Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment
Abstract
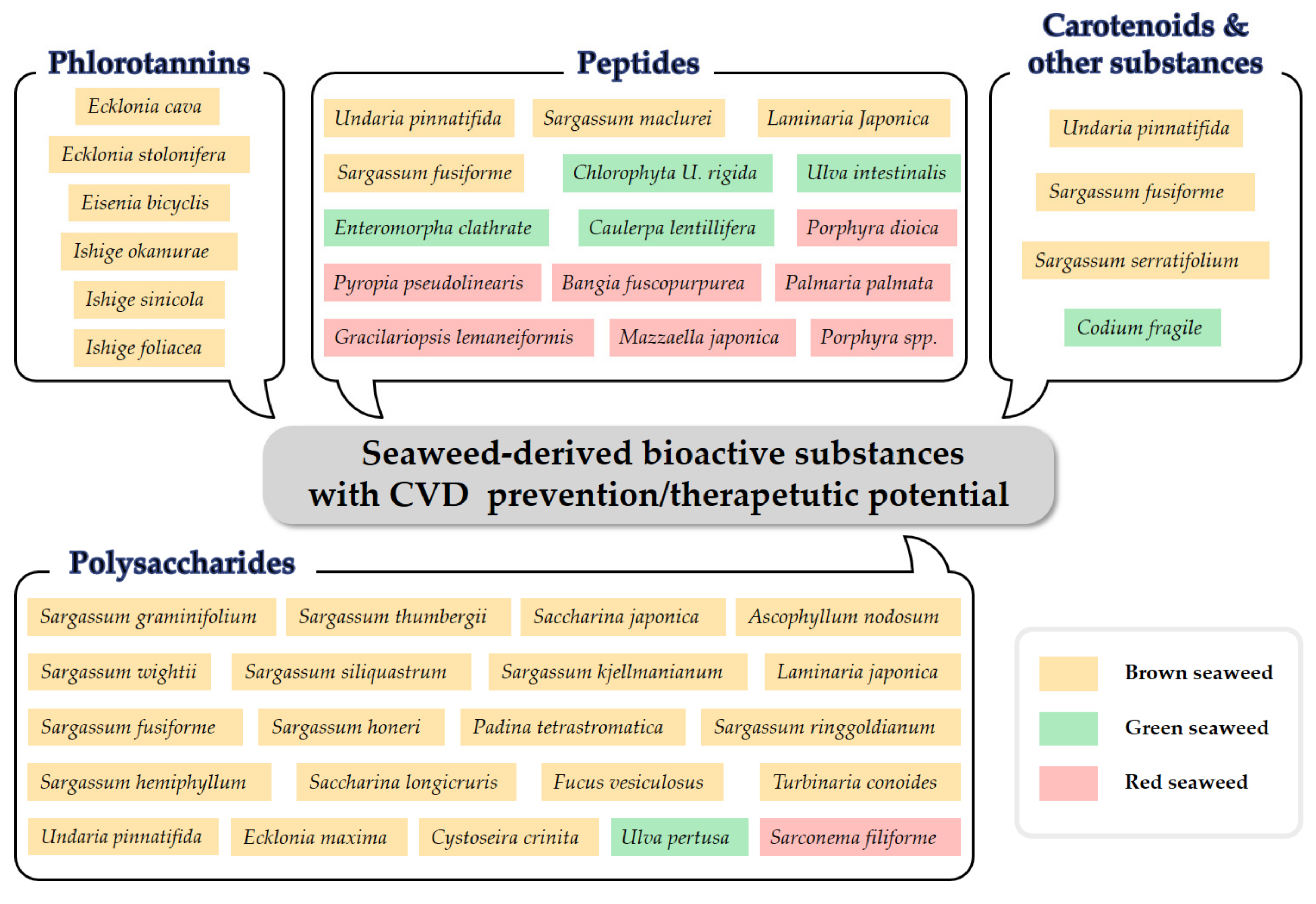
1. Introduction
2. Seaweed-Derived Phlorotannins with Therapeutic Potential against CVD
3. Seaweed-Derived Polysaccharides with Therapeutic Potential against CVD
4. Seaweed-Derived Peptides with Therapeutic Potential against CVD
5. Seaweed-Derived Carotenoids and Other Components with Therapeutic Potential against CVD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.; Brazeau, A.-S.; Meltzer, S.; Rahme, E.; Dasgupta, K. Conjoint Associations of Gestational Diabetes and Hypertension with Diabetes, Hypertension, and Cardiovascular Disease in Parents: A Retrospective Cohort Study. Am. J. Epidemiol. 2017, 186, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Leggio, M.; Lombardi, M.; Caldarone, E.; Severi, P.; D’emidio, S.; Armeni, M.; Bravi, V.; Bendini, M.G.; Mazza, A. The relationship between obesity and hypertension: An updated comprehensive overview on vicious twins. Hypertens. Res. 2017, 40, 947–963. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Alloubani, A.; Mari, M.; Alzaatreh, M. Cardiovascular disease risk factors: Hypertension, diabetes mellitus and obesity among Tabuk citizens in Saudi Arabia. Open Cardiovasc. Med. J. 2018, 12, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.H.H.; Nwachuku, C.E.; Black, H.R.; Cushman, W.C.; Davis, B.R.; Simpson, L.M.; Alderman, M.H.; Atlas, S.A.; Basile, J.N.; Cuyjet, A.B.; et al. Chlinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2006, 48, 374–384. [Google Scholar] [CrossRef]
- Haller, H. Effective management of hypertension with dihydropyridine calcium channel blocker-based combination therapy in patients at high cardiovascular risk. Int. J. Clin. Pract. 2008, 62, 781–790. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, L.; Jing, R.; Trexler, C.; Wang, H.; Li, Y.; Tang, H.; Huang, F.; Zhang, F.; Fang, X.; et al. Inositol 1,4,5-trisphosphate receptors in endothelial cells play an essential role in vasodilation and blood pressure regulation. J. Am. Heart Assoc. 2019, 8, e011704. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising plant of the millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Ryu, B.; Ahn, G.; Yeo, I.-K.; Jeon, Y.-J. Therapeutic potential of algal natural products against metabolic syndrome: A review of recent developments. Trends Food Sci. Technol. 2020, 97, 286–299. [Google Scholar] [CrossRef]
- Samarakoon, K.; Jeon, Y.-J. Bio-vunctionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.-J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus Siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant. Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Wikesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2005, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Meng, W.; Sun, T.M.H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavalability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ko, S.-C.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-convering enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, M.C.; Kang, N.; Kim, H.-S.; Lee, S.-H.; Ahn, G.; Jung, W.-K.; Jeon, Y.-J. Effect of angiotensin I-convering enzyme (ACE) inhibition and nitric oxide (NO) production of 6,6’-bieckol, a marine algal polyphenol and its anti-hypertensive effect in spontaneously hypertensive rats. Process. Biochem. 2017, 58, 326–332. [Google Scholar] [CrossRef]
- Ko, S.-C.; Jung, W.-K.; Kang, S.-M.; Lee, S.-H.; Kang, M.C.; Heo, S.-J.; Kang, K.-H.; Kim, Y.-T.; Park, S.-J.; Jeong, Y.; et al. Angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO)-mediated antihypertensive effect of oxtaphorethol A isolated from Ishige sinicola: In vitro molecular mechanism and in vivo SHR model. J. Funct. Foods 2015, 18, 289–299. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef]
- Devika, N.T.; Ali, B.M.J. Analysing calcium dependent and independent regulation of eNOS in endothelium triggered by extracelluar signalling events. Mol. BioSystems 2013, 9, 2653–2664. [Google Scholar] [CrossRef]
- Dudzinski, D.M.; Michel, T. Life history of eNOS: Partners and pathways. Cardiovasc. Res. 2007, 75, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Raouf, A.K. Modulators of the vascular endothelin receptor in blood pressure regulation and hypertension. Curr. Mol. Pharmacol. 2011, 4, 176–186. [Google Scholar]
- Nakamura, K.; Koga, Y.; Sakai, H.; Homma, K.; Ikebe, M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylatin of myosin phophatase. Circ. Res. 2007, 101, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-A.; Je, J.-G.; Hwang, J.; Jeon, Y.-J.; Ryu, B. Ecklonia cava extract and its derivative dieckol promote vasodilation by modulating calcium signaling and PI3K/AKT/eNOS pathway in in vitro and in vivo model. Biomedicines 2021, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.A.; Jiang, Y.; Yang, H.-W.; Hwang, J.; Jeon, Y.-J.; Ryu, B. Diphlorethohydroxycarmalol isolated from Ishige okamurae exerts vasodilatory effects via calcium signaling and PI3K/Akt/eNOS pathway. Int. J. Mol. Sci. 2021, 22, 1610. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Lee, H.S.; Kim, H.-S.; Jeon, Y.-J.; Byun, K. Protective effect of pyrogallol-phloroglucinol-6,6-bieckol from Ecklonia cava on monocyte-associated vascular dysfunction. Mar. Drugs 2018, 16, 441. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.-J.; Byun, K. Pyrogallol-phloroglucinol-6,6’-bieckol from Ecklonia cava improved blood circulation in diet-induced obese and diet-induced hypertension mouse model. Mar. Drugs 2019, 17, 272. [Google Scholar] [CrossRef]
- Gross, M. Flavonoids and cardiovascular disease. Pharm. Biol. 2004, 42, 21–35. [Google Scholar] [CrossRef]
- Hata, Y.; Nakajima, K.; Uchida, J.-I.; Hidaka, H.; Nakano, T. Clinical effects of brown seaweed, Undaria pinnatifida (wakame), on blood pressure in hypertensive subjects. J. Clin. Biochem. Nutr. 2001, 30, 43–53. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The impact of a single dose of a polyphenol-rich seaweed extract on postprandial glycaemic control in healthy adults: A randomised cross-over trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef]
- Danaei, G.; Lawes, C.M.; Hoorn, S.V.; Murray, C.J.; Ezzati, M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: Comparative risk assessment. Lancet 2006, 368, 1651–1659. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Asgar, A. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Reappraisal and perspectives of clinical drug-drug interaction potential of α-glucosidase inhibitors such as acarbose, voglibose and miglitol in the treatment of type 2 diabetes mellitus. Xenobiotica 2018, 48, 89–108. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.H.; Jang, H.D.; Yang, S.Y.; Kim, Y.H. Inhibitory activity of minor phlorotannins from Ecklonia cava on α-glucosidase. Food Chem. 2018, 257, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Karadeniz, F.; Kim, M.M.; Kim, S.K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2008, 89, 1552–1558. [Google Scholar] [CrossRef]
- Lee, H.-A.; Lee, J.-H.; Han, J.-S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm. Biol. 2017, 55, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, M.-H.; Heo, S.-J.; Kang, S.-M.; Ko, S.-C.; Han, J.-S.; Jeon, Y.-J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef]
- Moon, H.E.; Islam, M.N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Jiang, Y.; Kim, H.-S.; Hyun, J.-M.; Lim, S.-B.; Li, Y.; Jeon, Y.-J. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar. Drugs 2018, 16, 436. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Moon, S.-H.; Jeon, B.-T.; Lee, D.H.; Jeon, Y.-J. Octaphlorethol A: A potent α-glucosidase inhibitor isolated from Ishige foliacea shows an anti-hyperglycemic effect in mice with streptozotocin-induced diabetes. Food Funct. 2014, 5, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Han, J.-S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Doland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in β-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Viollet, B.; Mounier, R.; Leclerc, J.; Yazigi, A.; Foretz, M.; Andreelli, F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007, 33, 395–402. [Google Scholar] [CrossRef]
- Lee, H.-A.; Lee, J.-H.; Han, J.-S. 2,7”-Phloroglucinol-6,6’-bieckol pretects INS-1 cells against high glucose-induced apoptosis. Biomed. Pharmacother. 2018, 103, 1473–1481. [Google Scholar] [CrossRef]
- Kang, M.-C.; Wijesinghe, W.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type Ⅱ diabetes in db/db/ mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef]
- Yang, H.-W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.-J.; Byun, K.; Ryu, B. Effect of ishophloroglucin A, a component of Ishige okamurae, on glucose homeostasis in the pancreas and muscle of high fat diet-fed mice. Mar. Drugs 2019, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, S.-C.; Kang, M.-C.; Lee, D.H.; Jeon, Y.-J. Octaphlorethol A, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem. Toxicol. 2016, 91, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.H.N.; Yang, H.-W.; Jiang, Y.; Jeon, Y.-J.; Ryu, B. Diphlorethohydroxycarmalol isolated from Ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar. Drugs 2018, 16, 375. [Google Scholar] [CrossRef]
- Fernando, K.H.N.; Yang, H.-W.; Jiang, Y.; Jeon, Y.-J.; Ryu, B. Ishige okamurae extract and its constituent ishophloroglucin A attenuated in vitro and in vivo high glucose-induced angiogenesis. Int. J. Mol. Sci. 2019, 20, 5542. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itoh, M.; Ogawa, Y.; Suganami, T. Molecular mechanism of obesity-induced ‘metabolic’ tissue remodeling. J. Diabetes Investig. 2017, 9, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Kuri-harcuch, W.; Velez-delValle, C.; Vazquez-Sandoval, A.; Hernández-Mosqueira, C.; Fernandez-Sanchez, V. A cellular perspective of adipogenesis transcriptional regulation. J. Cell. Physiol. 2019, 234, 1111–1129. [Google Scholar] [CrossRef]
- Choi, K.-M.; Jeon, Y.S.; Kim, W.; Lee, A.; Kim, Y.-G.; Lee, J.H.; Kang, Y.E.; Jung, J.-C.; Lee, J.; Min, B.; et al. Xanthigen attenuates high-fat diet-induced obesity through down-regulation of PPARγ and activation of the AMPK pathway. Food Sci. Biotechnol. 2014, 23, 931–935. [Google Scholar] [CrossRef]
- Karadeniz, F.; Ahn, B.-N.; Kim, J.-A.; Seo, Y.; Jang, M.-S.; Nam, K.-H.; Kim, M.; Lee, S.-H.; Kong, C.-S. Phlorotannins suppress adipogensis in pre-adipocytes while enhancing osteoblastogenesis in pre-osteoblasts. Arch. Pharmacal Res. 2015, 38, 2172–2182. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, M.; Lee, J.-H.; Lee, S.-H.; Lim, Y.; Jeon, Y.-J. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ. Toxicol. Pharmacol. 2013, 36, 1253–1260. [Google Scholar] [CrossRef]
- Choi, H.-S.; Jeon, H.J.; Lee, O.H.; Lee, B.Y. Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid accumuation in the adipocytes of high-fat diet-fed zebrafish and mice: Inhibition of early adipogensis via cell-cycle arrest and AMPKα activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Wu, Y.X.; Kim, J.S.; Woo, J.H.; Park, K.T.; Kwon, O.J.; Seo, H.-J.; Kim, T.; Park, N.-H. 6,6’-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food Agric. 2014, 95, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jeon, Y.J.; Kim, H.J.; Han, J.S. Effect of diphlorethohydroxycarmalol isolated from Ishige okamurae on apoptosis in 3T3-L1 preadipocytes. Phytother. Res. 2013, 27, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Song, S.; Kim, H.; Cheon, Y.-P. Diphlorethohydroxycarmalol of Ishige okamurae and caffeine modified the expression of extracellular fibrillars during adipogenesis of mouse subcutaneous adipose derived stem cell. Dev. Reprod. 2013, 17, 275–287. [Google Scholar] [CrossRef]
- Kang, M.-C.; Ding, Y.; Kim, H.-S.; Jeon, Y.-J.; Lee, S.-H. Inhibition of adipogenesis by diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.-H.; Lee, M.S.; Lee, E.W.; Kim, Y.M.; Kim, T.H. Pancreatic lipase inhibitory activity of phlorotannins isolated from Eisenia bicyclis. Phytother. Res. 2013, 27, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Choi, J.; Choi, C.H.; Park, K.Y.; Son, K.H. Phlorotannins from Ecklonia cava attenuates plamitate-induced endoplasmic reticulum stress and leptin resistance in hypothalamic neurons. Mar. Drugs 2019, 17, 570. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Ahn, B.R.; Jung, H.A.; Choi, J.S. Inhibitory activity of Ecklonia stolonifera and its isolated phlorotannins against Cu2+-induced low-density lipoprotein oxidation. Fish. Sci. 2012, 78, 927–934. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on polyxamer 407-induced hyperlipidemic and choloesterol-fed rats. Arch. Pharmacal Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef]
- Yeo, A.-R.; Lee, J.; Tae, I.H.; Park, S.-R.; Cho, Y.H.; Lee, B.H. Anti-hyperlipidemic effect of polyphenol extract (SeapolynolTM) and dieckol isolated from Ecklonia cava in in vivo and in vitro models. Prev. Nutr. Food Sci. 2012, 17, 1–7. [Google Scholar] [CrossRef]
- Shibata, T.; Yamaguchi, K.; Nagayama, K.; Kawaguchi, S.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against glycosidase from the viscera of the turban shell Turbo cornutus. Eur. J. Phycol. 2002, 37, 493–500. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Lee, S.-H.; Park, P.-J.; Kang, D.-H.; Oh, C.; Kim, D.-W.; Han, J.-S.; Jeon, Y.-J.; et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2010, 48, 1448–1454. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, J.-I.; Heo, S.-J.; Park, M.-H.; Park, P.-J.; Jeon, B.-T.; Se-Kwon Kim, S.-K.; Han, J.-S.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Pae (Ishige okamurae) protects high glucose0induced damage in RINm5F pancreatic β cells via its antioxidant effects. Food Sci. Biotechnol. 2012, 21, 239–246. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Im, S.; Hwang, O.; Kim, H.-S.; Kang, M.-C.; Lee, S.-H. Anti-obesity effect of diphlorethohydroxycarmalol isolated from brown alga Ishige okamurae in high-fat diet-induced obese mice. Mar. Drugs 2019, 17, 637. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kang, N.; Kim, E.-A.; Heo, S.-J.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J. Antidiabetogenic and antioxidant effect of octaphlorethol A isolated from the brown algae Ishige foliacea in streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2014, 23, 1261–1266. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Kang, M.-C.; Jeon, Y.-J. Octaphlorethol A, a novel phenolic compound isolated from Ishige foliacea, protects against streptozotocin-induced pancreatic β cell damage by reducing oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 59, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds inseaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Mo’o, F.R.C.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from Macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. Fucoidan and its health benefits. Bioact. Seaweeds Food Appl. Nat. Ingred. Healthy Diets 2018, 223–238. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Chapter Seven-Biological Activities of Carrageenan. Adv. Food Nutr. Res. 2014, 72, 113–124. [Google Scholar] [PubMed]
- Maneesh, A.; Chakraborty, K. Pharmacological potential of sulfated polygalactopyranosyl-fucopyranan from the brown seaweed Sragassum wightii. J. Appl. Phycol. 2018, 30, 1971–1988. [Google Scholar] [CrossRef]
- Senthil, S.L.; Kumar, T.V.; Geetharmani, D.; Suja, G.; Yesudas, R.; Chacko, A. Fucoidan-An α-amylase inhibitor from Sargassum wightii with relevance to NIDDM. Int. J. Biol. Marcromol. 2015, 81, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan-A α-D-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Marcromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Molecular weight and sulfate content modulate the inhibition of α-amylase by fucoidan relevant for type 2 diabetes management. PharmaNutrition 2015, 3, 108–114. [Google Scholar] [CrossRef]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Baub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Eclonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Marcromol. 2020, 151, 412–420. [Google Scholar]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Marcromol. 2016, 82, 249–255. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Zhang, W.; Hou, Y.; Zhang, H.; Zhang, Q. Hypoglycemic property of acidic polysaccharide extracted from Saccharina japonica and its potential mechanism. Carbohydr. Polym. 2013, 95, 143–147. [Google Scholar] [CrossRef]
- Senthil, S.L.; Chandrasekaran, R.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleivation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Marcromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme fucoidan alleviates high-fat diet-induced obesity and insulin resistance associated with the improvement of hepatic oxidative stress and gut microbiota profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modified gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Arun, A.; Rauf, G.; Muraleedhara, K. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica attenuates isoproterenol-induced oxidative damage via activation of PI3K/Akt/Nrf2 signaling pathway-An in vitro and in vivo approach. Chem. -Biol. Interact. 2019, 308, 258–268. [Google Scholar]
- Cui, W.; Zheng, Y.; Zhang, Q.; Wang, J.; Wang, L.; Yang, W.; Guo, C.; Gao, W.; Wang, X.; Luo, D. Low-molecular-weight fucoidan protects endothelial function and ameliorates basal hypertension in diabetic Goto-Kakizaki rats. Lab. Investig. 2014, 94, 382–393. [Google Scholar] [CrossRef]
- Gara, A.B.; Kolsi, R.B.A.K.; Jardak, N.; Chaaben, R.; El-Feki, A.; Fki, L.; Belghith, H.; Belghith, K. Inhibitory activities of Cystoseira crinita sulfated polysaccharide on key enzymes related to diabetes and hypertension: In vitro and animal study. Arch. Physiol. Biochem. 2017, 123, 31–42. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Z.; Sang, Y.; Niu, Y.; Zhang, Q.; Ding, H.; Yin, S. Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the L-NAME-induced hypertensive rat model. Food Funct. 2016, 7, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Preez, R.D.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metablic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef]
- Qi, H.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.-H.; Auger, C.; Belcastro, E.; Park, S.-H.; Lee, H.-H.; Shini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Azuma, Y.; Ojima, T.; Hashimoto, T.; Mizuno, M.; Nishitani, Y.; Yoshida, M.; Azuma, T.; Kanazawa, K. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br. J. Nutr. 2013, 110, 880–890. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Morabito, M.; Minicante, S.A.; Arfuso, F.; Genovese, G. In vitro assessment of the effect of Undaria pinnatifida extracts on erythrocytes membrane integrity and blood coagulation parameters of Equus Caballus. J. Coast. Life Med. 2014, 2, 614–616. [Google Scholar]
- Favaloro, E.J.; Lippi, G.; Koutts, J. Laboratory testing of anticoagulants: The present and the future. Pathology 2011, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Giannetto, C.; Giudice, E.; Marafioti, S.; Fazio, F.; Assenza, A.; Piccione, G. ADP-induced platelet aggregation after addition of tramadol in vitro in fed and fasted horses plasma. Res. Vet. Sci. 2013, 94, 325–330. [Google Scholar] [CrossRef]
- Yu, W.-C.; Chen, Y.-L.; Hwang, P.-A.; Chen, T.-H.; Chou, T.-C. Fucoidan ameliorates pancreatic β -cell death and impaired insulin synthesis in streptozotocin-treated β cells and mice via a Sirt-1-dependent manner. Mol. Nutr. Food Res. 2017, 61, 1700136. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Yoon, J.-Y.; Lee, B.-Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef]
- Sim, S.-Y.; Shin, Y.-E.; Kim, H.-K. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res. Pract. 2019, 65, 54–62. [Google Scholar] [CrossRef]
- Kui-Jin; Lee, B.-Y. Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differentiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutr. Res. 2012, 32, 439–447. [Google Scholar]
- Juang, X.; Yu, J.; Ma, Z.; Zhang, H.; Xie, F. Effects of fucoidan on insulin stimulation and pancreatic protection via the cAMP signaling pathway in vivo and in vitro. Mol. Med. Rep. 2015, 12, 4501–4507. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, V.S.; Kurup, G.M. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica protects heart by ameliorating hyperlipidemia, endothelial dysfunction and inflammation in isoproterenol induced experimental myocardial infarction. J. Funct. Foods 2019, 54, 22–31. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, X. Separation and nanoencapsulation of antitumor polypeptide from Spirulina platensis. Biotechnol. Prog. 2013, 29, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, L.; Yang, G.; Piao, L.; Jin, M.; Cheng, X. Dipeptidyl peptidase-IV inhibition for the treatment of cardiovascular disease-Recent insights focusing on angiogenesis and neovascularization. Circ. J. 2017, 81, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, Y.; Wang, J.; Wu, S.; Geng, L.; Sui, Z.; Zhang, Q. Antihypertensive Effects of Two Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Gracilariopsis lemaneiformis (Rhodophyta) in Spontaneously Hypertensive Rats (SHRs). Mar. Drugs 2018, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; Lucia, M.D.; Madonna, M.; et al. Novel potent decameric peptide of Spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS-dependent mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Suetsuna, K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar]
- Suetsuna, K. Separation and identification of angiotensin I-converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein. Nippon Suisan Gakkaishi 1998, 64, 862–866. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sung, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Toji, K.; Katsukura, S.; Morikawa, R.; Uji, T.; Yasui, H.; Shimizu, T.; Kishimura, H. Characterization of ACE Inhibitory Peptides Prepared from Pyropia pseudolinearis Protein. Mar. Drugs 2021, 19, 200. [Google Scholar] [CrossRef]
- Chen, J.C.; Wang, J.; Zheng, B.-D.; Pang, J.; Chen, L.-J.; Lin, H.-T.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in L aminaria japonica Hydrolysates by RP-HPLC Method. J. Food Process. Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, Q.-F.; Yoshida, A.; Sun, L.-C.; Liu, Y.-X.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification and characterization of two novel angiotensin I-converting enzyme inhibitory peptides derived from r-phycoerythrin of red algae (Bangia fusco-purpurea). Eur. Food Res. Technol. 2017, 243, 779–789. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Joel, C.H.; Sutopo, C.C.Y.; Prajitno, A.; Su, J.-H.; Hsu, J.-L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathy, S.; Sumathi, P. Evaluation of antigenotoxic effects of carotenoids from green algae Chlorococcum humicola using human lymphocytes. Asian Pac. J. Trop. Biomed. 2012, 2, 109–117. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Phan, N.N.; Lu, W.-J.; Hieu, B.T.N.; Lin, Y.-C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory activities of microalgal fucoxanthin against α-amylase, α-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Gonen, A.; Gerber, Y.; Ben-Amotz, A.; Shaish, A. A 9-cis β-carotene–enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Gonen, A.; Ben-Shushan, D.; Kamari, Y.; Ben-Amotz, A.; Shaish, A. Supplementation with 9-cis β-carotene-rich alga Dunaliella improves hyperglycemia and adipose tissue inflammation in diabetic mice. J. Appl. Phycol. 2013, 25, 687–693. [Google Scholar] [CrossRef]
- Li, Z.-S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. α-Glucosidase inhibitory activities of lutein and zeaxanthin purified from green alga Chlorella ellipsoidea. J. Ocean Univ. China 2018, 17, 983–989. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharmacal Res. 2003, 26, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-F.; Yi, Y.-H.; Yao, X.-S.; Xu, Q.-Z.; Zhang, S.-Y.; Lin, H.-W. Bioactive steroids from the brown alga Sargassum carpophyllum. J. Asian Nat. Prod. Res. 2002, 4, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24 (S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Nguyen, D.H.; Wagle, A.; Woo, M.H.; Jug, H.A.; Choi, J.S. Experimental and computational study to reveal the potential of non-polar constituents from Hizikia fusiformis as dual protein tyrosine phosphatase 1B and α-glucosidase inhibitors. Mar. Drugs 2019, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kim, D.H.; Seong, S.H.; Kim, H.-R.; Jung, H.A.; Choi, J.S. α-Glucosidase and protein tyrosine phosphatase 1B inhibitory activity of plastoquinones from marine brown alga Sargassum serratifolium. Mar. Drugs 2017, 15, 368. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the bioactive diet components on the gene expression regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef]
| Source | Target Disease/Mechanism | Bioactive Component | Model Used | Biological Effect | Reference |
|---|---|---|---|---|---|
| Ecklonia cava | Diabetes mellitus/α-glucosidase and α-amylase inhibition | Fucodiphloroethol G, dieckol, 6,6′-bieckol, 7-phloroeckol, phlorofucofuroeckol A | in vitro | – α-Glucosidase inhibitory activity (IC50): fucodiphloroethol G (19.52 μM/L−1), dieckol (10.79 μM/L−1), 6,6′-bieckol (22.22 μM/L−1), 7-phloroeckol G (49.49 μM/L−1), phlorofucofuroeckol A (19.71 μM/L−1) – α-Amylase inhibitory activity (IC50): fucodiphloroethol G (>500 μM/L−1), dieckol (124.98 μM/L−1), 6,6′-bieckol (>500 μM/L−1), 7-phloroeckol G (250.02 μM/L−1), phlorofucofuroeckol A (>500 μM/L−1) | [49] |
| Diabetes mellitus/α-glucosidase inhibition | Eckol, 2-phloroeckol, 8,8′-bieckol, 6,8′-bieckol, 2-O-(2,4,6-trihydroxyphenyl)-6,6′-bieckol | in vitro | – α-Glucosidase inhibitory activity (IC50): Eckol (59.8 ± 0.8 μM), 2-phloroeckol (32.5 ± 2.1 μM), 8,8′-bieckol (12.5 ± 3.1 μM, competitive), 6,8′-bieckol (2.3 ± 1.2 μM, competitive), 2-O-(2,4,6-trihydroxyphenyl)-6,6′-bieckol (123.1 ± 2.4 μM, competitive) | [48] | |
| Diabetes mellitus/α-glucosidase and α-amylase inhibition, INS-1 cell protection against glucotoxicity | 2,7″-phloroglucinol-6,6′-bieckol | in vitro and in vivo | – α-Glucosidase inhibitory activity (IC50): 2,7″-phloroglucinol-6,6′-bieckol (23.35 μM) – α-Amylase inhibitory activity (IC50): 2,7″-phloroglucinol-6,6′-bieckol (6.94 μM) - Glucose response curve: 2,7″-phloroglucinol-6,6′-bieckol (2349.3 mmol·min/L) – 2,7″-phloroglucinol-6,6′-bieckol protects pancreatic β cells against high glucose-induced apoptosis | [50,60] | |
| Diabetes mellitus/α-glucosidase and α-amylase inhibition, postprandial hyperglycemia inhibition | Dieckol | in vitro and in vivo | – α-Glucosidase inhibitory activity (IC50): dieckol (0.24 mM) – α-amylase inhibitory activity (IC50): dieckol (0.66 mM) – Area under the curve of postprandial glucose responses in streptozotocin-induced diabetic mice: diabetic mice (483 mmol·min/L), dieckol 100 mg/kg body weight (259 mmol·min/L) | [51] | |
| Ecklonia cava | Diabetes mellitus/activation of both AMPK and Akt signaling pathways | Dieckol | in vivo | – Administration of 20 mg/kg body weight dieckol was reduced blood glucose, serum insulin level, and body weight | [61] |
| Obesity/anti-adipogenesis | Triphlorethol-A, eckol, dieckol | in vitro | – Triphlorethol-A, eckol, dieckol (20 μM): decreased intracellular lipid accumulation and increased intracellular calcification with an intervention in differentiation pathways at 3T3-L1 and MC3T3-E1 cell lines, respectively | [70] | |
| Obesity/inhibition of adipogenesis | Dieckol, 6,6′-bieckol, phlorofucoeckol A | in vitro | – Phlorotannin compounds such as dieckol, 6,6′-bieckol, and phlorofucoeckol A in Ecklonia cava inhibit intracellular lipid accumulation. In particular, dieckol suppressed adipogenesis in 3T3-L1 cells by suppressing the expression of PPARγ, C/EBPα, SREBP-1, and FABP4. | [71] | |
| Obesity/suppresses lipid accumulation and adipogenesis | Dieckol | in vitro and in vivo | – Dieckol inhibits early adipogenic events by suppressing cell cycle progression, and plays important roles in regulating AMPKα, ERK, and AKT signaling to inhibit lipid accumulation on high-fat diet-fed zebrafish, mice and 3T3-L1 models | [72] | |
| Obesity/reduced leptin resistance | Dieckol, 2,7-phloroglucinol-6,6-bieckol, pyrogallol-phloroglucinol-6,6-bieckol, phlorofucofuroeckol A | in vitro | – Phlorotannin compounds such as dieckol, 2,7-phloroglucinol-6,6-bieckol, pyrogallol-phloroglucinol-6,6-bieckol, and phlorofucofuroeckol A isolated from Ecklonia cava had the most potent effect on attenuating leptin resistance | [79] | |
| Hypertension/ACE inhibition | 6,6′-Bieckol | in vitro | – ACE inhibitory activity (IC50): 0.42 mM | [28] | |
| Hypertension/ACE inhibition | Phloroglucinol, Triphlorethol-A, eckol, dieckol, eckstolonol | in vitro | – ACE inhibitory activity (IC50): phloroglucinol (2.57 mM), triphlorethol-A (2.01 mM), eckol (2.27 mM), dieckol (1.47 mM), eckstolonol (2.95 mM) | [26] | |
| Hypertension/vascular smooth muscle cell proliferation and migration | Dieckol, 2,7-phloroglucinol-6,6-bieckol, phlorofucofuroeckol A, pyrogallol-phloroglucinol-6,6-bieckol | in vitro | – Inhibits monocyte migration and differentiation to inflammatory macrophages and monocyte associated vascular cell dysfunction | [37] | |
| Ecklonia cava | Hypertension/improved blood circulation | Pyrogallol-phloroglucinol-6,6′-bieckol (PPB) | in vitro and in vivo | – PPB improved blood circulation, including reduced adhesion molecule expression, endothelial cell death, excessive vascular smooth muscle cell proliferation, and migration– PPB remarkably reduced blood pressure, serum cholesterol, and lipoprotein levels in vivo | [38] |
| Hypertension/promotion of vasodilation | Dieckol | in vitro and in vivo | – Dieckol effectively promoted endothelial-dependent NO production by activating the PI3K/Akt/eNOS pathway and [Ca2+]cytosol regulation – Dieckol promotes vasodilation by increasing the DA diameter, further regulating blood-flow velocity in a zebrafish model | [35] | |
| Hyperlipidemia/reduction of total cholesterol, triglyceride, low-density lipoprotein | Dieckol | in vitro and in vivo | – In vitro: 200 μg/mL dieckol inhibited adipocyte differentiation, intracellular triglyceride accumulation, and lipid accumulation in 3T3-L1 cells – In vivo: administration of dieckol reduced total cholesterols, triglycerides and low-density lipoproteins in the serum of high-fat diet mice | [82] | |
| Ecklonia stolonifera | Diabetes mellitus/protein tyrosine phosphatase 1B, α-glucosidase inhibition | Phloroglucinol, dioxinodehydroeckol, eckol, phlorofucofuroeckol-A, dieckol, 7-phloroeckol | in vitro | – Protein tyrosine phosphatase 1B inhibitory activity (IC50): phloroglucinol (55.48 μM), dioxinodehydroeckol (29.97 μM), eckol (2.64 μM), phlorofucofuroeckol-A (0.56 μM), dieckol (1.18 μM), 7-phloroeckol (2.09 μM) – α-Glucosidase inhibitory activity (IC50): phloroglucinol (141.18 μM), dioxinodehydroeckol (34.60 μM), eckol (22.78 μM), phlorofucofuroeckol-A (1.37 μM), dieckol (1.61 μM), 7-phloroeckol (6.13 μM) | [52] |
| Obesity/inhibition of lipid accumulation and adipocyte differentiation, modulation of adipocyte marker gene expression | Phloroglucinol, eckol, dieckol, dioxinodehydroeckol, phlorofucofuroeckol A | in vitro | – Phlorotannins, such as phloroglucinol, eckol, dieckol, dioxinodehydroeckol, and phlorofucofuroeckol A isolated from Ecklonia stolonifera was reduced lipid accumulation in 3T3-L1 cell line – These phlorotannin compounds suppressed adipocyte differentiation through inhibiting C/EBPα and PPARγ expression | [73] | |
| Hypertension/ACE inhibition | Phloroglucinol, eckstolonol, eckol, phlorofucofuroeckol A, dieckol, Triphlorethol-A, fucosterol | in vitro | – ACE inhibitory activity (IC50) of phloroglucinol: (N.A.), eckstolonol (410.12 μM), eckol (70.82 μM), phlorofucofuroeckol A (12.74 μM), dieckol (34.25 μM), Triphlorethol-A (700.9 μM), fucosterol (N.A.) | [27] | |
| Ecklonia stolonifera | Hyperlipidemia/reduction of Cu2+-induced LDL oxidation | Phloroglucinol, dioxinodehydroeckol, eckol, phlorofucofuroeckol-A, dieckol, 7-phloroeckol | in vitro | – Cu2+-induced LDL oxidation inhibitory activity (IC50): phloroglucinol (87.30 μM), dioxinodehydroeckol (16.57 μM), eckol (7.47 μM), phlorofucofuroeckol-A (4.34 μM), dieckol (3.10 μM), 7-phloroeckol (9.07 μM) | [80] |
| Hyperlipidemia/reduction of total cholesterol, triglyceride, low-density lipoprotein-cholesterol, and atherogenic index | Eckol, dieckol | in vivo | – Poloxamer 407-induced hyperlipidemic rats model: 20 mg/kg BW eckol—TC level (255.6 mg/dL → 157.0 mg/dL), TG level (240.2 mg/dL → 174.9 mg/dL), LDL-C level (145.1 mg/dL →63.1 mg/dL), AI (3.47 → 1.77) 20 mg/kg BW dieckol—TC level (255.6 mg/dL → 144.7 mg/dL), TG level (240.2 mg/dL → 165.7 mg/dL), LDL-C level (145.1 mg/dL →35.5 mg/dL), AI (3.47 → 0.95) – High-cholesterol diet rats model: 20 mg/kg BW eckol—TC level (239.9 mg/dL → 226.3 mg/dL), TG3 level (271.1 mg/dL → 256.7 mg/dL), LDL-C3 level (160.6 mg/dL → 146.8 mg/dL), AI (7.55 → 7.14) 20 mg/kg BW dieckol—TC level (239.9 mg/dL → 200.7 mg/dL), TG level (271.1 mg/dL → 219.8 mg/dL), LDL-C level (160.6 mg/dL → 125.4 mg/dL), AI (7.55 → 5.53) | [81] | |
| Eisenia bicyclis | Diabetes mellitus/α-fucosidase, β-galactosidase, β-mannosidase inhibition | Phloroglucinol, phloroglucinol tetramer, eckol, phlorofucofuroeckol A, dieckol, 8,8′-bieckol | in vitro | – Among the 6 phlorotannin compounds isolated from Eisenia bicyclis, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol showed α-fucosidase, β-galactosidase, and β-mannosidase inhibitory activity. On the other hand, phloroglucinol, phloroglucinol tetramer, and eckol showed a weak activity of inhibiting these enzymes. – Dieckol was exhibited as a competitive inhibitor of α-fucosidase with an inhibition constant (K1) of 0.12 mM | [83] |
| Obesity/pancreatic lipase inhibitory activity | Eckol, fucofuroeckol A, 7-phloroeckol, dioxindehydroeckol, phlorofucofuroeckol A, dieckol | in vitro | – Pancreatic lipase inhibitory activity (IC50): eckol (76.6 μM), fucofuroeckol A (37.2 μM), 7-phloroeckol (12.7 μM), dioxindehydroeckol (>200 μM), phlorofucofuroeckol A (>200 μM), dieckol (99.3 μM) | [78] | |
| Obesity/inhibition of lipid accumulation and adipocyte differentiation | 6,6′-bieckol, 6,8′-bieckol, 8,8′-bieckol, dieckol, phlorofucofuroeckol-A | in vitro | – Phlorotannin compounds such as 6,6′-bieckol, 6,8′-bieckol, 8,8′-bieckol, dieckol, and phlorofucofuroeckol-A isolated from Eisenia bicyclis showed suppressed differentiation of 3T3-L1 adipocyte through downregulation of adipogenesis and lipogenesis | [74] | |
| Ishige okamurae | Diabetes mellitus/improve glucose homeostasis | Ishophloroglucin A (IPA) | in vivo | – Administration of 1.35 mg/kg BW IPA improved glucose homeostasis in high-fat diet-fed mice – IPA ameliorated glucose intolerance, reducing fasting glucose levels and 2 h glucose levels in high-fat diet-fed mice – IPA protect pancreatic function in high-fat diet-fed mice through pancreatic β-cells and C-peptide – Administration of IPA improves glucose homeostasis by increasing glucose transporter 4 levels in the muscles of high-fat diet-fed mice | [62] |
| Diabetes mellitus/human umbilical vein endothelial cell protection against high glucose-induced oxidative stress | Diphlorethohydroxycarmalol (DPHC) | in vitro | – DPHC prevented human umbilical vein endothelial cells from high glucose-induced damage through restoring cell viability, suppressed lipid peroxidation, reduced intracellular reactive oxygen species, and nitric oxide level | [84] | |
| Diabetes mellitus/α-glucosidase and α-amylase inhibition | Diphlorethohydroxycarmalol (DPHC) | in vitro and in vivo | – α-Glucosidase inhibitory activity (IC50): 0.16 mM – α-Amylase inhibitory activity (IC50): 0.53 mM – Administration of 100 mg/kg DPHC was reduced blood glucose level in streptozotocin-induced diabetic mice – Postprandial glucose response: normal mice (965 mmol·min/L), diabetic mice (2210 mmol·min/L), DPHC treated mice (1964 mmol·min/L) | [55] | |
| Diabetes mellitus/α-glucosidase inhibition | Ishophloroglucin A (IPA), diphlorethohydroxycarmalol (DPHC) | in vitro | – α-Glucosidase inhibitory activity (IC50): IPA (54.97 μM), DPHC (175.78 μM) | [53] | |
| Diabetes mellitus/inhibition of abnormal angiogenesis, vascular dysfunction | Diphlorethohydroxycarmalol (DPHC) | in vitro and in vitro | – DPHC treatment suppressed the phosphorylation of VEGFR-2 and down-regulation of angiogenesis-related key mechanisms | [64] | |
| Diabetes mellitus/protect RINm5F pancreatic β cells from high glucose-induced damage | Diphlorethohydroxycarmalol (DPHC) | in vivo | – DPHC treatment inhibited the apoptotic cell death of RINm5F pancreatic β cell via decrease of thiobarbituric acid reactive substances, intracellular reactive oxygen species generation, and nitric oxide level | [85] | |
| Ishige okamurae | Diabetes mellitus/anti-angiogenic effect | Ishophloroglucin A (IPA) | in vitro | – IPA effectively inhibited high glucose-induced endothelial cell proliferation, migration, and capillary formation, and exhibited an anti-angiogenic effect by interfering with the VEGFR-2 signaling pathway | [65] |
| Obesity/reduction of total cholesterol, triglyceride, low-density lipoprotein-cholesterol, and atherogenic index | Diphlorethohydroxycarmalol (DPHC) | in vivo | – Triglyceride levels: high-fat diet mice 137.88 mg/dL → 50 mg/kg BW 86.73 mg/dL – High-density lipoprotein cholesterol levels: high-fat diet mice 50.49 mg/dL → 50 mg/kg BW 72.71 mg/dL – Low-density lipoprotein cholesterol levels: high-fat diet mice 22.24 mg/dL → 50 mg/kg BW 16.82 mg/dL – Leptin levels: high-fat diet mice 2.04 ng/dL → 50 mg/kg BW 1.23 ng/dL | [86] | |
| Obesity/induces apoptosis in 3T3-L1 preadipocytes | Diphlorethohydroxycarmalol (DPHC) | in vitro | – DPHC treatment increased the number of early and late apoptotic cells in 3T3-L1 pre-adipocytes – DPHC mediated apoptotic cell death via the activation of caspase-3, caspase-8, and Bax | [75] | |
| Obesity/anti-adipogenesis | Diphlorethohydroxycarmalol (DPHC) | in vitro | – DPHC showed an anti-adipogenic effect via regulation of ECM during adipogenesis – DPHC treatment positively affects normal adipose tissue generation and acts as a suppressor of abnormal ECM structures | [76] | |
| Obesity/inhibition of lipid accumulation and suppressed adipogenesis via AMPK activation | Diphlorethohydroxycarmalol (DPHC) | in vitro | – DPHC treatment inhibited the fat accumulation by activating AMPK and ACC phosphorylation in 3T3-L1 adipocytes | [77] | |
| Hypertension/vasodilatory effect through increasing calcium intake level | Diphlorethohydroxycarmalol (DPHC) | in vitro and in vivo | – DPHC stimulated NO production by increasing calcium levels and endothelial nitric oxide synthase expression – DPHC modulated Ca2+ levels by activating AchR and VEGFR2 – DPHC modulated calcium transit through AchR and VEGFR2, increasing endothelial-dependent NO production in a zebrafish model | [36] | |
| Ishige foliacea | Diabetes mellitus/anti-diabetogenic effect | Octaphlorethol A (OPA) | in vivo | – Administration of 10 mg/kg BW OPA increased anti-apoptotic (Bcl-xL) and pro-apoptotic (Bax) protein expression level and increased antioxidant enzymes (SOD, CAT, GSH). | [87] |
| Ishige foliacea | Diabetes mellitus/impaired glucose tolerance improvement | Octaphlorethol A (OPA) | in vivo | – OPA treatment significantly decreased postprandial blood glucose levels in db/db mice – OPA supplements significantly improved fasting blood glucose levels and impaired glucose tolerance, decreased serum insulin levels, augmented the activation of AMPK, and increased the expression of GLUT4 in skeletal muscle | [63] |
| Diabetes mellitus/pancreatic β cells protection | Octaphlorethol A (OPA) | in vitro | – Pretreatment with 50 μg/mL OPA decreased the streptozotocin-induced pancreatic β cells damage by reducing the thiobarbituric acid reactive substances and intracellular ROS generation – OPA treatment increased the activity of antioxidant enzymes such as CAT, SOD, GSH in STZ-treated pancreatic β cells | [88] | |
| Diabetes mellitus/α-glucosidase inhibition | Octaphlorethol A (OPA) | in vitro and in vivo | – α-glucosidase inhibitory activity (IC50): OPA (0.11 mM) – α-glucosidase molecular docking: binding energy (−140.98 kcal mol−1) – OPA interacts with Phe575, His600, Arg526, Met444, Asp542, Tyr605, Ser448, Asp203, Lys480, and Phe450 – OPA treatment suppressed increases in postprandial blood glucose levels | [54] | |
| Ishige sinicola | Hypertension/ACE inhibition | Octaphlorethol A (OPA) | in vitro | – ACE inhibitory activity (IC50): OPA (59 μM) – OPA exhibited an anti-hypertensive effect via AMPK and Akt activation in endothelial cells | [29] |
| Source | Target Disease/Mechanism | Bioactive Component | Model Used | Biological Effect | Reference |
|---|---|---|---|---|---|
| Sargassum wightii | Diabetes mellitus/α-glucosidase and α-amylase inhibition Hypertension/ACE inhibition | Sulfated polygalactofucan | in vitro | – α-Glucosidase activity (IC50): 1.48 mg/mL – α-Amylase activity (IC50): 0.93 mg/mL – ACE inhibitory activity (IC50): 0.22 mg/mL | [94] |
| Diabetes mellitus/α-amylase inhibition | Fucoidan | in vitro | – α-Amylase activity (IC50): 103.83 μg | [95] | |
| Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-Glucosidase activity (IC50): 132 μg | [96] | |
| Sargassum fusiforme | Diabetes mellitus/inhibition of hyperglycemia, altered the composition of gut microbiota | Fucoidan | in vivo | – Administration of fucoidan decreased fasting blood glucose levels, dietary and water intake, and alleviated pathological changes in the heart and liver – Fucoidan supplements altered the composition of gut microbiota in STZ-induced diabetic mice | [103] |
| Diabetes mellitus/inhibition of hyperglycemia, altered the composition of gut microbiota | Fucoidan | in vivo | – Administration of fucoidan decreased fasting blood glucose, food consumption, water intake, and serum lipid levels in high-fat diet/STZ-induced diabetic mice – Administration of fucoidan altered the composition of gut microbiota and increased the levels of carnitine and choline in the colon | [105] | |
| Diabetes mellitus/improved insulin sensitivity, altered the composition of gut microbiota | Fucoidan | in vivo | – Administration of fucoidan reduced fasting blood glucose and insulin resistance indexes and improved glucose tolerance – Administration of fucoidan increased the abundance and diversity of gut microbiota in obese mice and improved intestinal integrity | [104] | |
| Sargassum hemiphyllum | Diabetes mellitus/prevention of pancreatic β cell damage and dysfunction | Fucoidan | in vitro and in vivo | – Fucoidan treatment attenuated pancreatic β cell death, pancreatic islet mass loss, and dysfunction – Fucoidan treatment increased insulin synthesis via activation of Sirt-1-dependent upregulation of PDX and GLP-1R | [117] |
| Undaria pinnatifida | Diabetes mellitus/regulation of blood glucose homeostasis | Fucoidan | in vivo | – Serum insulin (μIU/mL): db/db mice (41.6) → db/db mice + fucoidan (37.7) – Fasting blood glucose (mg/dL): db/db mice (445) → db/db mice + fucoidan (257) | [118] |
| Diabetes mellitus/improved insulin-stimulated glucose uptake | Fucoidan | in vitro | – Fucoidan treatment stimulated glucose uptake and inhibited basal lipolysis in hypertrophied insulin resistance | [119] | |
| Hypertension/vascular dysfunction prevention | Fucoidan | in vivo | – Fucoidan treatment induce NO release and eNOS activation – In L-NAME-induced hypertensive rats, administration of fucoidan attenuated elevated blood pressure, increased endothelium-dependent vasodilation, and improved vascular elasticity | [109] | |
| Undaria pinnatifida | Obesity/inhibition of adipocyte differentiation | Fucoidan | in vitro | – Fucoidan treatment suppressed adipogenesis by inhibiting proliferator-activated receptor γ, CCAAR/enhancer-binding protein α, adipocyte protein 2, and lipid accumulation in 3T3-L1 cells | [120] |
| Saccharina longicruris | Diabetes mellitus/α-amylase inhibition | Fucoidan | in vitro | – α-amylase activity (%):fucoidan (1 mg/mL) inhibited α-amylase activity by 80.3% | [97] |
| Fucus vesiculosus | Diabetes mellitus/α-amylase inhibition | Fucoidan | in vitro | – α-amylase activity (%): harvested in October 2002: NA, commercial fucoidan from F. vesiculosus: NA | [97] |
| Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase activity (IC50): 0.049 mg/mL | [98] | |
| Diabetes mellitus/insulin stimulation and pancreatic protection | Fucoidan | in vitro and in vivo | – Fucoidan supplements increase insulin secretion and provide pancreatic protection via the cAMP signaling pathway | [121] | |
| Diabetes mellitus/α-amylase inhibition | Fucoidan | in vitro | – α-amylase inhibitory activity (IC50): 0.04 mg/mL | [99] | |
| Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 67.9 μg/mL | [100] | |
| Ascophyllum nodosum | Diabetes mellitus/α-amylase inhibition | Fucoidan | in vitro | – α-amylase activity (%):fucoidan (1 mg/mL) inhibited α-amylase activity by 83.2% | [97] |
| Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase activity according to harvest seasons (IC50): May (0.047 mg/mL), June (0.037 mg/mL), July (0.014–0.036 mg/mL), August (0.017–0.046 mg/mL), September (0.026–0.029 mg/mL), October (0.013 mg/mL), and November (0.014 mg/mL) | [98] | |
| Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 165.4 μg/mL | [100] | |
| Cystoseira crinita | Diabetes mellitus/α-amylase inhibition | Sulfated polysaccharide | in vitro and in vivo | – α-amylase inhibitory activity (IC50): 39.16 μg/mL – α-amylase inhibitory activity (IC50): in serum (23%), in pancreas (44.38%), and intestine (45%) | [108] |
| Hypertenstion/ACE inhibition | Sulfated polysaccharide | in vitro and in vivo | – ACE inhibitory activity (IC50): 58.35 μg/mL | [108] | |
| Saccharina japonica | Diabetes mellitus/reduced hyperglycemia | Fucoidan | in vivo | – Administration of 1200 mg/kg BW fucoidan reduced the blood glucose level by 34% – Increased serum insulin levels – Fucoidan supplements alter plasma lipid levels by lowering cholesterol, triglyceride, and low-density lipoprotein concentrations | [101] |
| Ecklonia maxima | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-amylase inhibitory activity (IC50): 0.29 mg/mL | [99] |
| Sargassum thumbergii | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 376.7 μg/mL | [100] |
| Sargassum honeri | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 351.0 μg/mL | [100] |
| Sargassum ringgoldianum | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 172.9 μg/mL | [100] |
| Sargassum siliquastrum | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 399.6 μg/mL | [100] |
| Sargassum graminifolium | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 271.7 μg/mL | [100] |
| Sargassum kjellmanianum | Diabetes mellitus/α-glucosidase inhibition | Fucoidan | in vitro | – α-glucosidase inhibitory activity (IC50): 415.2 μg/mL | [100] |
| Turbinaria conoides | Diabetes mellitus/α-amylase and α-d-glucosidase inhibition | Fucoidan | in vitro | – α-amylase inhibitory activity (IC50): 1.07 μM – α-d-glucosidase inhibitory activity (IC50): 0.68 μM | [102] |
| Laminaria japonica | Diabetes mellitus/protects endothelial function | Low-molecular-weight fucoidan | in vivo | – Administration of 200 mg/kg/day fucoidan protects vasoendothelial function and reduces basal blood pressure in type 2 diabetes rats | [107] |
| Padina tetrastromatica | Hypertension/cardioprotective effect | Sulfated polysaccharides | in vitro and in vivo | – Treatment of sulfated polysaccharides isolated from Padina tetrastromatica reduced isoproterenol-induced cardiac damage via activation of PI3K/Akt/Nrf2 signaling pathway | [106] |
| Atherosclerosis/reduced hyperlipidemia, endothelial dysfunction | Sulfated polysaccharides | in vitro and in vivo | – Sulfated polysaccharides treatment maintained lipid homeostasis by regulating the expressions of SREBP-2 and LDL-R – Administration of sulfated polysaccharides normalized ISO-induced oxidative damage, hyperlipidemia, endothelial dysfunction, and inflammation in a rat model. | [122] | |
| Sarconema filiforme | Modulation of cardiovascular and metabolic health parameters | Carrageenan | in vivo | – Administration of 5% carrageenan in high-fat diet-fed rats attenuated cardiovascular diseases and metabolic health parameters | [110] |
| Ulva Pertusa | Hyperlipidemia/modulating hyperlipidemia related parameters | Ulvan | in vivo | – Ulvan decreased total cholesterol and low-density lipoprotein levels in high cholesterol-fed rats – Ulvan treatment improved lipid profiles via regulating FXR, PPARγ, and LXR expression levels | [111] |
| Source | Target Disease/Mechanism | Bioactive Component | Model Used | Biological Effect | Reference |
|---|---|---|---|---|---|
| Undaria pinnatifida | Hypertension/ACE inhibition, lower SBP | Val-Tyr, Ile-Tyr, Ala-Trp, Phe-Tyr, Val-Trp, Ile-Trp, and Leu-Trp, Val-Tyr, Ile-Tyr, Phe-Tyr, and Ile-Tyr | in vitro | – ACE inhibitory activity (IC50): Val-Tyr (35.2 μM), Ile-Tyr (6.1 μM), Ala-Trp (18.8 μM), Phe-Tyr (42.3 μM), Val-Trp (3.3 μM), Ile-Trp (1.5 μM) and Leu-Trp (23.6 μM) – Lower SBP (Val-Tyr; 206.7 mmHg, Ile-Tyr;184.3 mmHg, Phe-Tyr; 193.0 mmHg, and Ile-Trp;199.5 mmHg) | [127] |
| Hypertension/ACE inhibition | Ala-Ile-Tyr-Lys, Tyr-Lys-Tyr-Tyr, Lys-Phe-Tyr-Gly, and Tyr-Asn-Lys-Leu | in vitro | – ACE inhibitory activity (IC50): Ala-Ile-Tyr-Lys (213 μM), Tyr-Lys-Tyr-Tyr (64.2 μM), Lys-Phe-Tyr-Gly (90.5 μM) and Tyr-Asn-Lys-Leu (21 μM) | [128] | |
| Undaria pinnatifida | Hypertension/ACE inhibition | Tyr-His, Lys-Tyr, Phe-Tyr, and Ile-Tyr | in vitro | – ACE inhibitory activity (IC50): Tyr-His (5.1 μM), Lys-Tyr (7.7 μM), Phe-Tyr (3.7 μM) and Ile-Tyr (2.7 μM) | [129] |
| Gracilariopsis lemaneiformis | Hypertension/ACE inhibition | FQIN [M(O)] CILR and TGAPCR | in vitro and in vivo | – ACE inhibitory activity (IC50): FQIN [M(O)] CILR (9.64 μM) and TGAPCR(23.94 μM) – Decrements in SBP: FQIN [M(O)] CILR (27 mmHg) and TGAPCR (25 mmHg) | [133] |
| Hypertension/ACE inhibition | Gln-Val-Glu-Tyr | in vitro | – ACE inhibitory activity (IC50): Gln-Val-Glu-Tyr (474.36 μM) | [134] | |
| Chlorophyta U. rigida | Hypertension/ACE inhibition | Ile-Pro and Ala-Phe-Leu | in vitro | – ACE inhibitory activity (IC50): Ile-Pro (87.6 μM), Ala-Phe-Leu (65.9 μM) | [135] |
| Sargassum maclurei | Hypertension/ACE inhibition | RWDISQPY | in vitro | – ACE inhibitory activity (IC50): 72.24 μM – Endothelin-1 suppressing capacity: 26.21% at 1.5 mg/mL | [136] |
| Mazzaella japonica | Hypertension/ACE inhibition | YRD | in vitro | – ACE inhibitory activity (IC50): YRD (320 μM) | [137] |
| Sargassum fusiforme | Hypertension/ACE inhibition | ly-Lys-Tyr, Ser-Val-Tyr and Ser-Lys-Thr-Tyr | in vitro | – ACE inhibitory effect (IC50): Gly-Lys-Tyr (3.92 μM) Ser-Val-Tyr (8.12 μM) and Ser-Lys-Tyr-Tyr (11.07 μM) | [138] |
| Ulva intestinalis | Hypertension/ACE inhibition | Phe-Gly-Met-Pro-Leu-Asp-Arg and Met-Glu-Leu-Val-Leu-Arg | in vitro | – ACE inhibitory activity (IC50): Phe-Gly-Met-Pro-Leu-Asp-Arg (219.35 μM) and Met-Glu-Leu-Val-Leu-Arg (236.85 μM) | [139] |
| Enteromorpha clathrate | Hypertension/ACE inhibition | Pro-Ala-Phe-Gly | in vitro | – ACE inhibitory activity (IC50): Pro-Ala-Phe-Gly(35.9 μM) | [140] |
| Pyropia Pseudolinearis | Hypertension/ACE inhibition | LRM | in vitro | – ACE inhibitory activity (IC50): LRM (0.15 μM) | [141] |
| Palmaria palmata | Hypertension/ACE inhibition | α subunits of PE (rPEα and rPEβ) | in vitro | – ACE inhibitory effect (%): rPEα (94.4%) and rPEβ (87.0%) | [130] |
| Diabetes mellitus/DPP-Ⅳ inhibitory activity | Ile-Leu-Ala-Pro, Leu-Leu-Ala-Pro and Met-Ala-Gly-Val-Asp-His-Ile | in vitro | – DPP-Ⅳ inhibitory activity (IC50): Ile-Leu-Ala-Pro (17.90 μg/mL), Leu-Leu-Ala-Pro (22.14 μg/mL), and Met-Ala-Gly-Val-Asp-His-Ile (118.23 μg/mL) | [131] | |
| Laminaria Japonica | Hypertension/ACE inhibition | KY, GKY, SKTY, AKY, AKYSY, KKFY, FY and KFKY | in vitro | – ACE inhibitory activity (IC50): KY (5.24 μM), GKY (7.94 μM), SKTY (20.63 μM), AKY (7.52 μM), AKYSY (2.42 μM), KKFY (15.33 μM), FY (4.83 μM), and KFKY (10.73 μM) | [142] |
| Bangia fusco-purpurea | Hypertension/ACE inhibition | r-phycoerythrin, ALLAGDPSVLEDR and VVGGTGPVDEWGIAGAR | in vitro | – ACE inhibitory activity (IC50): r-phycoerythrin (191.1 μg/mL), ALLAGDPSVLEDR (57.2 μg/mL), and VVGGTGPVDEWGIAGAR (66.2 μg/mL) | [143] |
| Porphyra spp. Caulerpa lentillifera | Diabetes mellitus/ | Gly-Gly-Ser-Lys and Glu-Leu-Ser | in vitro | – α-amylase inhibitory activity (IC50): Gly-Gly-Ser-Lys (2.58 mM) and Glu-Leu-Ser (2.62 mM) | [144] |
| Hypertension/ACE inhibition | FDGIP and AIDPVRA | in vitro | – ACE inhibitory activity (IC50): FDGIP (58.89 μM) and AIDPVRA (65.76 μM) | [145] | |
| Porphyra dioica | Hypertension/ACE inhibition | Thr-Tyr-Ile-Ala and Tyr-Leu-Val-Ala | in vitro | – ACE inhibitory activity (IC50): Thr-Tyr-Ile-Ala (197.5 μM), Tyr-Leu-Val-Ala (259.7 μM), and Asp-Tyr-Tyr-Lys-Arg (628.9 μM) – DPP-Ⅳ inhibitory activity (IC50): Tyr-Leu-Val-Ala (439.5 μM) | [146] |
| Source | Target Disease/Mechanism | Bioactive Component | Model Used | Biological Effect | Reference |
|---|---|---|---|---|---|
| Undaria Pinnatifida | Atherosclerosis/decreased in serum TG levels | Fucoxanthin | in vivo | – Decreased tryglycerides in plasma: 19.4 mg/100 mL – Enhancement of HDL-cholesterol in plasma: 7.5 mg/100 mL | [153] |
| Codium fragile | Obesity/altered in serum lipid | Siphonaxanthin | in vivo | – Decreased serum lipid: total-C (21 mg/dL) and tryglyceride (16 mg/dL) | [157] |
| Phaeodactylum tricornutum | Diabetes mellitus/α-amylase and α-glucosidase inhibition | Fucoxanthin | in vitro | – Enzyme inhibitory activity (Ki): α-amylase (0.13 mM) and α-glucosidase (0.05 mM) | [154] |
| Sargassum fusiforme | Atherosclerosis/LXRβ agonist activity | 24(S)-Saringosterol | in vitro | – LXRβ-mediated transactivation: 3.50-fold vs 1.63-fold compared with control | [162] |
| Diabetes mellitus/α-glucosidase inhibition | 18α-glycyrrhetinic acid,18β-glycyrrhetinic acid, FAs C20:4 (Δ7,9,11,13), C17:3 (Δ8,11,14), neolignan, and trace | in vitro | – α-glucosidase inhibitory activity (IC50): 18α-glycyrrhetinic acid (113.30 μM), 18β-glycyrrhetinic acid (128.72 μM), FAs C20:4 (Δ7,9,11,13) (34.85 μM), C17:3 (Δ8,11,14) (43.90 μM), neolignan (133.84 μM), and trace amine (273.23 μM) | [163] | |
| Sargassum Serratifolium | Diabetes mellitus/α-glucosidase inhibition | Sargachromenol and sargaquinoic acid | in vitro | – α-glucosidase inhibitory activity (IC50): sargachromenol (42.41 μM) and sargaquinoic acid (96.17 μM) | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.-H.; Lu, Y.-A.; Kim, M.-Y.; Jeon, Y.-J.; Lee, S.-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025. https://doi.org/10.3390/app12031025
Cho C-H, Lu Y-A, Kim M-Y, Jeon Y-J, Lee S-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Applied Sciences. 2022; 12(3):1025. https://doi.org/10.3390/app12031025
Chicago/Turabian StyleCho, Chi-Heung, Yu-An Lu, Ming-Yeong Kim, You-Jin Jeon, and Sang-Hoon Lee. 2022. "Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment" Applied Sciences 12, no. 3: 1025. https://doi.org/10.3390/app12031025
APA StyleCho, C.-H., Lu, Y.-A., Kim, M.-Y., Jeon, Y.-J., & Lee, S.-H. (2022). Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Applied Sciences, 12(3), 1025. https://doi.org/10.3390/app12031025








