Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Extraction Conditions
2.3. HPLC/DAD Conditions
2.4. Total Phenol (TPC) and Flavonoid (TFC) Contents
2.5. Antioxidant Activity
2.6. Acetylcholinesterase Inhibition Assay
2.7. Lipase Inhibitory effect
2.8. Inhibition Assay for α-Glucosidase Activity
2.9. Antimicrobial Potential
2.10. FT-Raman Spectral Acquisition
2.11. Statistical Analysis
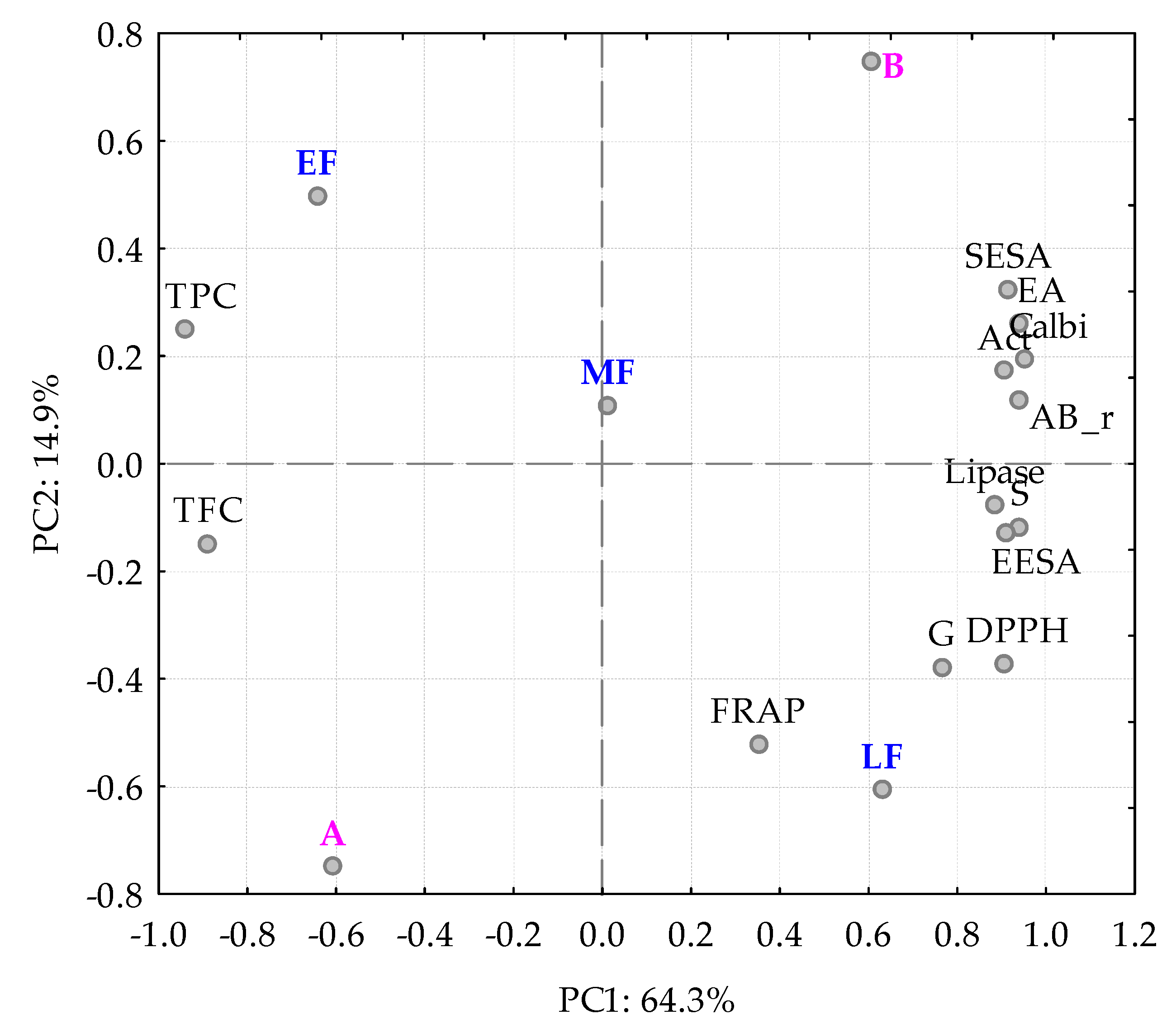
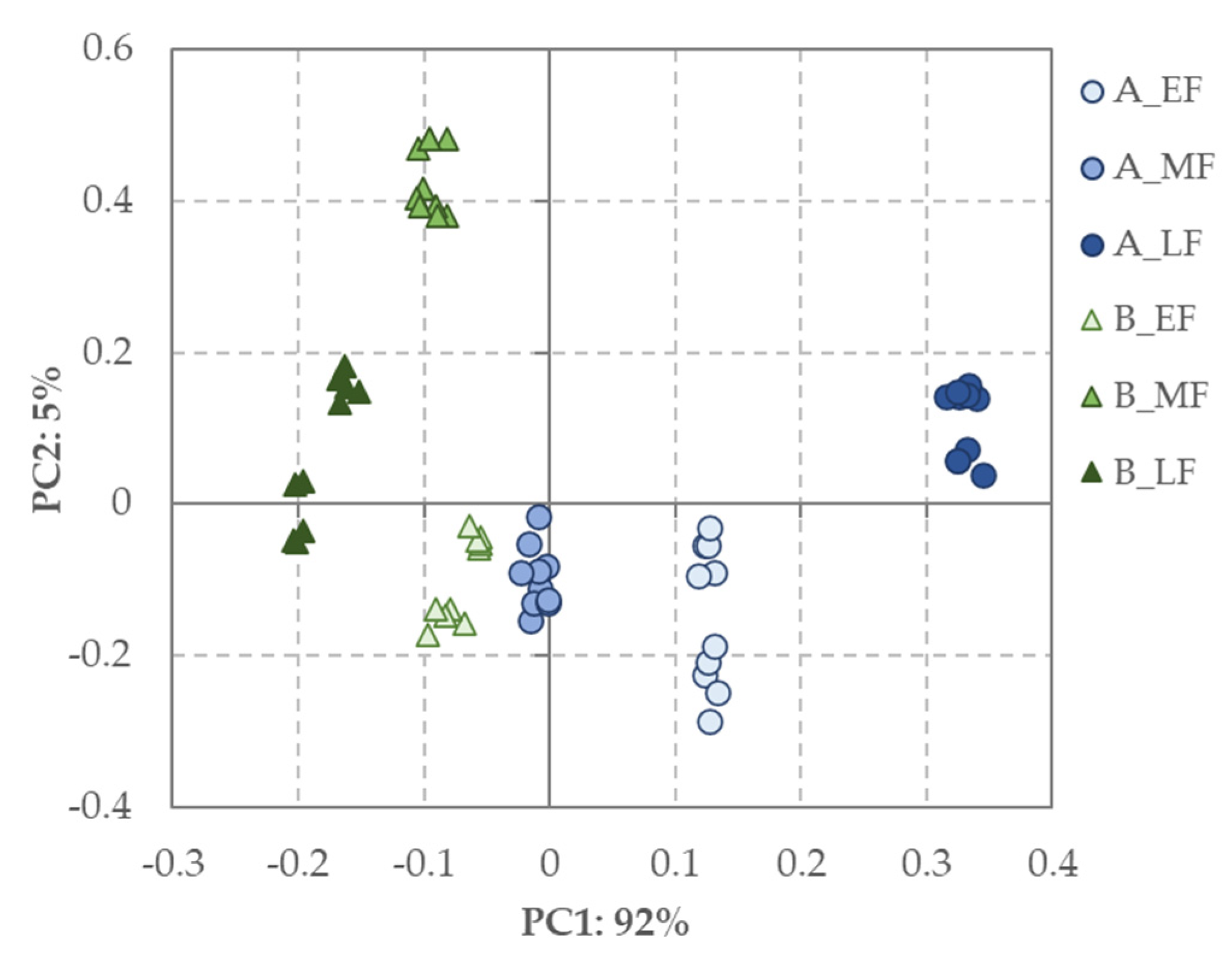
3. Results and Discussion
3.1. Characterization of the Extract
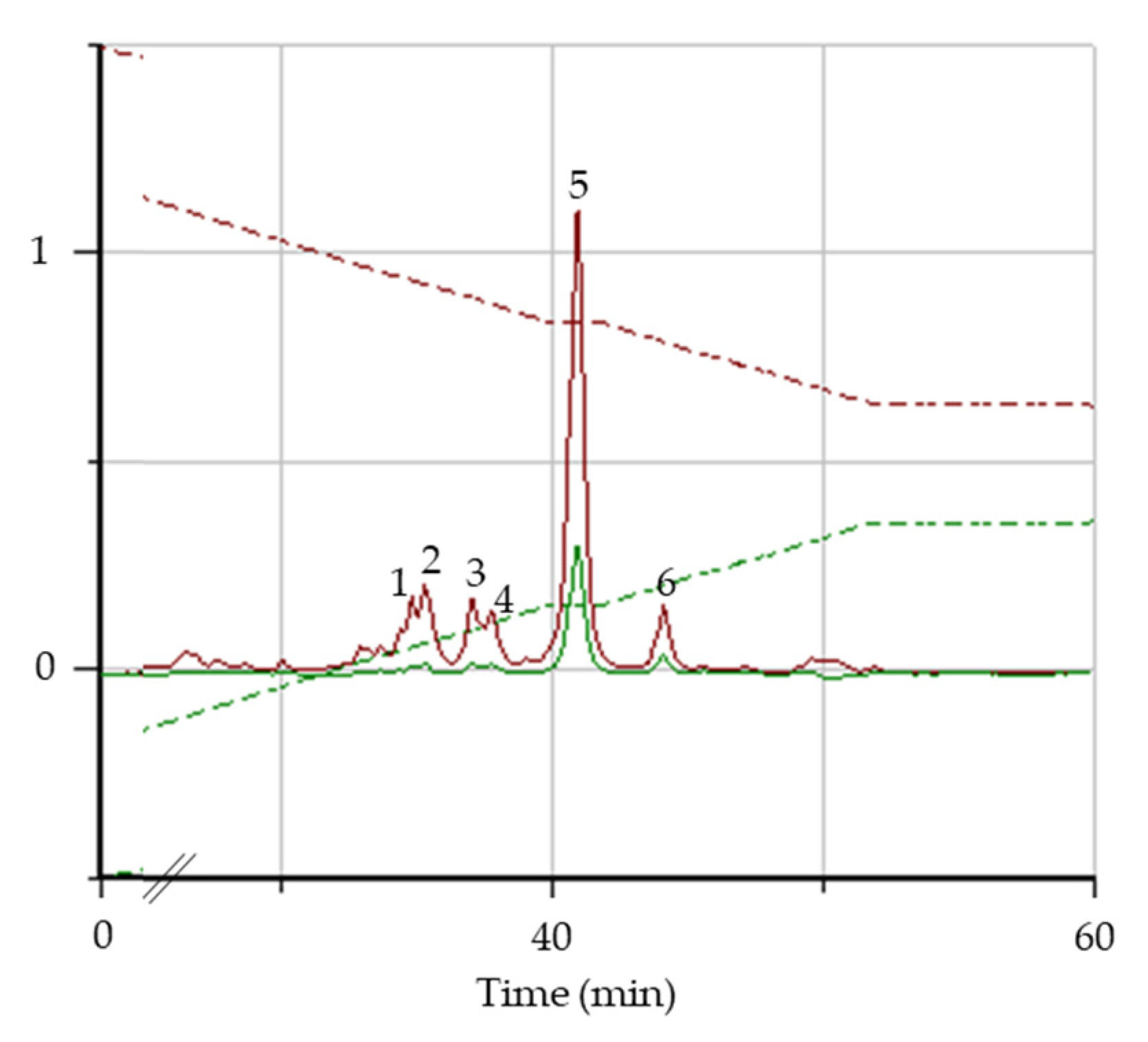
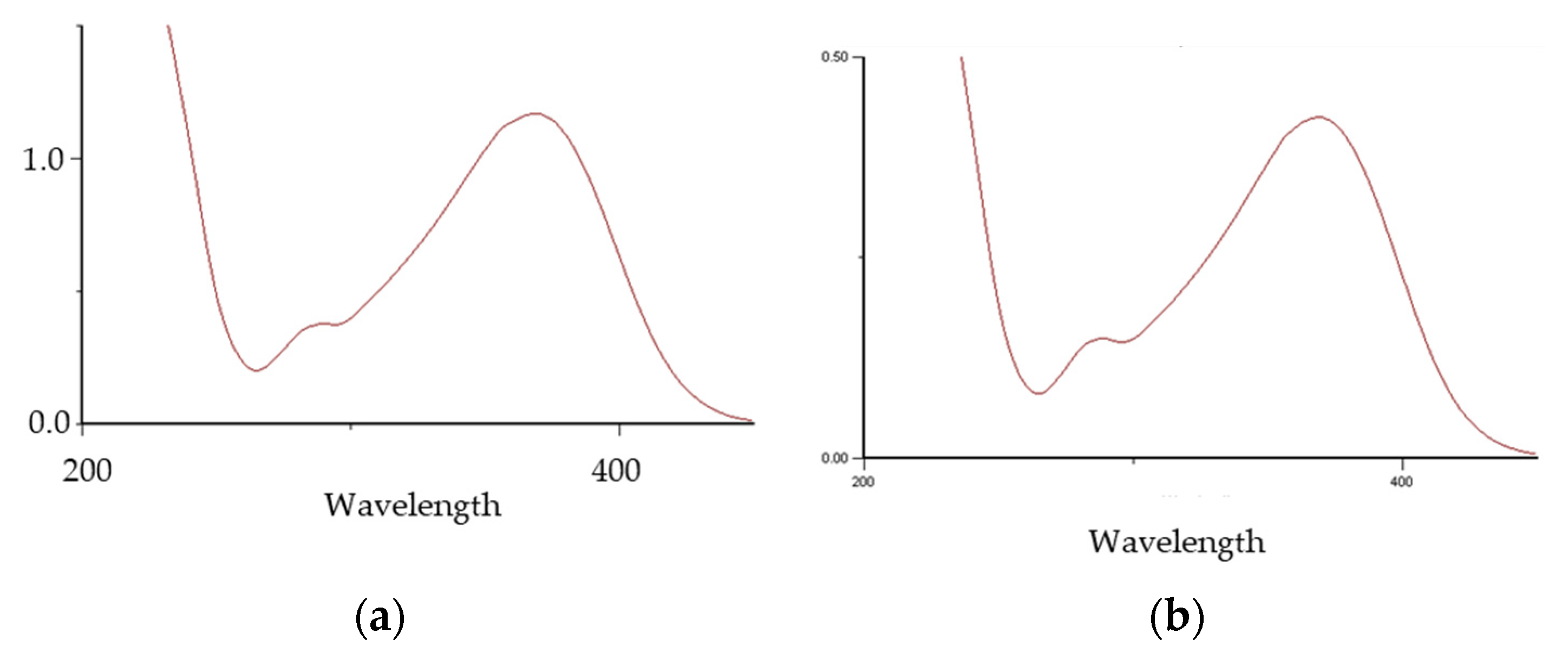
3.1.1. HPLC/DAD
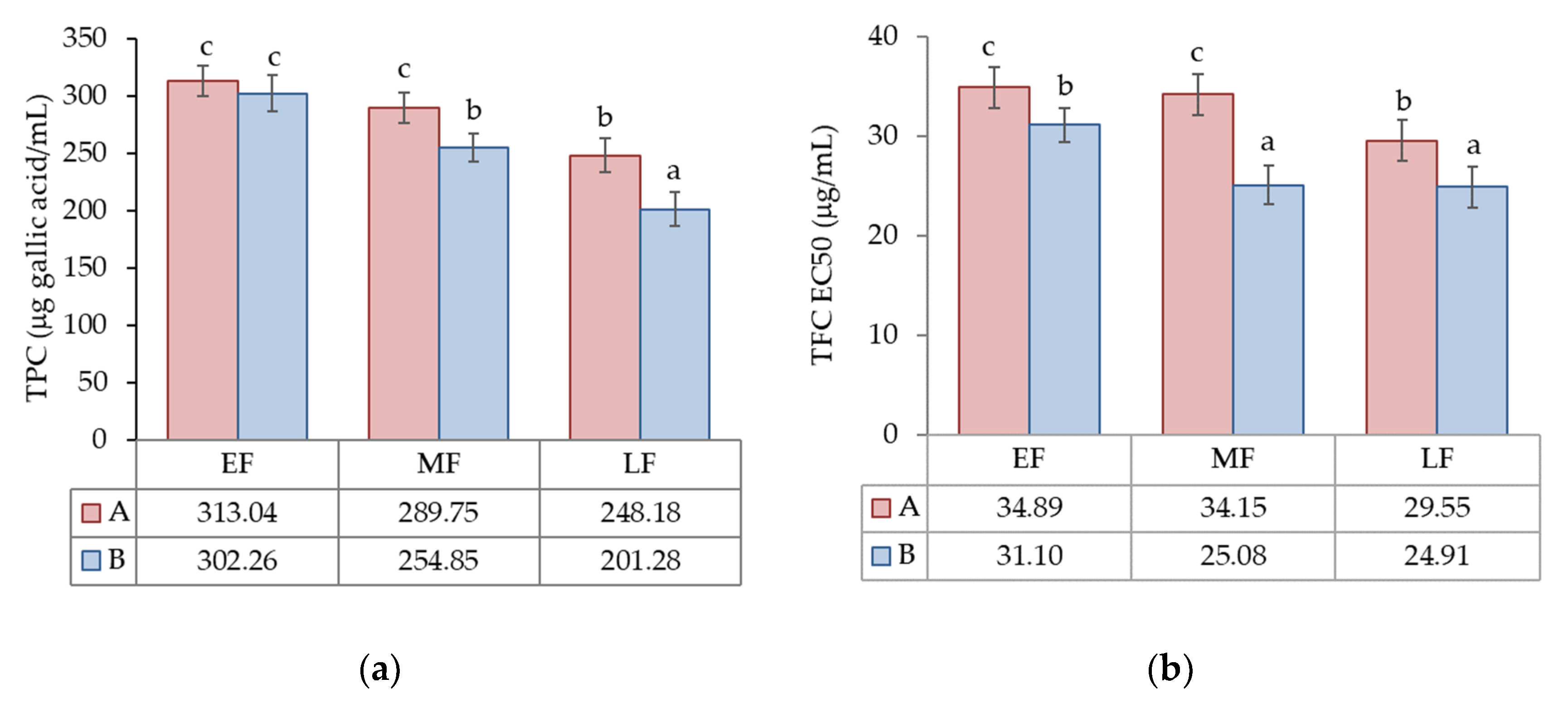
3.1.2. Total Phenol (TPC) and Flavonoid (TFC) Contents
3.2. Bioactivities
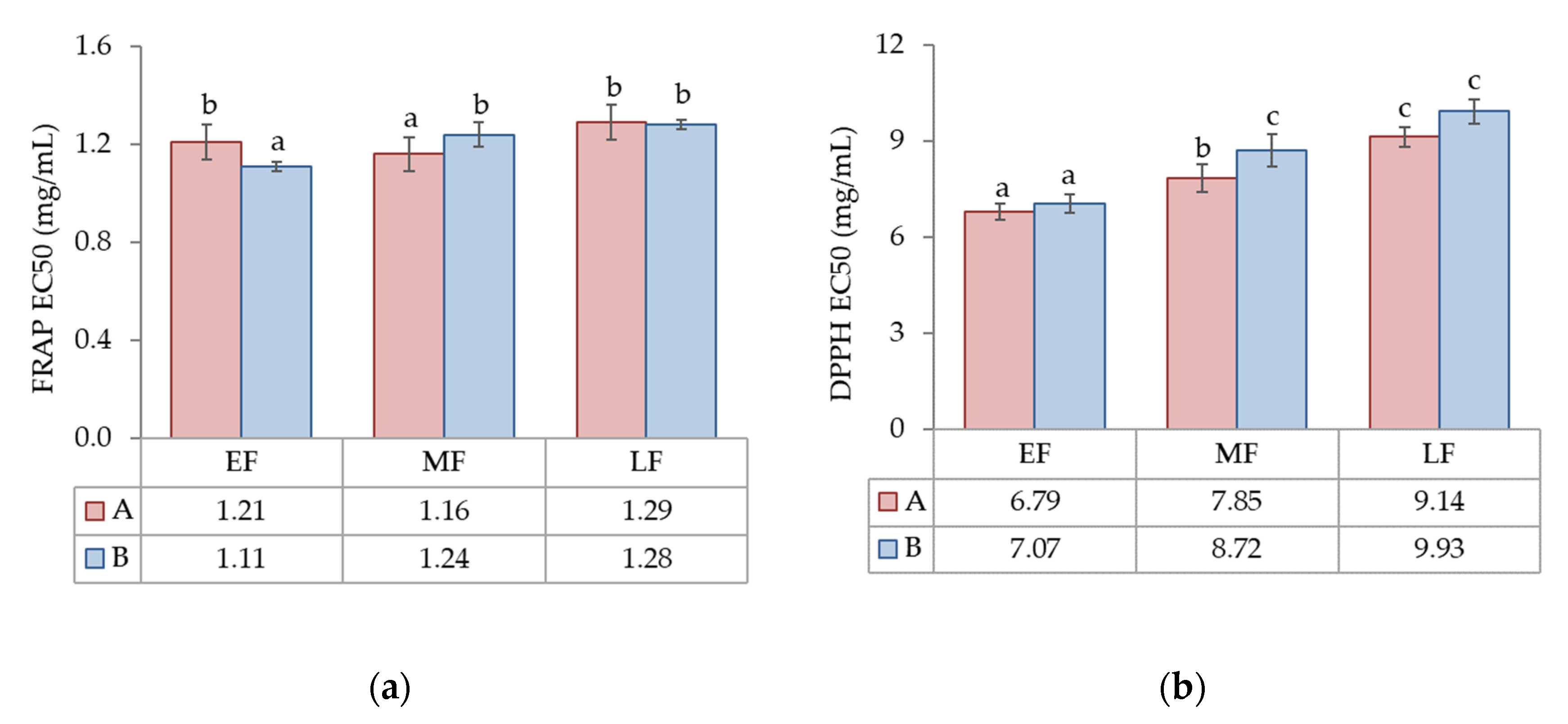
3.2.1. Antioxidant Activity
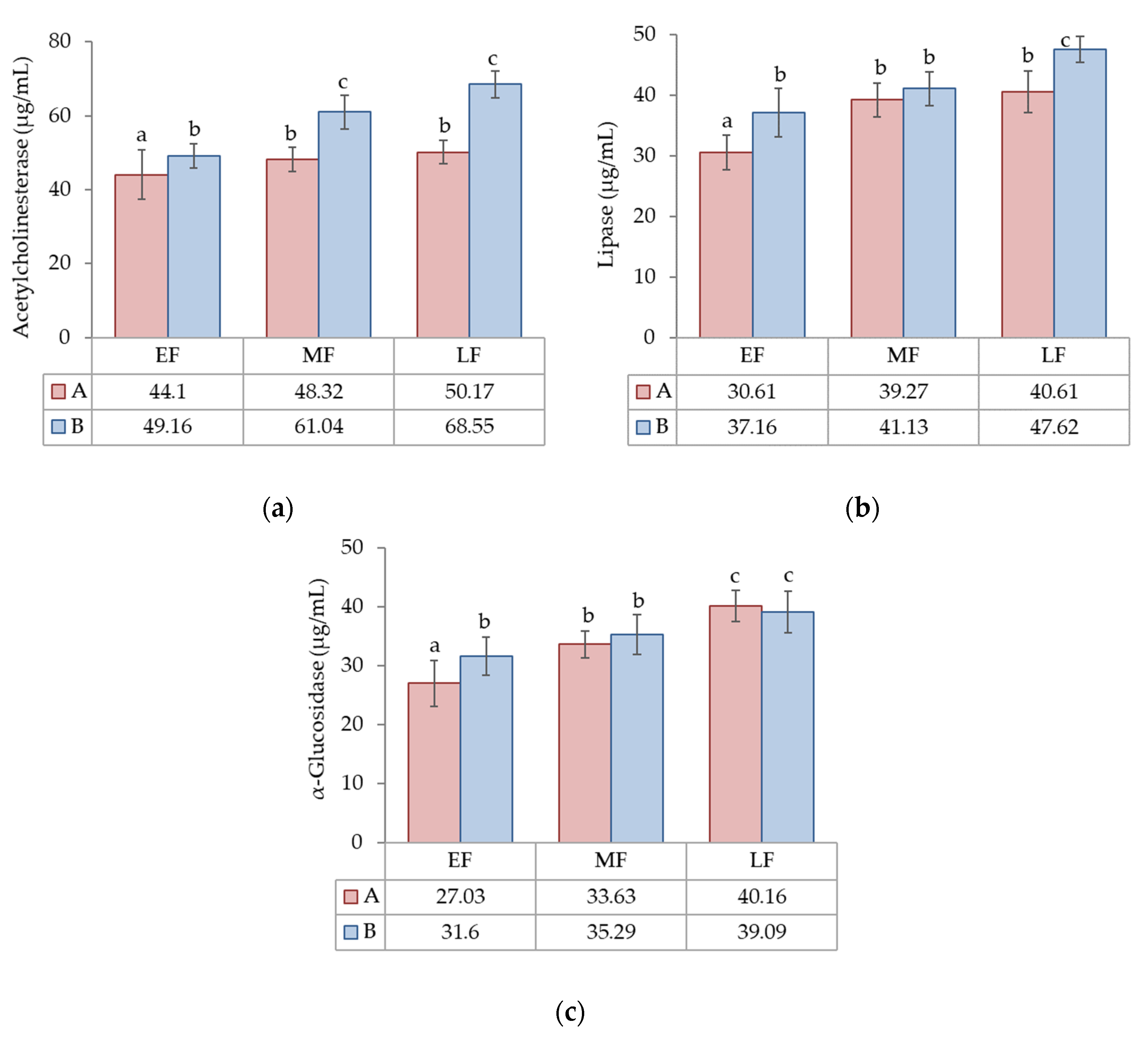
3.2.2. Enzyme Inhibitory Activity (Acetylcholinesterase, Lipase and α-Glucosidase Enzymes)
3.2.3. Antimicrobial Bioactivity
3.3. FT-Raman Spectral Acquisition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammad, D.; Chan, P.; Bradley, J.; Lanctôt, K.; Herrmann, N. Acetylcholinesterase inhibitors for treating dementia symptoms—A safety evaluation. Expert Opin. Drug Saf. 2017, 16, 1009–1019. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Marchante, E.; Freitas, H.; Marchante, H. Guia Prático para a Identificação de Plantas Invasoras de Portugal Continenta; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2008; ISBN 9789892603988. [Google Scholar]
- Lorenzo, P.; González, L.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef] [Green Version]
- Domingues De Almeida, J.; Freitas, H. Exotic naturalized flora of continental Portugal—A reassessment. Bot. Complut. 2006, 30, 117–130. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; Cavaleiro, C. Ambient has Become Strained. Identification of Acacia dealbata Link Volatiles Interfering with Germination and Early Growth of Native Species. J. Chem. Ecol. 2014, 40, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.P.; Conde, E.; Ribeiro, D.; Fernandes, E.; Domínguez, H.; Torres, M.D. Bioactive properties of Acacia dealbata flowers extracts. Waste Biomass Valorization 2020, 11, 2549–2557. [Google Scholar] [CrossRef]
- Pinto, F.; Silva, F.; Barbosa, A. Evaluation of Haemolytic Activity of Leaves from Acacia podalyriifolia. Eur. J. Med. Plants 2016, 17, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Determination of selected phenolic compounds in quince jams by solid-phase extraction and HPLC. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 2861–2872. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chang, T.C.; Chang, S.T. A review of antioxidant and pharmacological properties of phenolic compounds in Acacia confusa. J. Tradit. Complement. Med. 2018, 8, 443–450. [Google Scholar] [CrossRef]
- Li, X.-C.; Liu, C.; Yang, L.-X.; Chen, R.-Y. Phenolic compounds from the aqueous extract of Acacia catechu. J. Asian Nat. Prod. Res. 2011, 13, 826–830. [Google Scholar] [CrossRef]
- De Andrade, C.A.; Miguel, M.D.; Miguel, O.G.; Ferrnato, M.d.L.; Peitz, C.; Cunico, M.; Dias, J.d.F.G.; Balestrin, L.; Kerber, V.A. Efeitos Alelopáticos das Flores da Acacia podalyriaefolia A. Cunn. Visão Acadêmica 2003, 4, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Farid, I.B.; Sheded, M.G.; Mohamed, E.A. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J. Biol. Sci. 2014, 21, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Ziani, B.E.C.; Carocho, M.; Abreu, R.M.V.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Talhi, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddiana extracts. Food Biosci. 2020, 36, 100616. [Google Scholar] [CrossRef]
- Saini, M.L.; Saini, R.; Roy, S.; Kumar, A. Comparative pharmacognostical and antimicrobial studies of Acacia species (Mimosaceae). J. Med. Plants Res. 2008, 2, 378–386. [Google Scholar]
- Banso, A. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J. Med. Plants Res. 2009, 3, 82–85. [Google Scholar]
- Mahesh, B.; Satish, S. Antimicrobial Activity of Some Important Medicinal Plant Against Plant and Human Pathogens. World J. Agric. Sci. 2008, 4, 839–843. [Google Scholar]
- Paula, V.B.; Delgado, T.; Campos, M.G.; Anjos, O.; Estevinho, L.M. Enzyme inhibitory potential of ligustrum lucidum aiton berries. Molecules 2019, 24, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; da Cunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Campos, M.d.G.; Markham, K.R. Structure Information from HPLC and On-Line Measured Absorption Spectra: Flavones, Flavonols and Phenolic Acids; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2007; ISBN 9789892604800. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Alencar, S.M.; Oldoni, T.L.C.; Castro, M.L.; Cabral, I.S.R.; Costa-Neto, C.M.; Cury, J.A.; Rosalen, P.L.; Ikegaki, M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007, 113, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Chee, C.; Viswanathan, G.; Buckle, M.; Othman, R.; Abd Rahman, N.; Chung, L. Synthesis, Biological Evaluation and Molecular Modelling of 2′-Hydroxychalcones as Acetylcholinesterase Inhibitors. Molecules 2016, 21, 955. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.M.; Kheadr, E.; Ibrahim, W.H. Inhibitory effects of honey from arid land on some enzymes and protein damage. Food Chem. 2021, 364, 130415. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Anjos, O.; Caldeira, I.; Pedro, S.I.; Canas, S. FT-Raman methodology applied to identify different ageing stages of wine spirits. LWT 2020, 134, 110179. [Google Scholar] [CrossRef]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem. 2021, 108, 104681. [Google Scholar] [CrossRef]
- Isla, M.I.; Salas, A.; Danert, F.C.; Zampini, I.C.; Ordoñez, R.M. Analytical methodology optimization to estimate the content of non-flavonoid phenolic compounds in Argentine propolis extracts. Pharm. Biol. 2014, 52, 835–840. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, C.A.; Costa, C.K.; Bora, K.; Miguel, M.D.; Miguel, O.G.; Kerber, V.A. Determinação do conteúdo fenólico e avaliação da atividade antioxidante de Acacia podalyriifolia A. Cunn. ex G. Don, Leguminosae-mimosoideae. Rev. Bras. Farmacogn. 2007, 17, 231–235. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Wang, S.-Y.; Shyur, L.-F.; Kuo, Y.-H.; Chang, S.-T. Phenolic Antioxidants from the Heartwood of Acacia confusa. J. Agric. Food Chem. 2005, 53, 5917–5921. [Google Scholar] [CrossRef] [PubMed]
- Faujdar, S.; Sharma, S.; Sati, B.; Pathak, A.K.; Paliwal, S.K. Comparative analysis of analgesic and anti-inflammatory activity of bark and leaves of Acacia ferruginea DC. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and Lignans in Legumes: Nutritional and Health Aspects in Humans 11The method development and synthesis of the standards and deuterium-labelled compounds was supported by National Institutes of Health Grants No. 1 R01 CA56289-01 and No. 2 R0. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hertog, M.G.; Hollman, P.C.; Hertog, M.G.; Katan, M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Merino, E.N.; Sendin, M.A.C.; Osorio, J.A.V. Enfermedad de Alzheimer. Med. Programa Form. Médica Contin. Acreditado 2015, 11, 4306–4315. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [Green Version]
- Pierpaoli, W. Neurodegenerative Diseases: A Common Etiology and a Common Therapy. Ann. N. Y. Acad. Sci. 2005, 1057, 319–326. [Google Scholar] [CrossRef]
- Jin, Z.; Chu, Q.; Xu, W.; Cai, H.; Ji, W.; Wang, G.; Lin, B.; Zhang, X. All-Fiber Raman Biosensor by Combining Reflection and Transmission Mode. IEEE Photonics Technol. Lett. 2018, 30, 387–390. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Sakhamuri, S.; Irudayaraj, J. Comparison of FTIR, FT-Raman, and NIR Spectroscopy in a Maple Syrup Adulteration Study. J. Food Sci. 2002, 67, 2009–2015. [Google Scholar] [CrossRef]
- Nickless, E.M.; Holroyd, S.E.; Stephens, J.M.; Gordon, K.C.; Wargent, J.J. Analytical FT-Raman spectroscopy to chemotype Leptospermum scoparium and generate predictive models for screening for dihydroxyacetone levels in floral nectar. J. Raman Spectrosc. 2014, 45, 890–894. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 9, ISBN 9780123869845. [Google Scholar]



| Site (S) | Maturation Status (M) | S × M | Residual | |
|---|---|---|---|---|
| TPC (µg gallic acid/mL) | 24.4 *** | 55.5 *** | 11.7 ** | 8.4 |
| TFC EC50 (µg/mL) | 37.4 *** | 27.3 *** | n.s. | 35.3 |
| Site (S) | Maturation Status (M) | S × M | Residual | |
|---|---|---|---|---|
| FRAP EC50 (mg/mL) | n.s. | 54.3 *** | n.s. | 45.7 |
| DPPH EC50 (mg/mL) | 10.0 ** | 83.3 *** | n.s. | 6.7 |
| Site (S) | Maturation Status (M) | S × M | Residual | |
|---|---|---|---|---|
| ACET (µg/mL) | 51.9 *** | 27.7 *** | 7.6 ** | 12.9 |
| Lipase (µg/mL) | 25.7 *** | 52.6 *** | n.s. | 21.7 |
| GLUC (µg/mL) | n.s. | 68.5 * | n.s. | 31.5 |
| MS | EcoliA (µg/mL) | Saur (µg/mL) | Calbi (µg/mL) | EcoliESA (µg/mL) | SaurESA (µg/mL) | AB_r (µg/mL) | ||
|---|---|---|---|---|---|---|---|---|
| Ethanol | A | EF | 4.57 ± 0.29 a | 0.79 ± 0.11 a | 8.37 ± 0.50 a | 9.30 ± 0.34 a | 2.63 ± 0.24 a | 15.00 ± 1.08 a |
| MF | 4.96 ± 0.35 c | 0.89 ± 0.09 a | 8.90 ± 0.55 a | 10.22 ± 0.61 a,b | 2.85 ± 0.1 a,b | 19.16 ± 1.57 b | ||
| LF | 5.67 ± 0.21 b | 1.21 ± 0.12 b | 10.34 ± 0.50 b | 11.19 ± 0.79 a,b | 3.02 ± 0.22 a,b | 19.89 ± 3.48 b | ||
| B | EF | 6.00 ± 0.26 b | 0.87 ± 0.08 a | 10.53 ± 0.58 b | 9.72 ± 1.15 a,b | 3.21 ± 0.24 b | 19.43 ± 1.43 b | |
| MF | 7.28 ± 0.43 d | 1.32 ± 0.16 b | 11.48 ± 0.74 b | 11.47 ± 1.51 b | 3.83 ± 0.25 c | 22.59 ± 1.68 b,c | ||
| LF | 8.46 ± 0.29 e | 1.57 ± 0.21 c | 13.40 ± 0.71 c | 13.77 ± 1.00 c | 4.05 ± 0.24 c | 25.61 ± 0.87 c | ||
| Gentamicin (µg/mL) | Gentamicin (µg/mL) | Anfotericin B (µg/mL) | Gentamicin (µg/mL) | Gentamicin (µg/mL) | Anfotericin B (µg/mL) | |||
| 6.16 ± 0.08 | 1.11 ± 0.04 | 10.50 ± 0.005 | 10.95 ± 0.21 | 3.27 ± 0.08 | 20.20 ± 0.22 |
| Site (S) | Maturation Status (M) | S × M | Residual | |
|---|---|---|---|---|
| EcoliA (µg/mL) | 68.3 *** | 22.6 *** | 6.3 *** | 2.8 |
| Saur (µg/mL) | 27.9 *** | 50.5 *** | 9.2 ** | 12.4 |
| Calbi (µg/mL) | 64.2 *** | 28.8 *** | n.s. | 7.0 |
| EcoliESA (µg/mL) | 21.3 *** | 47.8 *** | 9.6 * | 21.2 |
| SaurESA (µg/mL) | 69.1 *** | 17.7 *** | 4.1 * | 9.1 |
| AB_r (µg/mL) | 47.3 *** | 36.0 *** | n.s. | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. https://doi.org/10.3390/app12031022
Paula V, Pedro SI, Campos MG, Delgado T, Estevinho LM, Anjos O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Applied Sciences. 2022; 12(3):1022. https://doi.org/10.3390/app12031022
Chicago/Turabian StylePaula, Vanessa, Soraia I. Pedro, Maria G. Campos, Teresa Delgado, Letícia M. Estevinho, and Ofélia Anjos. 2022. "Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments" Applied Sciences 12, no. 3: 1022. https://doi.org/10.3390/app12031022
APA StylePaula, V., Pedro, S. I., Campos, M. G., Delgado, T., Estevinho, L. M., & Anjos, O. (2022). Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Applied Sciences, 12(3), 1022. https://doi.org/10.3390/app12031022









