulation. J. Biotechnol. 2006, 125, 540–546. [Google Scholar] [CrossRef]
- Aflalo, C. On the Relative Efficiency of Two- vs. One-stage Production of Astaxanthin by the Green Alga Haematococcus Pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the Production of Astaxanthin by Mixotrophic Cultivation of Haematococcus Pluvialis in Open Raceway Ponds. Aquac. Int. 2020, 28, 625–638. Available online: https://link.springer.com/article/10.1007/s10499-019-00483-2 (accessed on 20 January 2022). [CrossRef]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome Analysis in Haematococcus Pluvialis: Astaxanthin Induction by High Light with Acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced astaxanthin accumulation in Haematococcus Pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef]
- Tocquin, P.; Fratamico, A.; Franck, F. Screening for a Low-Cost Haematococcus Pluvialis Medium Reveals an Unexpected Impact of a Low N/P Ratio on Vegetative Growth. J. Appl. Phycol. 2012, 24, 365–373. [Google Scholar] [CrossRef]
- Cerón, M.C.; García-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernandez, J.M.; del Rio, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef]
- González, M.A.; Cifuentes, A.S.; Gómez, P.I. Growth and total carotenoid content in four Chilean strains of Haematococcus pluvialis Flotow, under laboratory conditions. Gayana Bot. 2009, 66, 58–70. [Google Scholar] [CrossRef][Green Version]
- Butler, T.O.; McDougall, G.J.; Campbell, R.; Stanley, M.S.; Day, J.G. Media Screening for Obtaining Haematococcus Pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids. Biology 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of Stress on Astaxanthin Production in Haematococcus Pluvialis Grown under Different Culture Conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Méléder, V.; Barillé, L.; Launeau, P.; Carrère, V.; Rincé, Y. Spectrometric Constraint in Analysis of Benthic Diatom Biomass Using Monospecific Cultures. Remote Sens. Environ. 2003, 88, 386–400. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. (Eds.) Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; UNESCO Publishing: Paris, France, 1997. [Google Scholar]
- Clementson, L.A.; Wojtasiewicz, B. Dataset on the absorption characteristics of extracted phytoplankton pigments. Data Brief 2019, 24, 103875. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Pohnert, G. Rapid Estimation of Astaxanthin and the Carotenoid-to-Chlorophyll Ratio in the Green Microalga Chromochloris Zofingiensis Using Flow Cytometry. Mar. Drugs 2017, 15, 231. [Google Scholar] [CrossRef]
- Imamoglu, E.; Sukan, F.; Dalay, M.C. Effect of Different Culture Media and Light Intensities on Growth of Haematococcus Pluvialis. Int. J. Nat. Eng. Sci. 2007, 1, 5–9. [Google Scholar]
- Cifuentes, A.S.; González, M.A.; Vargas, S.; Hoeneisen, M.; González, N. Optimization of Biomass, Total Carotenoids and Astaxanthin Production in Haematococcus Pluvialis Flotow Strain Steptoe (Nevada, USA) under Laboratory Conditions. Biol. Res. 2003, 36, 343–357. [Google Scholar] [CrossRef]
- Göksan, T.; Ak, İ.; Kılıç, C. Growth Characteristics of the Alga Haematococcus Pluvialis Flotow as Affected by Nitrogen Source, Vitamin, Light and Aeration. Turk. J. Fish. Aquat. Sci. 2011, 11, 377–383. [Google Scholar] [CrossRef]
- The Dollar Business Bureau. Final Findings in the Anti-Dumping Investigation Concerning Imports of Sodium Nitrate Originating in or Exported from European Union, China PR, Ukraine and Korea RP-reg No.15/1009/2012. 2014. Available online: https://www.thedollarbusiness.com/news/anti-dumping-investigation-on-imports-of-sodium-nitrate-final-findings/9541 (accessed on 20 January 2022).
- Colusse, G.A.; Mendes, C.R.B.; Duarte, M.E.R.; de Carvalho, J.C.; Noseda, M.D. Effects of different culture media on physiological features and laboratory scale production cost of Dunaliella salina. Biotechnol. Rep. 2020, 27, e00508. [Google Scholar] [CrossRef]
- Rizal, A.; Yeasmin, F.; Hossain, A.; Akter, T.; Rahman, M. Replacement of sodium nitrate in Kosaric medium with urea for culture of Spirulina platensis. Intl. J. Fish. Aquat. Stud. 2017, 5, 403–408. [Google Scholar] [CrossRef]
- Uddin, A.F.M.J.; Ifaz, M.I.; Husna, M.A.; Sakib, I.; Rakibuzzaman, M. Comparative Growth Analysis of Spirulina platensis using Urea as a Nitrogen Substitute for NaNO3. Int. J. Bus. Soc. Sci. Res. 2020, 8, 76–80. [Google Scholar]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour. Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosynthesis and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Orosa, M. Analysis and enhancement of astaxanthin accumulation in Haematococcus Pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Liu, J. Exogenous sodium acetate enhances astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. J. Appl. Phycol. 2019, 31, 1001–1008. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Dong, C.D. Recent Advancements in Mixotrophic Bioprocessing for Production of High Value Microalgal Products. Bioresour. Technol. 2021, 320, 124421. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Lombardi, A.T. Growth, photosynthesis and biochemical composition of Haematococcus pluvialis at various pH. J. Algal Biomass Util. 2017, 8, 1–15. [Google Scholar]
- Sipaúba-Tavares, L.H.; Berchielli-Morais, F.A.; Scardoeli-Truzzi, B. Growth of Haematococcus pluvialis Flotow in alternative media. Braz. J. Biol. 2015, 75, 796–803. [Google Scholar] [CrossRef]
- Gwak, Y.; Hwang, Y.-S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.-G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef]
- Rastar, M. Effects of Iron and Zinc Concentrations on Growth Performance and Biochemical Composition of Haematococcus Pluvialis: A Comparison between Nanoparticles and Their Corresponding Metals Bulks. J. Algal Biomass Util. 2018, 9, 59–67. [Google Scholar]
- Cai, M.; Li, Z.; Qi, A. Effects of iron electrovalence and species on growth and astaxanthin production of Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2009, 27, 370. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current Trends and Future Prospects of Ammonia Removal in Wastewater: A Comprehensive Review on Adsorptive Membrane Development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological Production of Astaxanthin from the Microalga Haematococcus Pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef] [PubMed]
- Mascia, F.; Girolomoni, L.; Alcocer, M.J.P.; Bargigia, I.; Perozeni, F.; Cazzaniga, S.; Cerullo, G.; D’Andrea, C.; Ballottari, M. Functional Analysis of Photosynthetic Pigment Binding Complexes in the Green Alga Haematococcus Pluvialis Reveals Distribution of Astaxanthin in Photosystems. Sci. Rep. 2017, 7, 16319. [Google Scholar] [CrossRef]
- Elumalai, S.; Santhose, B.I.; Kanna, G.R. Extraction of Carotenoid and Thin Layer Chromatography (TLC), GC-MS, FT-IR and HPLC Analysis of Pharmaceutically Important Pigment Astaxanthin from a New Strain of Haematococcus Pluvialis. Wkly. Sci. Res. J. 2014, 2, 2321–7871. [Google Scholar] [CrossRef]
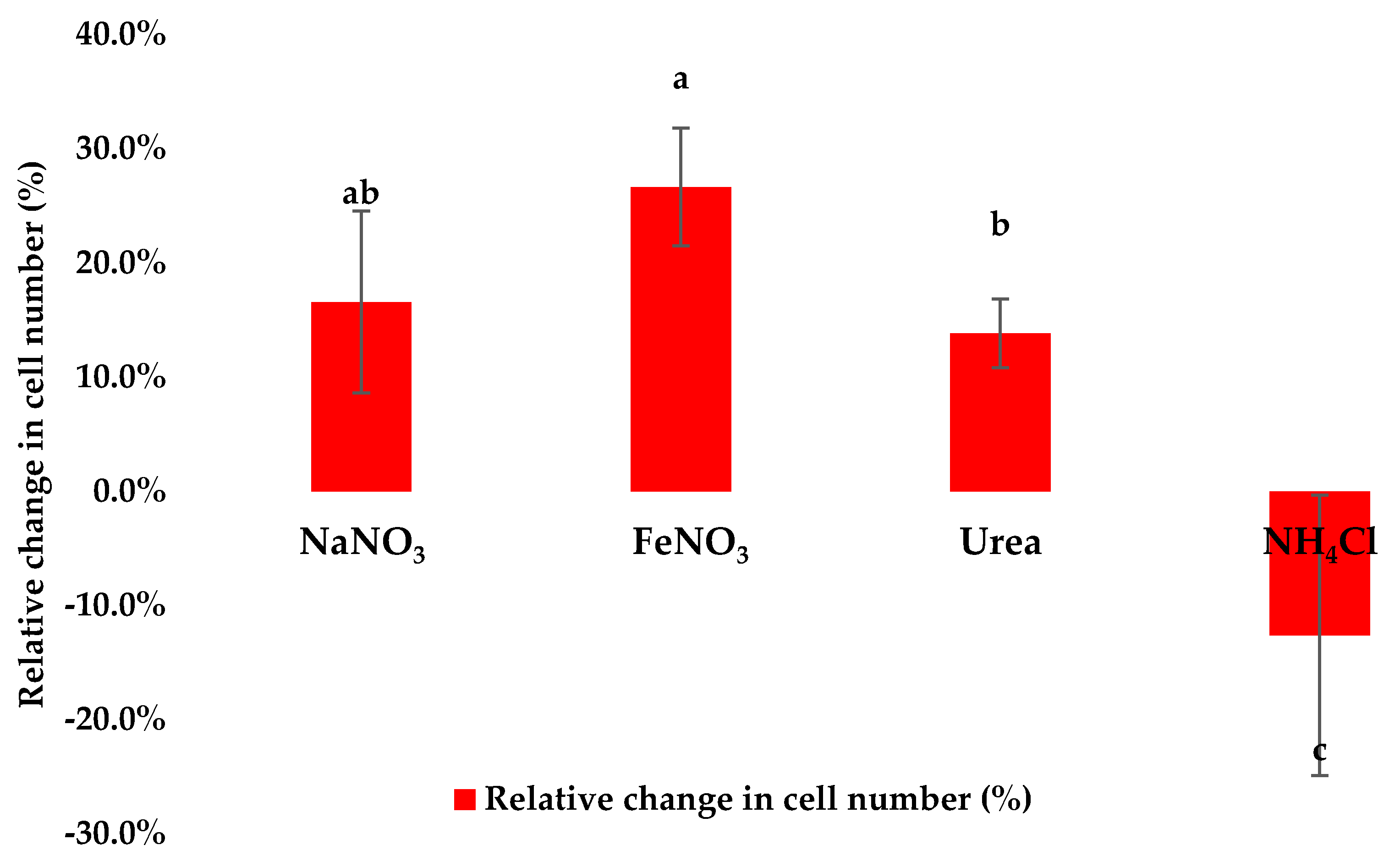
| Green | Red | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lutein † | Astaxanthin † | Chlorophyll a † | β-Carotene † | Pheophytin a † | Antheraxanthin † | Canthaxanthin | Carotenoid-like | Lutein † | Astaxanthin † | Chlorophyll a † | β-Carotene † | Pheophytin a † | Antheraxanthin † | |
| NaNO3 | 0.078 ± 0.022 a | 0.227 ± 0.177 a | 2682 ± 0.417 a,* | 0.017 ± 0.008 a | 0.376 ± 0.077 a | nd | 1.4 ± 0.85 a,* | 1.69 ± 1.16 a,* | 0.046 ± 0.022 a | 0.711 ± 0.143 a | 0.812 ± 0.115 a | 0.017 ± 0.008 a | 0.107 ± 0.018 a | 0.010 ± 0.003 a |
| FeNO3 | 0.068 ± 0.014 a | 2,714 ± 0.585 b,* | 2722 ± 0.353 a,* | 0.031 ± 0.000 a | 1083 ± 0.041 b,* | nd | 1.87 ± 0.68 a,* | 2.26 ± 0.32 a,* | 0.076 ± 0.03 a | 1353 ± 0.366 b | 1664 ± 0.347 b | 0.029 ± 0.013 a | 0.232 ± 0.056 a | nd |
| Urea | 0.278 ± 0.229 a,* | Nd * | 2212 ± 0.823 a,* | 0.090 ± 0.087 a | 0.325 ± 0.079 a | 0.294 ± 0.302 a | nd | 3.09 ± 0.63 a,* | 0.003 ± 0.002 a | 0.313 ± 0.008 a | 0.181 ± 0.068 c | 0.003 ± 0.000 a | nd | 0.015 ± 0.006 a |
| NH4Cl | 0.072 ± 0.016 a | 0.299 ± 0.055 a | 1132 ± 0.579 b | 0.013 ± 0.008 a | 0.165 ± 0.023 a | nd | nd | 0.39 ± 0 a,* | 0.043 ± 0.04 a | 0.486 ± 0.233 a | 1396 ± 0.996 b | 0.023 ± 0.011 a | 0.560 ± 0.518 a | 0.052 ± 0.033 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, A.; Ross, M.E.; Norici, A.; Jesus, B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Appl. Sci. 2022, 12, 1261. https://doi.org/10.3390/app12031261
Rizzo A, Ross ME, Norici A, Jesus B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Applied Sciences. 2022; 12(3):1261. https://doi.org/10.3390/app12031261
Chicago/Turabian StyleRizzo, Arianna, Michael E. Ross, Alessandra Norici, and Bruno Jesus. 2022. "A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis" Applied Sciences 12, no. 3: 1261. https://doi.org/10.3390/app12031261
APA StyleRizzo, A., Ross, M. E., Norici, A., & Jesus, B. (2022). A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Applied Sciences, 12(3), 1261. https://doi.org/10.3390/app12031261







