In Vitro Analysis of Organic Ester Functional Groups in Carious Dentine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Sample Collection
2.2. Study Design
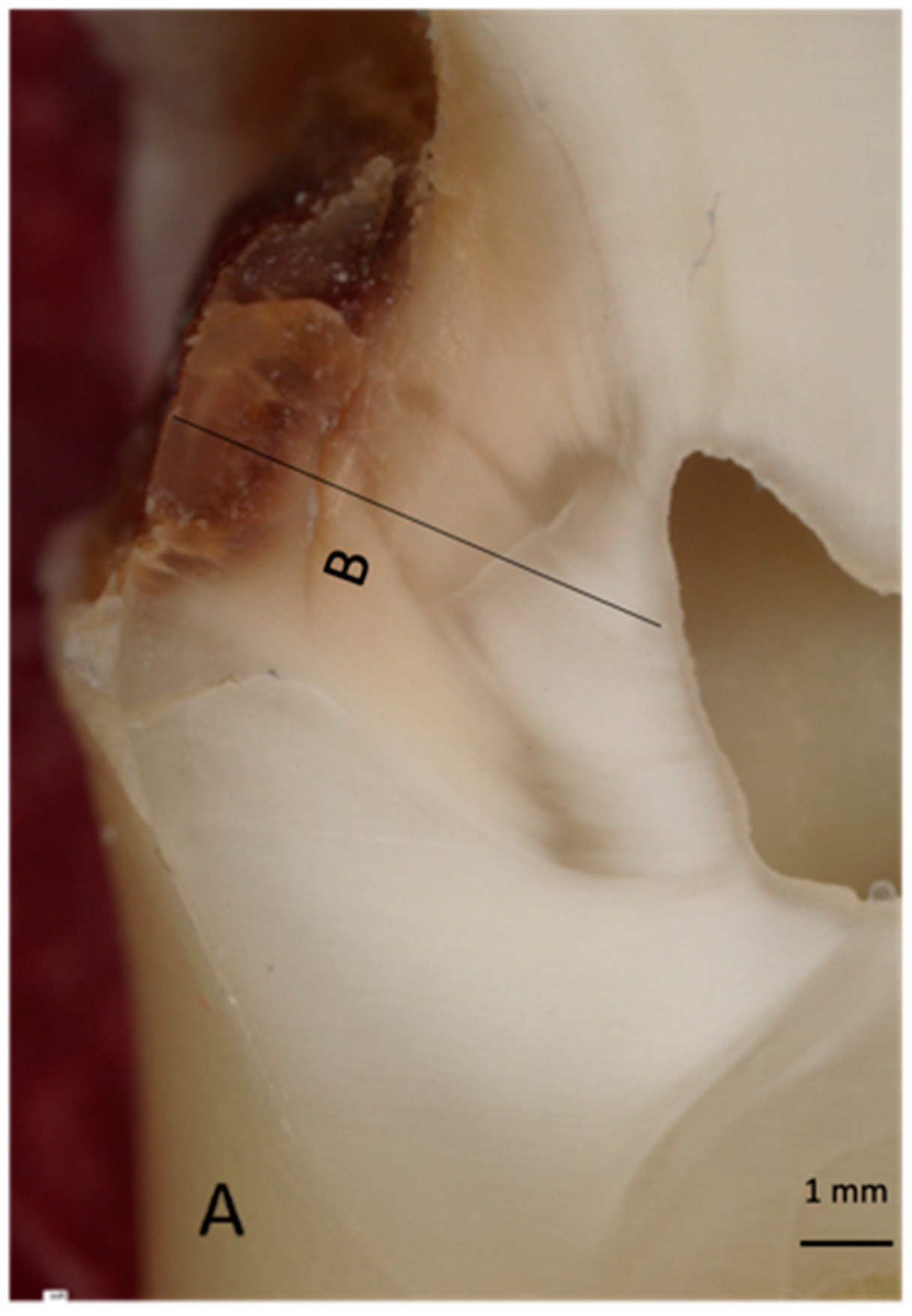
2.3. Raman Spectroscopy
2.4. Molecular Label Preparation
2.5. Data Collection and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, A. Selective Removal of Carious Dentin. In Management of Deep Carious Lesions; Schwendicke, F., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–70. ISBN 978-3-319-61370-3. [Google Scholar]
- Slimani, A.; Terrer, E.; Manton, D.J.; Tassery, H. Carious Lesion Detection Technologies: Factual Clinical Approaches. Br. Dent. J. 2020, 229, 432–442. [Google Scholar] [CrossRef]
- Ogushi, K.; Fusayama, T. Electron Microscopic Structure of the Two Layers of Carious Dentin. J. Dent. Res. 1975, 54, 1019–1026. [Google Scholar] [CrossRef]
- Fusayama, T. Two Layers of Carious Dentin; Diagnosis and Treatment. Oper Dent. 1979, 4, 63–70. [Google Scholar]
- Kidd, E.A.M. How “clean” Must a Cavity Be before Restoration? Caries Res. 2004, 38, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Dörfer, C.E.; Paris, S. Incomplete Caries Removal: A Systematic Review and Meta-Analysis. J. Dent. Res. 2013, 92, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.P.T.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Terminology. Adv. Dent. Res. 2016, 28, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendicke, F. Removing Carious Tissue: Why and How? Monogr. Oral. Sci. 2018, 27, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kidd, E.A.; Watson, T.F. In Vitro Evaluation of Five Alternative Methods of Carious Dentine Excavation. Caries Res. 2000, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Isolan, C.P.; Sarkis-Onofre, R.; Lima, G.S.; Moraes, R.R. Bonding to Sound and Caries-Affected Dentin: A Systematic Review and Meta-Analysis. J. Adhes. Dent. 2018, 20, 7–18. [Google Scholar] [CrossRef]
- Ogawa, K.; Yamashita, Y.; Ichijo, T.; Fusayama, T. The Ultrastructure and Hardness of the Transparent Layer of Human Carious Dentin. J. Dent. Res. 1983, 62, 7–10. [Google Scholar] [CrossRef]
- Banerjee, A.; Cook, R.; Kellow, S.; Shah, K.; Festy, F.; Sherriff, M.; Watson, T. A Confocal Micro-Endoscopic Investigation of the Relationship between the Microhardness of Carious Dentine and Its Autofluorescence. Eur. J. Oral Sci. 2010, 118, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F.; Lynch, R.J.M. Comparison of Knoop and Vickers Surface Microhardness and Transverse Microradiography for the Study of Early Caries Lesion Formation in Human and Bovine Enamel. Arch. Oral Biol. 2014, 59, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Almhöjd, U.S.; Norén, J.G.; Arvidsson, A.; Nilsson, Å.; Lingström, P. Analysis of Carious Dentine Using FTIR and ToF-SIMS. Oral. Health Dent. Manag. 2014, 13, 735–744. [Google Scholar]
- Alturki, M.; Koller, G.; Almhöjd, U.; Banerjee, A. Chemo-Mechanical Characterization of Carious Dentine Using Raman Microscopy and Knoop Microhardness. R. Soc. Open Sci. 2020, 7, 200404. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, 5th ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-44897-8. [Google Scholar]
- Pitts, N.B.; Ekstrand, K.R. International Caries Detection and Assessment System (ICDAS) and Its International Caries Classification and Management System (ICCMS)—Methods for Staging of the Caries Process and Enabling Dentists to Manage Caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef]
- Almhöjd, U.S.; Lingström, P.; Nilsson, Å.; Norén, J.G.; Siljeström, S.; Östlund, Å.; Bernin, D. Molecular Insights into Covalently Stained Carious Dentine Using Solid-State NMR and ToF-SIMS. Caries Res. 2017, 51, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Alturki, M.; Koller, G.; Warburton, F.; Almhöjd, U.; Banerjee, A. Biochemical Characterisation of Carious Dentine Zones Using Raman Spectroscopy. J. Dent. 2021, 105, 103558. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P.; Walker, M.P. Chemical Profile of Adhesive/Caries-Affected Dentin Interfaces Using Raman Microspectroscopy. J. Biomed. Mater. Res. A 2007, 81, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yao, X.; Liu, Y.W.; Wang, Y. A Fourier Transform Infrared Spectroscopy Analysis of Carious Dentin from Transparent Zone to Normal Zone. Caries Res. 2014, 48, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef]
- Maske, T.T.; Isolan, C.P.; van de Sande, F.H.; Peixoto, A.C.; Faria-E-Silva, A.L.; Cenci, M.S.; Moraes, R.R. A Biofilm Cariogenic Challenge Model for Dentin Demineralization and Dentin Bonding Analysis. Clin. Oral. Investig. 2015, 19, 1047–1053. [Google Scholar] [CrossRef]
- Lopes, C.D.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier Transform Infrared Spectroscopy (FTIR) Application Chemical Characterization of Enamel, Dentin and Bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Almhöjd, U.S.; Lingström, P.; Melin, L.; Nilsson, Å.; Norén, J.G. Staining of Carious Dentine Using Dyes with Covalent and Electrostatic Binding Properties—An in-Vitro Study. Oral. Health Dent. Manag. 2015, 14, 7. [Google Scholar]
- Rostand, K.S.; Esko, J.D. Cholesterol and Cholesterol Esters: Host Receptors for Pseudomonas Aeruginosa Adherence. J. Biol. Chem. 1993, 268, 24053–24059. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry (Second Edition). Biochem. Educ. 1995, 23, 104–105. [Google Scholar] [CrossRef] [Green Version]
- Gotliv, B.-A.; Veis, A. Peritubular Dentin, a Vertebrate Apatitic Mineralized Tissue without Collagen: Role of a Phospholipid-Proteolipid Complex. Calcif. Tissue Int. 2007, 81, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Gotliv, B.A.; Veis, A. The Composition of Bovine Peritubular Dentin: Matching TOF-SIMS, Scanning Electron Microscopy and Biochemical Component Distributions. New Light on Peritubular Dentin Function. Cells Tissues Organs 2009, 189, 12–19. [Google Scholar] [CrossRef]
- Tipson, R.S.S.; Horton, D. Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: San Diego, CA, USA, 1995; Volume 51, ISBN 978-0-12-007251-4. [Google Scholar]
- Goh, S.Y.; Tan, W.-S.; Khan, S.A.; Chew, H.P.; Kasim, N.H.A.; Yin, W.-F.; Chan, K.-G. Unusual Multiple Production of N-Acylhomoserine Lactones a by Burkholderia Sp. Strain C10B Isolated from Dentine Caries. Sensors 2014, 14, 8940–8949. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short-Chain N-Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, 86, e01941-19. [Google Scholar] [CrossRef]

| Infected Dentine ** | Mean | 3.39 * | |
| 95% Confidence Interval for Mean | Lower | 2.95 | |
| Upper | 3.84 | ||
| Std. Deviation | 2.11 | ||
| Affected Dentine ** | Mean | 0.87 | |
| 95% Confidence Interval for Mean | Lower | 0.76 | |
| Upper | 0.98 | ||
| Std. Deviation | 0.58 | ||
| Sound Dentine ** | Mean | 0.04 | |
| 95% Confidence Interval for Mean | Lower | 0.02 | |
| Upper | 0.06 | ||
| Std. Deviation | 0.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alturki, M.; Almhöjd, U.; Koller, G.; Warburton, F.; Banerjee, A. In Vitro Analysis of Organic Ester Functional Groups in Carious Dentine. Appl. Sci. 2022, 12, 1088. https://doi.org/10.3390/app12031088
Alturki M, Almhöjd U, Koller G, Warburton F, Banerjee A. In Vitro Analysis of Organic Ester Functional Groups in Carious Dentine. Applied Sciences. 2022; 12(3):1088. https://doi.org/10.3390/app12031088
Chicago/Turabian StyleAlturki, Mohammed, Ulrica Almhöjd, Garrit Koller, Fiona Warburton, and Avijit Banerjee. 2022. "In Vitro Analysis of Organic Ester Functional Groups in Carious Dentine" Applied Sciences 12, no. 3: 1088. https://doi.org/10.3390/app12031088
APA StyleAlturki, M., Almhöjd, U., Koller, G., Warburton, F., & Banerjee, A. (2022). In Vitro Analysis of Organic Ester Functional Groups in Carious Dentine. Applied Sciences, 12(3), 1088. https://doi.org/10.3390/app12031088






