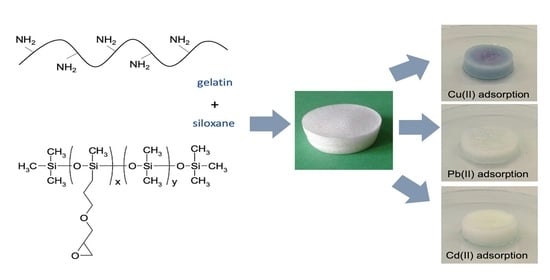
Gelatin–Siloxane Hybrid Monoliths as Novel Heavy Metal Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
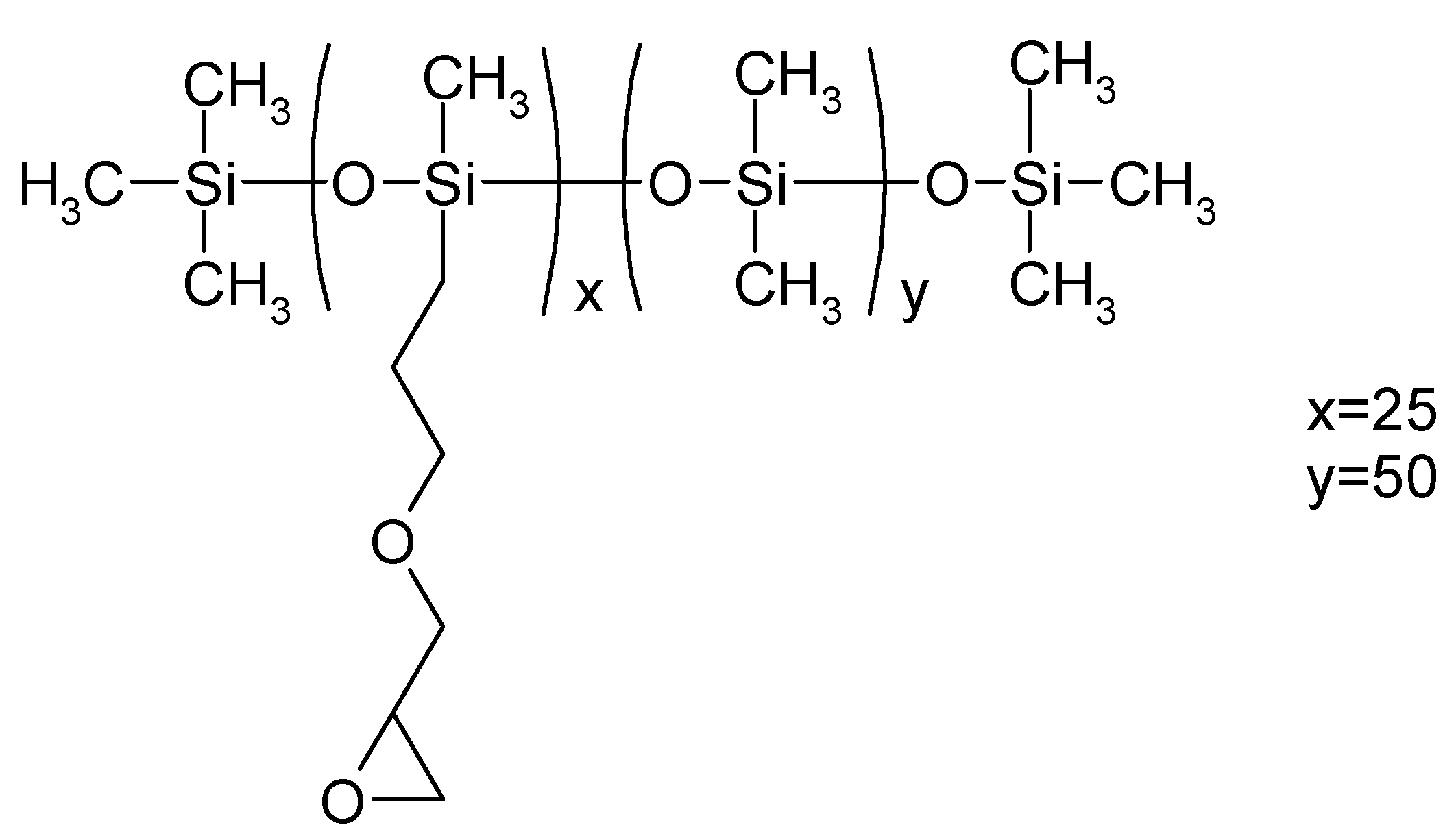
2.2. Gelatin–Siloxane Porous Hybrids Preparation
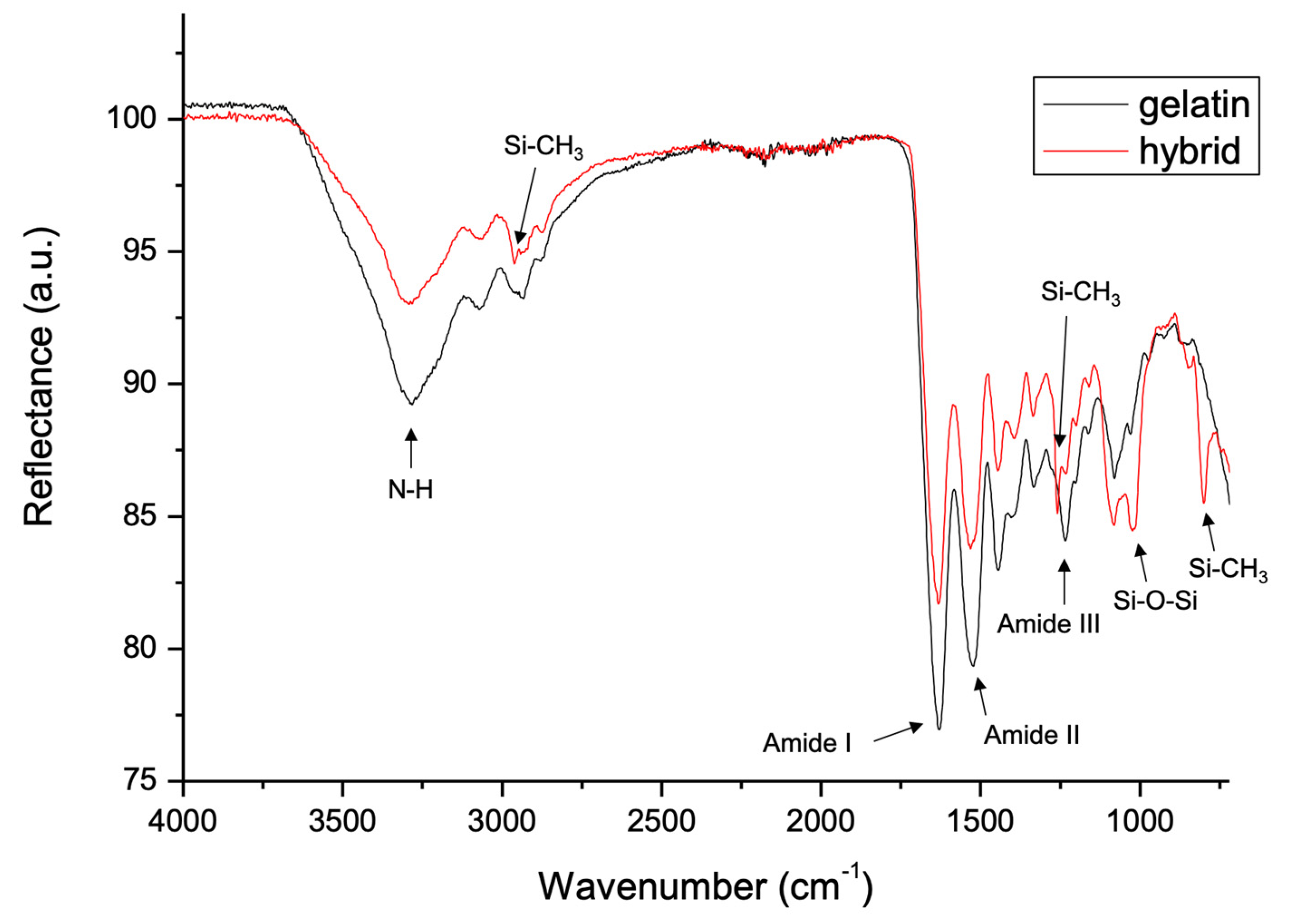
2.3. FT-IR Analysis
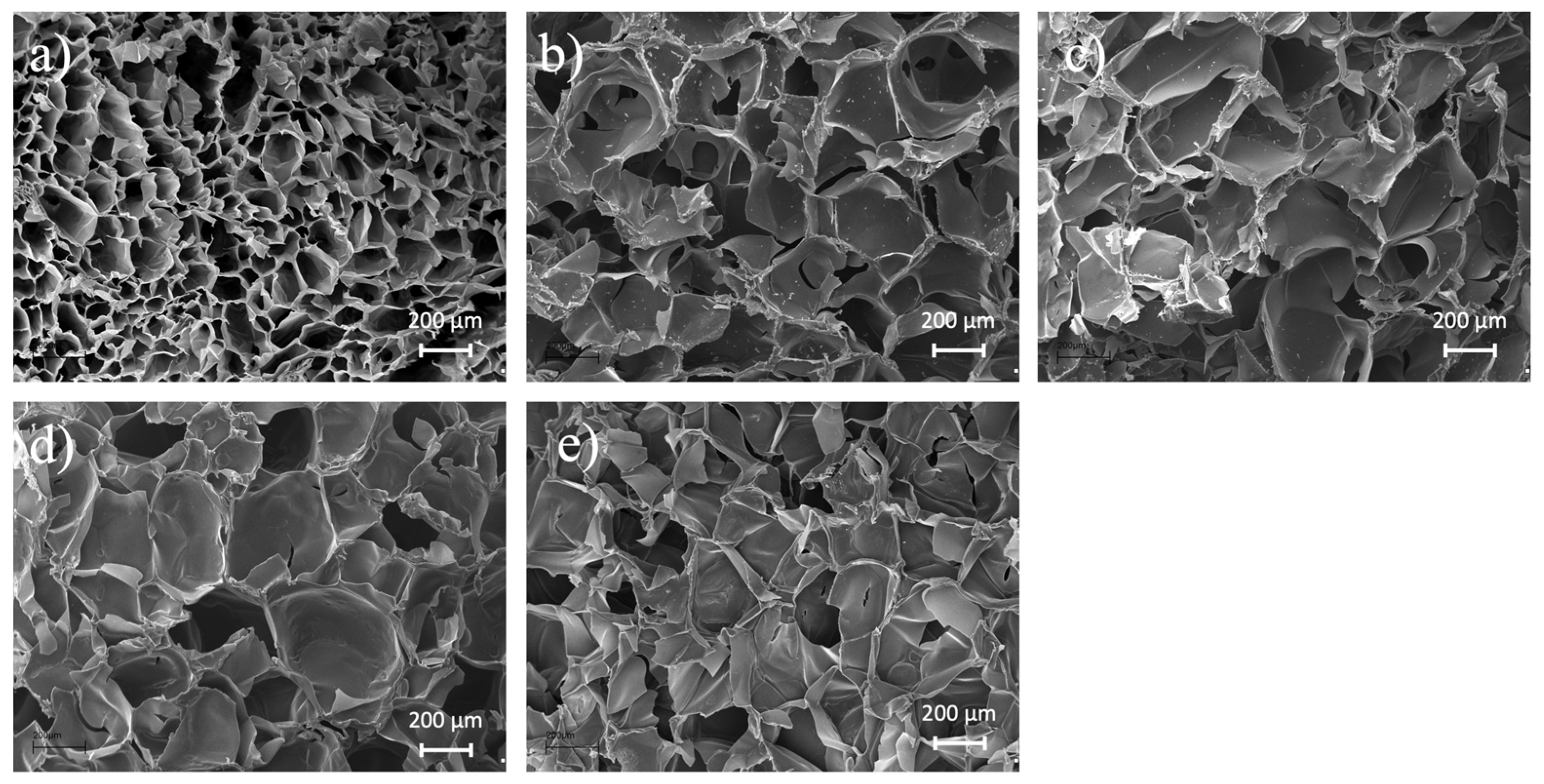
2.4. SEM Analysis
2.5. Compressive Strength of Gelatin and Gelatin–Siloxane Monoliths
2.6. The Water Absorption
2.7. Adsorption of Metal Ions
2.8. Investigation of pH Changes during Water Absorption
2.9. Desorption
3. Results and Discussion
3.1. FT-IR Analysis of Gelatin and Gelatin–Siloxane Monoliths
3.2. SEM Analysis of Gelatin and Gelatin-Siloxane Monoliths
3.3. Compressive Strength Results
3.4. The Water Absorption
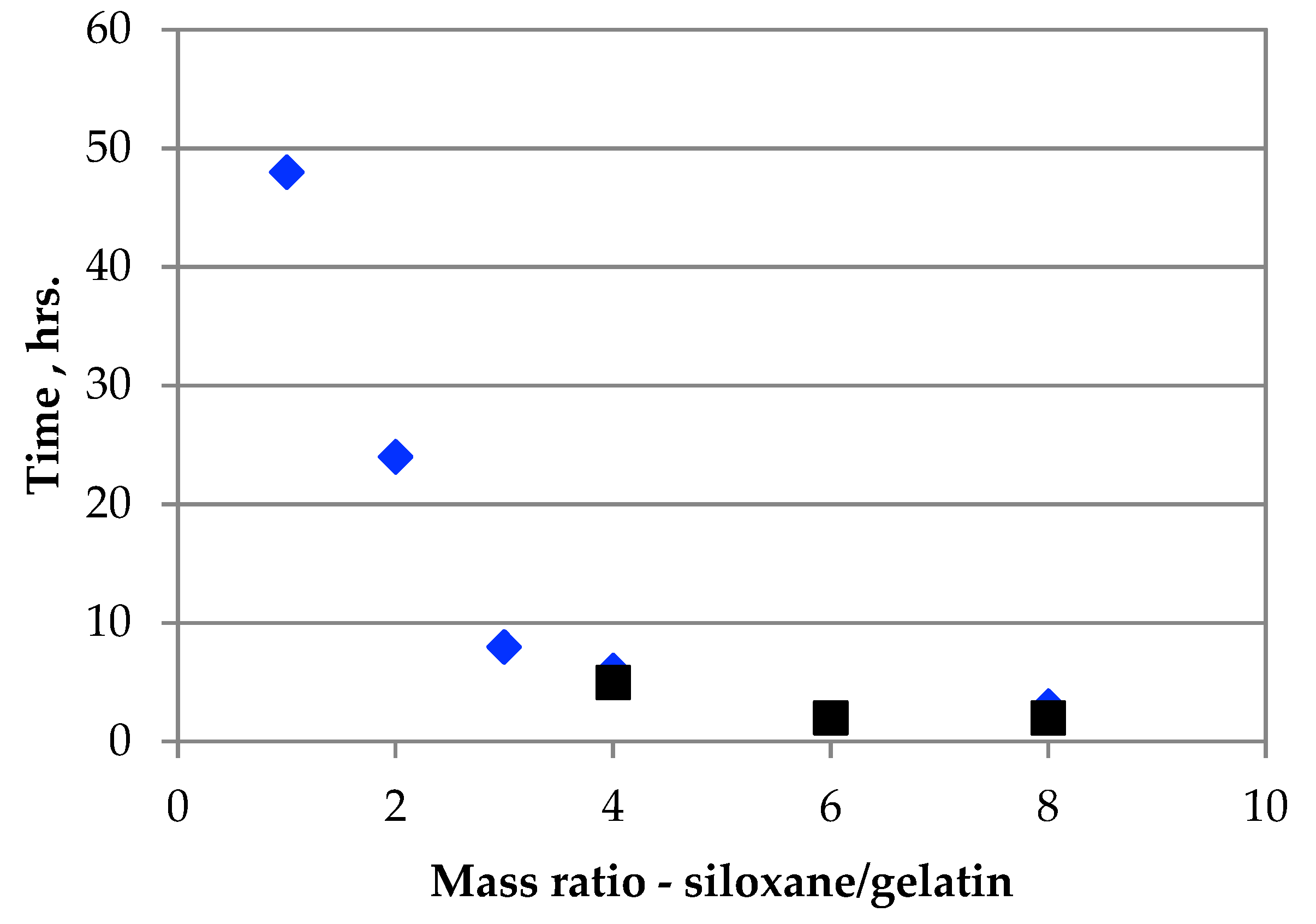
3.5. Resistance of Gelatin Beads to Shaking in Water Solutions
3.6. The Adsorption of Heavy Metals (Cu2+, Pb2+, Cd2+) on the Gelatin Monoliths
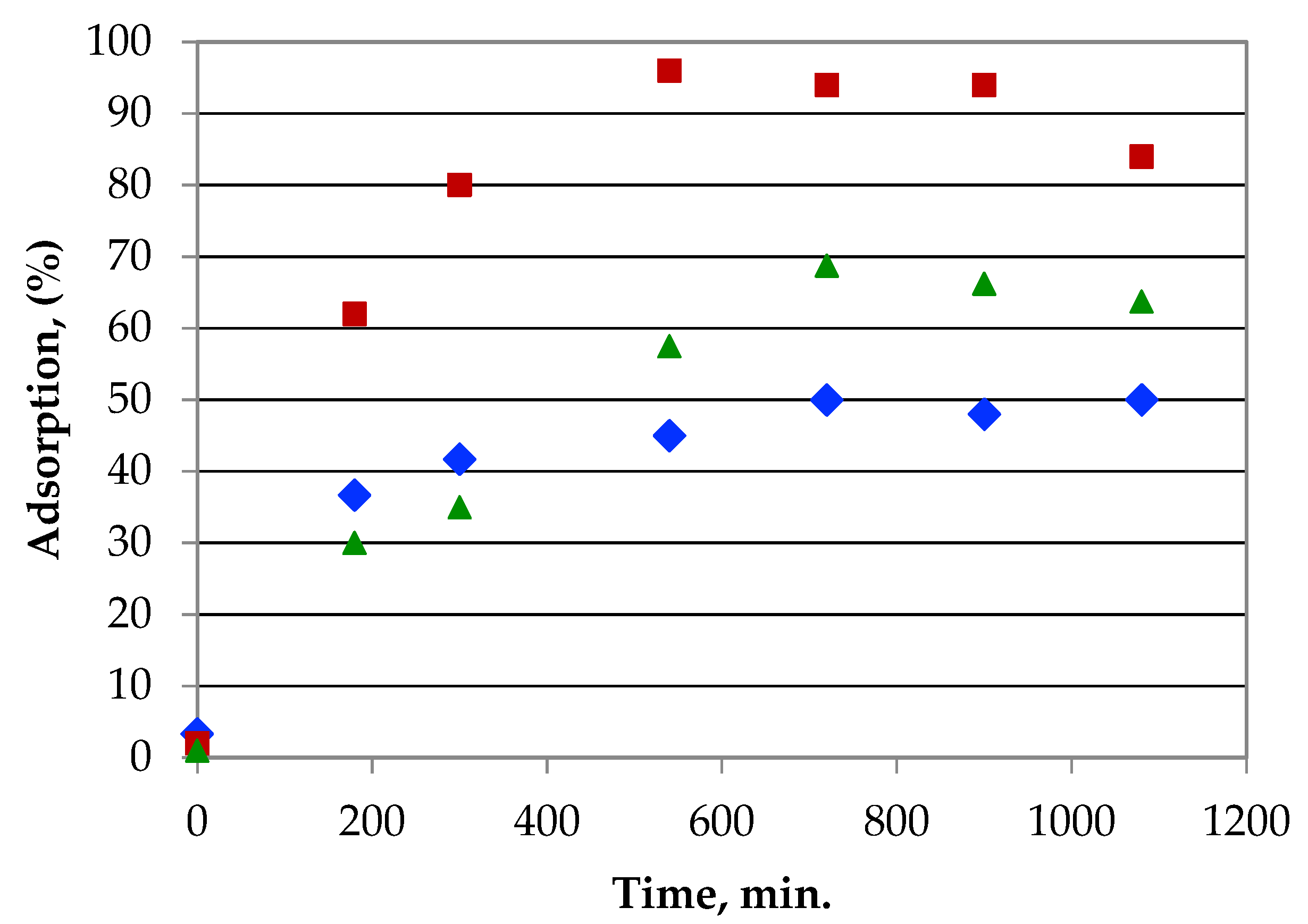
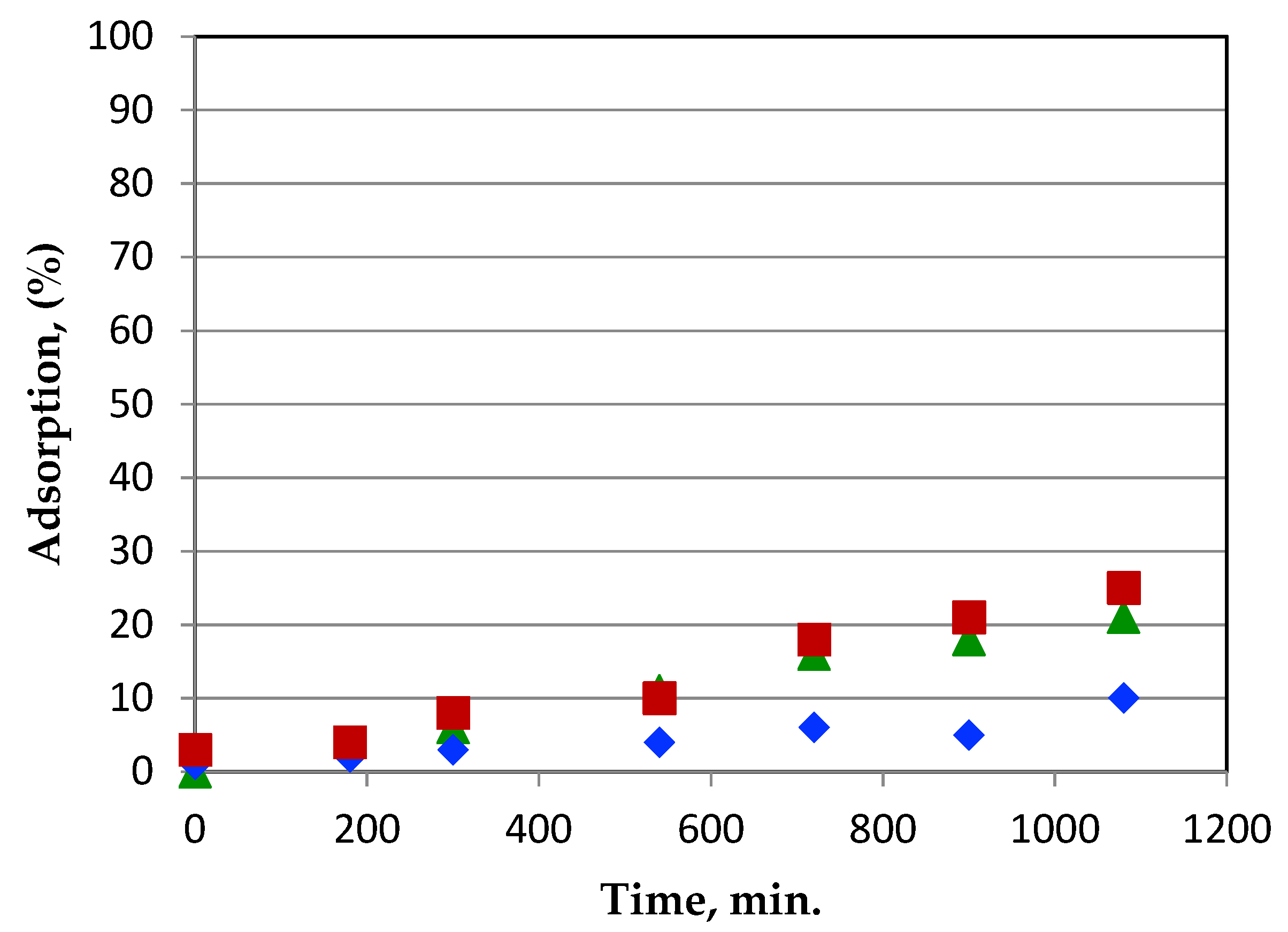
3.6.1. Effect of Contact Time
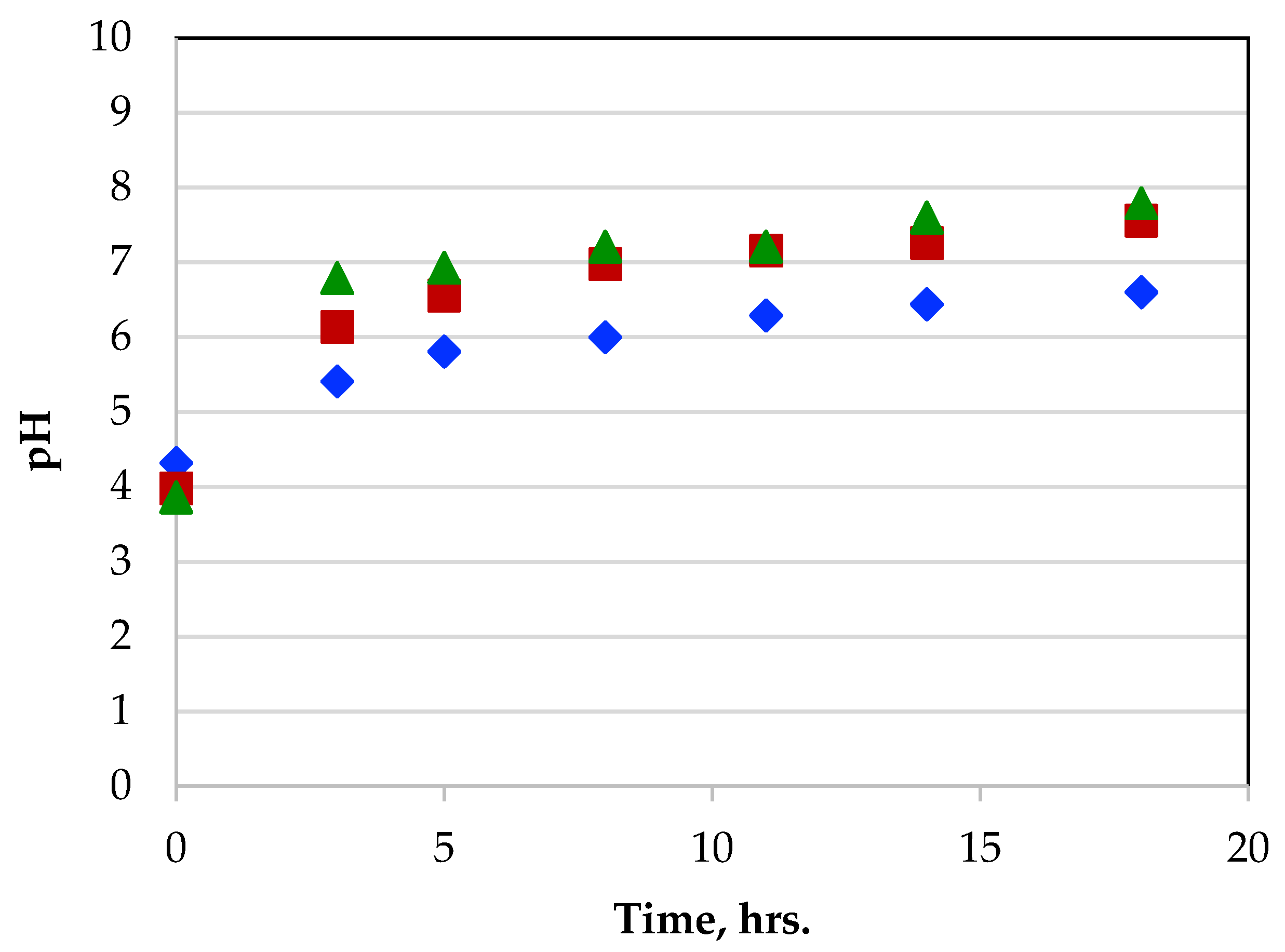
3.6.2. pH Changes during Metal Adsorption
3.6.3. Adsorption Kinetics of Metal Ions on Gelatin–Siloxane Monoliths
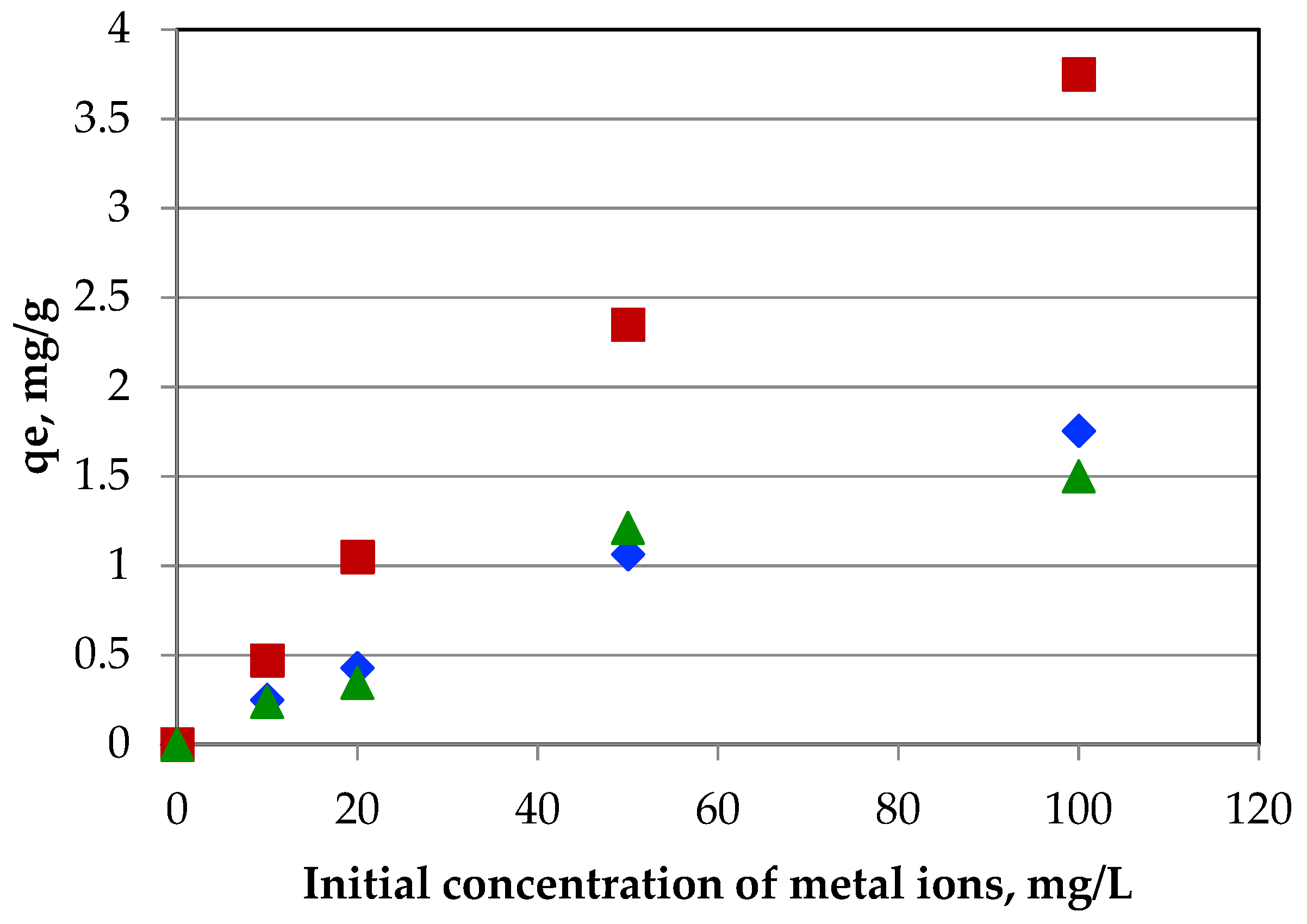
3.6.4. The Effect of Metal Ions Concentration
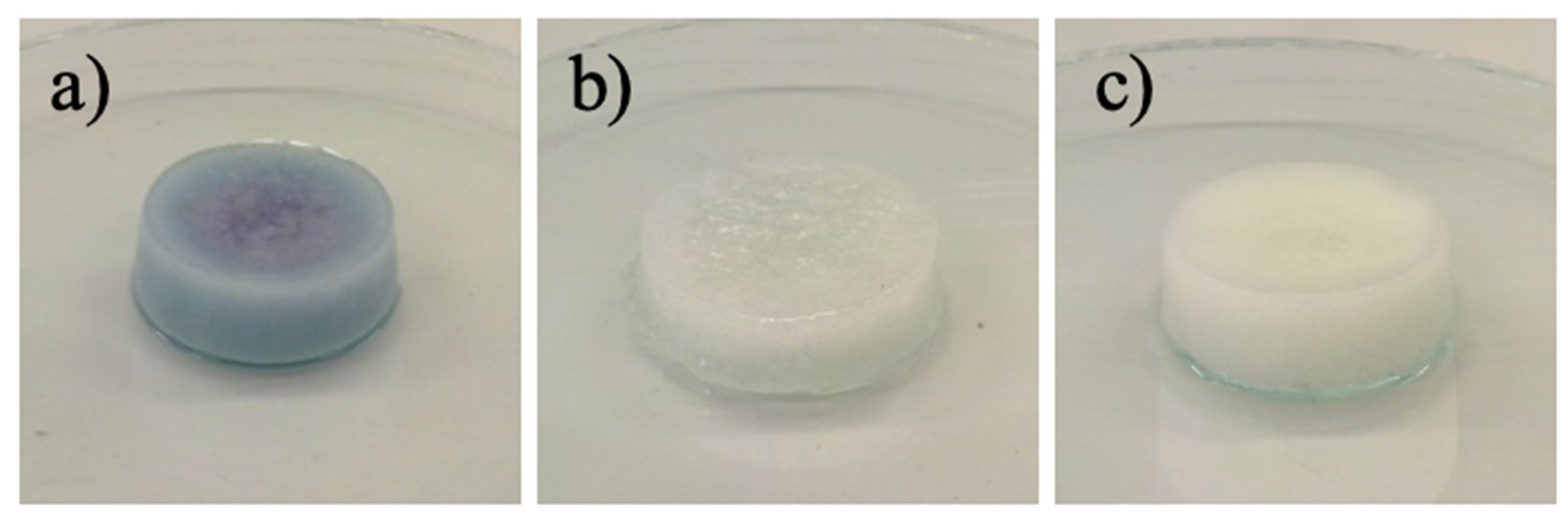
3.7. Desorption of Metals Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Moradi, O.; Tyagi, I.; Agarwal, S.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Makhlouf, A.S.H.; Goodarzi, M.; Garshasbi, A. Study on the removal of heavy metal ions from industry waste by carbon nanotubes: Effect of the surface modification: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 93–118. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mat. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ishanullah, A.A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Loa, W.-H.; Babel, S. Physico-Chemical Treatment Techniques for Wastewater Laden with Heavy Metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation strategies for heavy metals contaminated ecosystem: A review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of Heavy Metals: A Review. Inter. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Babel, S.; Kurniawan, T.A. Low Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mat. 2003, B97, 219–243. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Inter. Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Alsaedi, A.; Hayat, T.; Chen, C. Synthesis of highly porous inorganic adsorbents derived from metal-organic frameworks and their application in efficient elimination of mercury(II). J. Colloid Interf. Sci. 2018, 517, 61–71. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical Properties of Functional Hybrid Organic-Inorganic Nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol–gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; the “Gold Book”; McNaught, A.D., Wilkinson, A., Eds.; Online Version 2019; Chalk, S.J., Created; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Application of Hybrid Organic–Inorganic Nanocomposites. J. Mat. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Drisko, G.L.; Sanchez, C. Hybridization in Materials Science–Evolution, Current State, and Future Aspirations. Eur. J. Inorg. Chem. 2012, 32, 5097–5105. [Google Scholar] [CrossRef]
- Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis of Bioactive and Porous Organic-Inorganic Hybrids for Biomedical Applications. J. Sol-Gel Sci. Technol. 2004, 32, 201–205. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators B 2013, 182, 467–481. [Google Scholar] [CrossRef]
- Obanla, O.R.; Mohammed, F.U.; Ojewumi, M.E.; Odunlami, O.A.; Ikpotor, U.F. Kinetic Adsorption of Heavy Metal (Copper) On Rubber (Hevea Brasiliensis) Leaf Powder. S. Afr. J. Chem. Eng. 2021, 37, 74–80. [Google Scholar]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and T methylene blue from aqueous solution. Carbohydr. Polym. 2020, 249, 116841. [Google Scholar] [CrossRef]
- Marciano, J.S.; Ferreira, R.R.; de Souza, A.G.; Barbosa, R.F.S.; de Moura Junior, A.J.; Rosa, D.S. Biodegradable gelatin composite hydrogels filled with cellulose for chromium (VI) adsorption from contaminated water. Int. J. Biol. Macromol. 2021, 181, 112–124. [Google Scholar] [CrossRef]
- Kamal, O.; Pochat-Bohatier, C.; Sanchez-Marcano, J. Development and stability of gelatin cross-linked membranes for copper (II) ions removal from acid waters. Sep. Purif. Technol. 2017, 183, 153–161. [Google Scholar] [CrossRef]
- Wojciechowska, P.; Tichoniuk, M.; Gwiazdowska, D.; Maciejewski, H.; Nowicki, M. Antimicrobial activity of organic–inorganic hybrid films based on gelatin and organomodified silicones. Adv. Polym. Technol. 2018, 37, 2958–2970. [Google Scholar] [CrossRef]
- Wojciechowska, P.; Pietras, P.; Maciejewski, H. Synthesis, Characterization, and Thermal Properties of Organic–Inorganic Hybrids Based on Gelatin and Organomodified Silicones. Adv. Polym. Technol. 2014, 33, 21459. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Zhang, Q.; Xia, Y.; Li, T. Synthesis and characterization of gelatin-polydimethylsiloxane graft copolymers. J. Appl. Polym. Sci. 2011, 120, 2130–2137. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, J.; Shrivastava, R.; Bajpai, A.K. Dynamic and equilibrium studies on adsorption of Cr(VI) ions onto binary bio-polymeric beads of cross linked alginate and gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2004, 236, 81–90. [Google Scholar] [CrossRef]
- Murilo, Á.; Rodrigues, V.; Marangon, C.A.; Amaro Martins, V.D.C.; de Guzzi Plepis, A.N. Chitosan/gelatin films with jatobá resin: Control of properties by vegetal resin inclusion and degree of acetylation modification. Int. J. Biol. Macromol. 2021, 182, 1737–1745. [Google Scholar]
- Hui, B.; Zhang, Y.; Ye, L. Structure of PVA/gelatin hydrogel beads and adsorption mechanism for advanced Pb(II) removal. J. Ind. Eng. Chem. 2015, 21, 868–876. [Google Scholar] [CrossRef]
- Maciejewski, H.; Marciniec, B.; Kownacki, I. Sposób Modyfikacji Polisiloksanów PL 193 689 B1, 18 December 2000.
- Marciniec, B.; Chadyniak, D.; Pawluć, P.; Maciejewski, H.; Błażejewska-Chadyniak, P. Sposób Otrzymywania Poli(Metylo,Alkilo)Siloksanów PL 194 668, 10 July 2006.
- Fiedorow, R.; Dutkiewicz, M.; Marciniec, B.; Guliński, J.; Maciejewski, H. Sposób Otrzymywania Modyfikowanych (Poli)Siloksanów. PL 198290, 30 June 2008. [Google Scholar]
- González-Paz, R.J.; Lligadas, G.; Ronda, J.C.; Galià, M.; Ferreira, A.M.; Boccafoschi, F.; Ciardelli, G.; Cádiz, V. Study on the interaction between gelatin and polyurethanes derived from fatty acids. J. Biomed. Mater. Res. Part A 2013, 101A, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qiu, H.; Si, Y.; Wang, W.; Gao, J. Fabrication of highly porous biodegradable monoliths strengthened by graphene oxide and their adsorption of metal ions. Carbon 2011, 49, 827–837. [Google Scholar] [CrossRef]
- Dwivedi, P.; Sahu, J.N.; Mohanty, C.R.; Mohana, B.R.; Meikap, B.C. Column performance of granular activated carbon packed bed for Pb(II) removal. J. Hazard. Mater. 2008, 156, 596–603. [Google Scholar] [CrossRef]
- Kalak, T.; Kłopotek, A.; Cierpiszewski, R. Effective adsorption of lead ions using fly ash obtained in the novel circulating fluidized bed combustion technology. Microchem. J. 2019, 145, 1011–1025. [Google Scholar]
- Lee, Y.C.; Chang, S.-P. The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresour. Technol. 2011, 102, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Laus, R.; Costa, T.G.; Szpoganicz, B.; Fávere, V.T. Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J. Hazard. Mat. 2010, 183, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Ma, P.; Bai, H.; Dong, W.; Chen, M. Preparation of polyvinyl alcohol/chitosan hydrogel compounded with graphene oxide to enhance the adsorption properties for Cu(II) in aqueous solution. J. Polym. Res. 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.Q.; Han, Y. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Micropor. Mesopor. Mater. 2017, 252, 105–115. [Google Scholar] [CrossRef]
- Calero, M.; Hernainz, F.; Blazquez, G.; Martin-Lara, M.A.; Tenorio, G. Biosorption kinetics of Cd(II), Cr(III) and Pb(II) in aqueous solutions by olive stone. Braz. J. Chem. Eng. 2009, 26, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Gayathri, R. Adsorption of Pb2+ ions from aqueous solutions onto bael tree leaf powder: Isotherms, kinetics and thermodynamics study. J. Eng. Sci. Technol. 2009, 4, 381–399. [Google Scholar]
- El-Ashtoukhy, E.-S.Z.; Amin, N.K.; Abdelwahab, O. Removal of Lead(II) and Copper(II) from Aqueous Solution Using Pomegranate Peel as a New Adsorbent. Desal 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Blázquez, G.; Martín-Lara, M.A.; Dionisio-Ruiz, E.; Tenorio, G.; Calero, M. Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. J. Ind. Eng. Chem. 2011, 17, 824–833. [Google Scholar] [CrossRef]
- Łużny, R.; Ignasiak, M.; Walendziewski, J.; Stolarski, M. Heavy metal ions removal from aqueous solutions using carbon aerogels and xerogels. Chemik 2014, 68, 544–553. [Google Scholar]
- Magossi, M.S.; Maraldi, V.A.; Magossi, M.S.; Da Costa, F.M.; Alves, K.; Franco, F.D.S.; Do Carmo, D.R. A New Triazole-Thiol Compound Organofunctionalized on the Silica Gel Surface: Chemical Properties and Copper Sorption in Ethanol/Water Media. Silicon 2021, 13, 2243–2255. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.P. Adsorption–desorption of heavy metal ions. Curr. Sci. 2014, 107, 601–612. [Google Scholar]

| Sample | Compressive Strength (kPa) |
| G | 206.3 ± 18.6 |
| GS 1:1 | 157.1 ± 3.2 |
| GS 2:1 | 184.6 ± 13.5 |
| GS 3:1 | 190.2 ± 2.4 |
| GS 4:1 | 197.5 ± 5.2 |
| Type of Solution | Composition of Hybrid Monoliths (Gelatin:Siloxane) | pH | Disintegration Time [hours] | ||
|---|---|---|---|---|---|
| Initial | After 1 h | After 2 h | |||
| Cu(II) | 1:1 | 2.26 | - | - | >48 |
| Cu(II) | 2:1 | 2.26 | - | - | >24 |
| Cu(II) | 3:1 | 2.26 | 4.58 | 6.01 | 8 |
| Cu(II) | 4:1 | 2.26 | 4.47 | 5.82 | 6 |
| Cu(II) | 6:1 | 2.26 | 6.15 | 6.98 | 2 |
| Cu(II) | 8:1 | 2.26 | 4.42 | 5.78 | 3 |
| water | 4:1 | 5.05 | 9.64 | 9.57 | 5 |
| water | 6:1 | 5.05 | 9.77 | 9.69 | 2 |
| water | 8:1 | 5.05 | 9.73 | 9.70 | 2 |
| Metal Ions | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| k1 | q e | R2 | k2 | q e | R2 | |
| min−1 | mg/g | g/(mg min) | mg/g | |||
| GS 2:1 | ||||||
| Cu | 0.00568 | 0.893 | 0.915 | 4.389 | 1.045 | 0.993 |
| Pb | 0.00564 | 1.92 | 0.974 | 1.340 | 1.892 | 0.978 |
| Cd | 0.00231 | 1.33 | 0.968 | 1.910 | 1.584 | 0.915 |
| GS 1:1 | ||||||
| Cu | 0.00104 | 0.449 | 0.989 | 12.040 | 0.631 | 0.902 |
| Pb | 0.00216 | 0.726 | 0.968 | 9.398 | 0.902 | 0.856 |
| Cd | 0.00122 | 0.516 | 0.951 | 8.294 | 0.760 | 0.714 |
| Metal Ions | pH | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | n | R2 | ||
| mg/g | L/mg | mg/g | (L/mg)(1/n) | ||||
| Cu | 2.688 | 0.0193 | 0.938 | 0.4720 | 5.5889 | 0.971 | |
| Pb | 3.500 | 0.2507 | 0.849 | 0.7111 | 2.0151 | 0.909 | |
| Cd | 3.088 | 7.9000 | 0.877 | 0.1816 | 6.5908 | 0.817 | |
| Adsorbent | qm, mg/g | Metal | Reference |
|---|---|---|---|
| CGGO (chitosan-gelatin/graphene oxide) monoliths | about 100 | Pb(II) | [34] |
| about 130 | Cu(II) | ||
| Granular activated carbon | 26.546 | Pb(II) | [35] |
| Fly ash | 51.98 | Pb(II) | [36] |
| Green algae Spirogyra | 90.91 | Pb(II) | [37] |
| 38.61 | Cu(II) | ||
| Chitosan crosslinked with epichlorohydrin triphosphate | 130.72 | Cu(II) | [38] |
| 83.72 | Cd(II) | ||
| 166.94 | Pb(II) | ||
| PVA/gelatin hydrogel beads | 211.86 | Pb(II) | [29] |
| PVA/CS/GO hydrogel beads | 162 | Cu(II) | [39] |
| Fly-ash-based SBA-15 | 131.00 | Pb(II) | [40] |
| Olive stone | 5.88 | Pb(II) | [41] |
| 7.33 | Cd(II) | ||
| Bael tree leaf powder | 4.065 | Pb(II) | [42] |
| Pomegranate peel | 1.32 | Cu(II) | [43] |
| Pink bark | 11.94 | Cu(II) | [44] |
| Monolithic xerogel | 0.585 | Cu(II) | [45] |
| Silica gel with SGA | 0.22 mmol/g | Cu(II) | [46] |
| Magnetic nanocomposites | 4.11 | Cd(II) | [47] |
| Silica-Coated Magnetic Nanocomposites | 14.9 | Pb(II) | [48] |
| Gelatin-siloxane monoliths | 3.75 | Pb(II) | own research |
| 1.76 | Cu(II) | ||
| 1.5 | Cd(II) |
| Metal | Adsorption Process | Desorption Process | Mass Balance | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Initial Amount of Metal Ions | Amount of Metal ions after Adsorption | Amount of Metal Adsorbed(Difference between Column 1 and 2) | Amount of Metal Ions After Desorption | Amount of Metal Ions in Monolith | Sum of Column 4 and 5 | (3-6)/3 × 100% | |
| µg | µg | µg | µg | µg | µg | % | |
| Cd(II) | 192.6 | 30.2 | 162.4 | 38.6 | 110.5 | 149.1 | 8.2 |
| Cu(II) | 198.2 | 87.2 | 111.0 | 65.8 | 30.2 | 95.8 | 13.7 |
| Pb(II) | 200.2 | 18.2 | 182.0 | 107.4 | 58.3 | 165.7 | 9.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, P.; Cierpiszewski, R.; Maciejewski, H. Gelatin–Siloxane Hybrid Monoliths as Novel Heavy Metal Adsorbents. Appl. Sci. 2022, 12, 1258. https://doi.org/10.3390/app12031258
Wojciechowska P, Cierpiszewski R, Maciejewski H. Gelatin–Siloxane Hybrid Monoliths as Novel Heavy Metal Adsorbents. Applied Sciences. 2022; 12(3):1258. https://doi.org/10.3390/app12031258
Chicago/Turabian StyleWojciechowska, Patrycja, Ryszard Cierpiszewski, and Hieronim Maciejewski. 2022. "Gelatin–Siloxane Hybrid Monoliths as Novel Heavy Metal Adsorbents" Applied Sciences 12, no. 3: 1258. https://doi.org/10.3390/app12031258
APA StyleWojciechowska, P., Cierpiszewski, R., & Maciejewski, H. (2022). Gelatin–Siloxane Hybrid Monoliths as Novel Heavy Metal Adsorbents. Applied Sciences, 12(3), 1258. https://doi.org/10.3390/app12031258