Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems
Abstract
1. Introduction
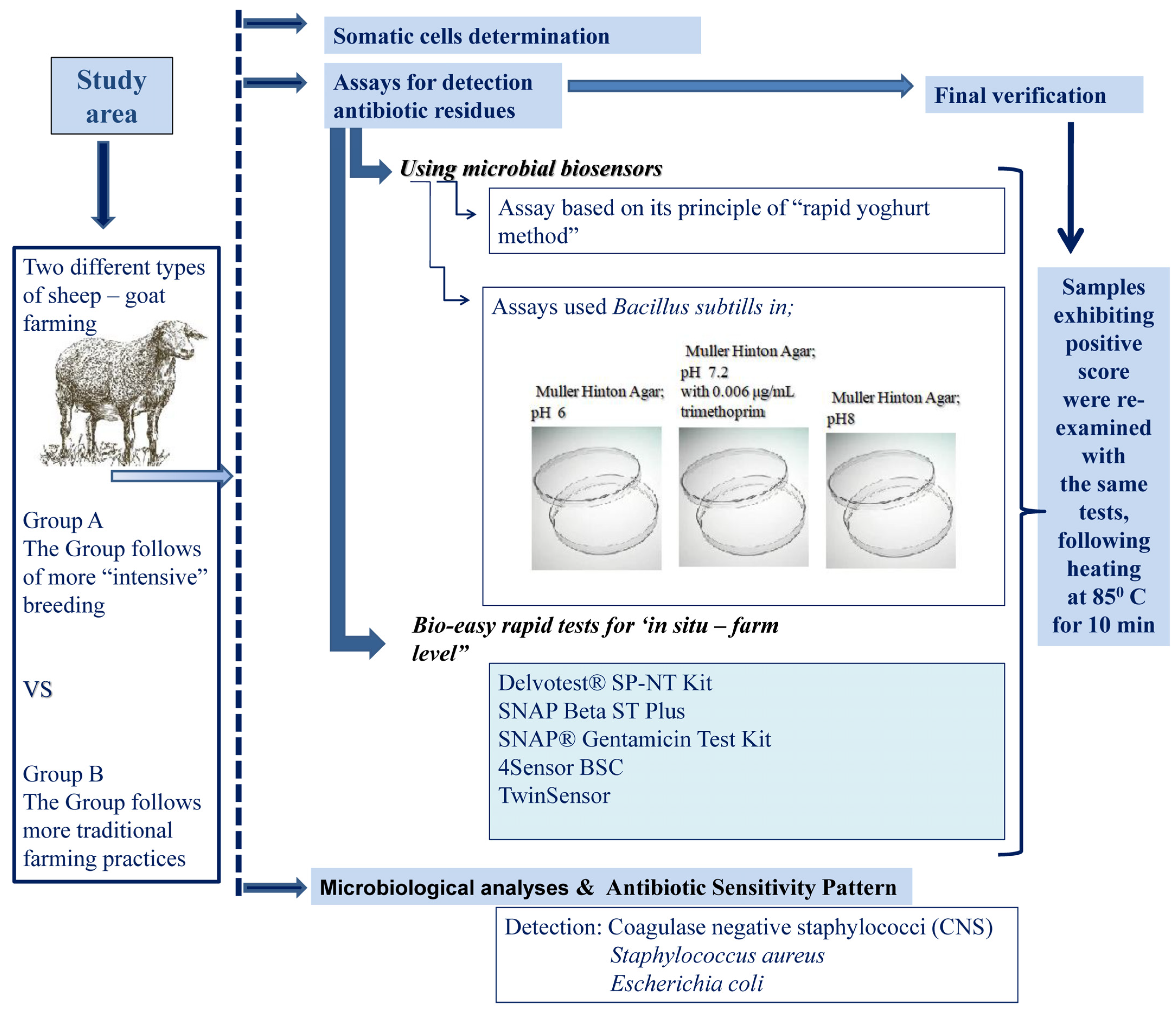
2. Materials and Methods
2.1. Study Area—Milk Samples
2.2. Screening of Milk for Somatic Cells
2.3. Assays for Detection Antibiotic Residues
2.3.1. Using Microbial Biosensors
Assay Based on Its Principle of the “Rapid Yoghurt Method”
Assays Used Bacillus Subtills
2.3.2. Bio-Easy Rapid Tests for ‘In Situ—Farm Level’
- -
- The Delvotest® SP-NT Kit (DSM, Food Specialties, The Netherlands), which is a non-specific microbial inhibitor test.
- -
- SNAP Beta ST Plus—Detection Level (at or below), ppb (Detects more beta-lactam residues including cephalexin at or below established maximum residue limits). The specific tests are enzyme-linked receptor binding assays that detect antibiotics in raw milk. (IDEXX Laboratories Inc., Westbrook, ME, USA).
- -
- SNAP® Gentamicin Test Kit (IDEXX B.V., Hoofddorp, The Netherlands). An enzyme-linked immunoassay designed to detect gentamicin residues in raw, commingled milk. In addition, the manufacturer ensures that residues from common mastitis antibiotic are within regulatory limits.
- -
- 4Sensor BSC [Unisensor Diagnostic E., Seraing (Ougrée)—Belgium]. A multiplex dipstick assay for the rapid and simultaneous detection of beta-lactam antibiotics, tetracyclines, streptomycin and chloramphenicol in milk. 4Sensor is a competitive test involving specific receptors and generic monoclonal antibodies in one single operation.
- -
- TwinSensor [Unisensor Diagnostic E., Seraing (Ougrée)—Belgium]. A competitive test involving specific receptors with high affinity for Betalactams and Tetracyclines molecules, in one single operation, specific to the European Union maximum residue limits (KIT020).
2.3.3. Final Verification of the Detection of Residues of Antibiotics in Milk
2.4. Microbiological Analyses
- -
- Detection of Staphylococcus sp. [31,32,33]: 1 mL of milk samples was enriched in 9 mL of Mueller–Hinton broth supplemented with 6.5% NaCl (Oxoid Ltd., Basingstoke, UK). Incubation at 37 °C for 20 h followed and 1 mL was enriched in 9 mL of Tryptone Soya broth with 10% NaCl and 1% sodium pyruvate (HiMedia Labs, Einhausen, Germany). Incubation followed at 35 °C for 20 h and then the enrichment broth was inoculated onto Baird-Parker agar (Oxoid Ltd., Basingstoke, UK) containing 30% egg yolk with 1% tellurite (Oxoid Ltd., Basingstoke, UK) and mannitol salt phenol red agar (Merck, Darmstadt, Germany) plates. Another quantity of milk sample was inoculated into 5.0% Sheep Blood Agar [SBA, (Becton Dickinson, Sparks, NV, USA)] plates. Anaerobic incubation followed for the SBA plates at 37 °C and were evaluated after 48 and 72 h. Taxonomic classification of the potentially Staphylococcus sp. isolates followed by using routine microbiological procedures such as: colony morphology, Gram staining, catalase and oxidase reactions and coagulase test [free coagulase (Coagulase plasma—EDTA, bioMérieux, Lyon, France) and bound coagulase production (Staphylase test, bioMérieux, Lyon, France)]. After the basic microbiology, the Staphylococcus sp. isolates were processed by using VITEK-2 (bioMérieux, Lyon, France), according to the manufacturer’s instructions. Finally, the strains were then frozen at −80 °C in BHI broth (Oxoid Ltd., Basingstoke, UK) with 20% glycerol.
- -
- Enumeration of E. coli was carried out according to ISO 16649-2:2001 [34]. A 1-mL aliquot of each sample and series of 10- fold dilutions were set using peptone tryptone water (Condalab, Madrid, Spain) and were transferred into petri dishes, to which Tryptone Bile X-glucuronide medium (TBX; Oxoid, Hampshire, UK) was immediately added. Incubation at 44 °C for 24 h followed, and then the presumptive colonies were enumerated. The quantification limit was 1 CFU/mL. The primary identification was carried out using API 20 E (bioMerieux) and the identification was completed using the Vitek®2 system (bioMerieux, Marcy l’Etoile, France).
Antimicrobial Susceptibility Testing
- -
- For S. aureus were used; ampicillin (20 μg), erythromycin (15 μg), tetracycline (30 μg), clindamycin (2 μg), ciprofloxacin (10 μg), ceftiofur (30 μg, 3rd generation cephalosporin), rifampicin (5 μg), teicoplanin (30 μg), pefloxacin (10 μg), amoxicillin-clavulanate (30 μg), streptomycin (30 μg), gentamicin(10 μg), cefuroxime (20 μg), norfloxacin (NX-10 µg), ceftriaxone (25 μg), nalidixic acid (NA-30 µg), vancomycin (VA-30 µg), sulphamethoxazole-trimethoprim (30 μg), chloramphenicol (30 μg) and Cef (cefoxitin, 30 μg).
- -
- For E. coli isolates, the panel consisted of: ampicillin (10 μg), amoxicillin-clavulanic acid (20–10 μg), aztreonam (30 μg), ceftiofur (30 μg), streptomycin (30 μg), cefotaxime (5 μg), ceftazidime (10 μg), chloramphenicol (30 µg), cefepime (30 μg), kanamycin (30 μg), oxytetracycline (30 μg), tetracycline (30 μg), sulfamethoxazole-trimethoprim (23.75–1.25 μg), enrofloxacin (5 μg), and imipenem (10 μg). In addition, E. coli isolates were tested for extended-spectrum beta-lactamase (ESBL) phenotype using a double-disk diffusion test, which is based on the synergy between third generation cephalosporins and clavulanate [37].
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- -
- The bulk tank milk should be monitored on a regular basis for residues of antibiotics. The monitoring process should include at least two different types of tests and should be performed after heating the milk samples to avoid false positive tests;
- -
- It seems that the type of small ruminant farming practice affects the CNS counts in the bulk tank milk. In the present study the group of traditional practice farms showed increased counts of CNS. Milk from such farms should be treated cautiously in the dairies, and instructions should be given to the farmers how to remedy the problem;
- -
- S. aureus and E. coli were isolated in very low frequencies from both groups, without any statistical correlation with other parameters and with most strains being found multi resistant to antibiotics. These finding suggests that perhaps even in low prevalence and in absence of disease, these bacteria retain the resistance acquired in the past and thus play the role of reservoir. Such tests for susceptibility to antibiotics should be regularly performed to various microorganisms-indicators to monitor the resistance reservoir in the herd;
- -
- CNS although incriminated as the most frequent cause of subclinical mastitis can also play a protective role depending on the strains and species involved. Such strains- if isolated and better understood- may have a role to play in the prevention and treatment of subclinical mastitis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pappa, E.C.; Kondyli, E.; Sotirakoglou, K.; Bosnea, L.; Mataragas, M.; Allouche, L.; Tsiplakou, E.; Pappas, A.C. Farmers profile and characterization of sheep and goat dairy chain in Northwestern Greece. Sustainability 2021, 13, 833. [Google Scholar] [CrossRef]
- Lai, G.; Pes, M.; Addis, M.; Pirisi, A. A cluster project approach to develop new functional dairy products from sheep and goat milk. Dairy 2020, 1, 154–168. [Google Scholar] [CrossRef]
- Morales, F.D.A.R.; Genís, J.M.C.; Guerrero, Y.M. Current status, challenges and the way forward for dairy goat production in Europe. Asian-Australas. J. Anim. Sci. 2019, 32, 1256–1265. [Google Scholar] [CrossRef]
- Balthazar, C.; Pimentel, T.; Ferrão, L.; Almada, C.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.; Nascimento, J.; Silva, M.; et al. Sheep milk: Physicochemical characteristics and relevance for functional food development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Csapó, Z.; Péntek, Á.; Riskó, T.C. Sensory evaluation and acceptance of goat yogurts in comparison with cow yogurts—An empirical study. In GSMAC 2019: Challenges and Opportunities to Develop Organizations Through Creativity, Technology and Ethics; Fotea, S., Fotea, I., Văduva, S., Eds.; Springer Proceedings in Business and Economics; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Flis, Z.; Molik, E. Importance of bioactive substances in sheep’s milk in human health. Int. J. Mol. Sci. 2021, 22, 4364. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Katsouri, E.; Magriplis, E.; Zampelas, A.; Nychas, G.J.; Drosinos, E.H. Nutritional characteristics of prepacked feta PDO cheese products in Greece: Assessment of dietary intakes and nutritional profiles. Foods 2020, 9, 253. [Google Scholar] [CrossRef]
- Katsouri, E.; Magriplis, E.; Zampelas, A.; Drosinos, E.H.; Nychas, G.-J. Dietary intake assessment of pre-packed graviera cheese in Greece and nutritional characterization using the nutri-score front of pack label scheme. Nutrients 2021, 13, 295. [Google Scholar] [CrossRef]
- Velčovská, Š.; Sadílek, T. Certification of cheeses and cheese products origin by EU countries. Br. Food J. 2015, 117, 1843–1858. [Google Scholar] [CrossRef]
- Guyomard, H.; Bureau, J.-C.; Chatellier, V.; Detang-Dessemdre, C.; Dupraz, P.; Jacquet, F.; Reboud, X.; Requillart, V.; Soler, L.G.; Tysebaert, M. Research for AGRI Committee—The Green Deal and the CAP: Policy Implications to Adapt Farming Practices and to Preserve the EU’s Natural Resources; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Leitner, G.; Merin, U.; Silanikove, N. Estimate of milk and curd yield loss of sheep and goats with intrammamary infection and its relation to somatic cell count. Small Rumin. Res. 2008, 74, 221–225. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Merin, U.; Leitner, G. On effects of subclinical mastitis and stage of lactation on milk quality in goats. Small Rumin. Res. 2014, 122, 76–82. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Piepers, S.; Leroy, F.; Van Coillie, E.; Haesebrouck, F.; De Vliegher, S. Invited review: Effect, persistence, and virulence of coagulase-negative Staphylococcus species associated with ruminant udder health. J. Dairy Sci. 2014, 97, 5275–5293. [Google Scholar] [CrossRef] [PubMed]
- Riggio, V.; Portolano. B. Genetic selection for reduced somatic cell counts in sheep milk: A review. Small Rumin. Res. 2015, 126, 33–42. [Google Scholar] [CrossRef]
- Beltrán Martínez, M.C. Analytical Strategy for the Detection of Antibiotic Residues in Milk from Small Ruminants. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2014. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Asadpour, R.; Alamoti, M.P.; Golchin, A.; Kiyani, R.; Mohammadpour, R.; Pour, R.M. Raw cow milk quality: Relationship between antibiotic residue and Somatic cell count. Int. F. Res. J. 2013, 20, 3347–3350. Available online: http://www.ifrj.upm.edu.my/20%20(06)%202013/54%20IFRJ%2020%20(06)%202013%20Rohman%20Mahmoudi%20217.pdf (accessed on 20 November 2021).
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Sachi, S.; Ferdous, J.; Sikder, M.H.; Hussani, S.A.K. Antibiotic residues in milk: Past, present, and future. J. Adv. Vet. Anim. Res. 2019, 6, 315–332. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández – Escámez, P.S.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; EFSA Panel on Biological Hazards (BIOHAZ); et al. Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA J. 2017, 27, e04665. [Google Scholar] [CrossRef]
- European Commission, Food Safety. Residues of Veterinary Medicinal Products. Available online: https://ec.europa.eu/food/safety/chemical-safety/residues-veterinary-medicinal-products_en (accessed on 2 December 2021).
- Prabha, M.A.; Dharini, V.; Aishwarya, B.; Jaswanth, M.; Allan, S.; Selvam, S.P.; Kumar, M.M. Detection of antibiotic residues in food using biosensors. Asian J. Chem. 2021, 33, 1699–1708. [Google Scholar] [CrossRef]
- Ndgung’u, T.W.; Omwamba, M.; Muliro, P.S. Evaluation of rapid beta-lactam antibiotic residues detection kits for raw milk. AJFS 2021, 15, 353–359. [Google Scholar] [CrossRef]
- Kurjogi, M.; Issa Mohammad, Y.H.; Alghamdi, S.; Abdelrahman, M.; Satapute, P.; Jogaiah, S. Detection and determination of stability of the antibiotic residues in cow’s milk. PLoS ONE 2019, 14, e0223475. [Google Scholar] [CrossRef]
- Pietschmann, J.; Dittmann, D.; Spiegel, H.; Krause, H.J.; Schröper, F. A novel method for antibiotic detection in milk based on competitive magnetic immunodetection. Foods 2020, 30, 1773. [Google Scholar] [CrossRef]
- Layada, S.; Benouareth, D.E.; Coucke, W.; Andjelkovic, M. Assessment of antibiotic residues in commercial and farm milk collected in the region of Guelma (Algeria). Int. J. Food Contam. 2016, 3, 19. [Google Scholar] [CrossRef]
- Yamani, M.I.; Al-Kurdi, L.M.; Haddadin, M.S.Y.; Robinson, R.K. A simple test for the detection of antibiotics and other chemical residues in ex-farm milk. Food Control 1999, 10, 35–39. [Google Scholar] [CrossRef]
- Tumini, E.; Herrera-Moyano, E.; San Martín-Alonso, M.; Barroso, S.; Galmarini, C.M.; Aguilera, A. The antitumor drugs trabectedin and lurbinectedin induce transcription-dependent replication stress and genome instability. Mol. Cancer Res. 2019, 17, 773–782. [Google Scholar] [CrossRef]
- Almashhadany, D.A. Screening of antibiotic residues in raw milk of cows and buffalos by diffusion assays. Italy J. Food Saf. 2021, 10, 9034. [Google Scholar] [CrossRef] [PubMed]
- Vyletělová, M.; Vlková, H.; Manga, I. Occurrence and characteristics of methicillin resistant Staphylococcus aureus and methicillin resistant coagulase-negative staphylococci in raw milk manufacturing. Czech J. Food Sci. 2011, 29, S11–S16. [Google Scholar] [CrossRef]
- Moura, G.S.; Gebreyes, W.A.; Marques, M.F.S.; Stipp, D.T.; Souza, F.N.; Da Costa, L.B.; Oliveira, C.J.B. Short communication: Occurrence of methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci in dairy goat herds in Ohio, United States. J. Dairy Sci. 2018, 101, 7804–7807. [Google Scholar] [CrossRef]
- Zangerl, P.; Asperger, H. Chapter 6 Media used in the detection and enumeration of Staphylococcus aureus. In Progress in Industrial Microbiology; Janet, E.L., Corry, G.D.W., Baird, C.R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 37, pp. 91–110. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide; International Organization for Standardization: Geneva, Switzerland, 2010; ISO 16649-2. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed.; CLSI Document M02-A11; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2012. [Google Scholar]
- Kali, A.; Stephen, S.; Umadevi, S. Laboratory evaluation of phenotypic detection methods of methicillin-resistant Staphylococcus aureus. Biomed J. 2014, 37, 411–414. [Google Scholar] [CrossRef]
- Rawat, D.; Nair, D. Extended-spectrum β-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2010, 2, 263–274. [Google Scholar] [CrossRef]
- Kivirand, K.; Kagan, M.; Rinken, T. Biosensors for the detection of antibiotic residues in milk. Biosens.-Micro Nanoscale Appl. 2015, 16, 425–456. [Google Scholar] [CrossRef]
- Kang, J.H.; Jin, J.H.; Kondo, F. False-positive outcome and drug residue in milk samples over withdrawal times. J. Dairy Sci. 2005, 88, 908–913. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Occurrence of false-positive results of inhibitor on milk samples using the Delvotest SP assay. J. Food Prot. 2001, 64, 1211–1215. [Google Scholar] [CrossRef]
- Cardoso, C.V.; de C Nunes, E.L.; Barbosa, E.V. Ribeiro, A.d. G.P.; Souza, G.d.M.; Liberal, M.H.T.; Castro, H.C. Farm test for rapid identification of antibiotic residues in raw milk. Adv. Biotech. Microbiol. 2019, 13, 555853. [Google Scholar] [CrossRef]
- Romero, T.; Beltrán, M.C.; Althaus, R.L.; Molina, M.P. Interference of non-specific detergents in microbial inhibitor test results for screening antibiotics in goat’s milk. J. Appl. Anim. Res. 2017, 45, 159–163. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Q.; Liu, Y.; Shabbir, M.A.B.; Sattar, A.; Peng, D.; Tao, Y.; Chen, D.; Wang, Y.; Yuan, Z. A microbiological inhibition method for the rapid, broad-spectrum, and high-throughput screening of 34 antibiotic residues in milk. J. Dairy Sci. 2019, 102, 10825–10837. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.; Lavon, Y.; Matzrafi, Z.; Benun, O.; Bezman, D.; Merin, U. Somatic cell counts, chemical composition and coagulation properties of goat and sheep bulk tank milk. Int. Dairy J. 2016, 58, 9–13. [Google Scholar] [CrossRef]
- Lianou, D.T.; Michael, C.K.; Vasileiou, N.G.C.; Petinaki, E.; Cripps, P.J.; Tsilipounidaki, K.; Katsafadou, A.; Fthenakis, G.C. Extensive countrywide field investigation of somatic cell counts and total bacterial counts in bulk-tank raw milk in sheep flocks in Greece. Foods 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Paape, M.J.; Wiggans, G.R.; Bannerman, D.D.; Thomas, D.L.; Sanders, A.H.; Contreras, A.; Moroni, P.; Miller, R.H. Monitoring goat and sheep milk somatic cell counts. Small Rumin. Res. 2007, 68, 114–125. [Google Scholar] [CrossRef]
- Nudda, A.; Atzori, A.S.; Correddu, F.; Battacone, G.; Lunesu, M.F.; Cannas, A.; Pulina, G. Effects of nutrition on main components of sheep milk. Small Rumin. Res. 2020, 184, 106015. [Google Scholar] [CrossRef]
- Mehdid, A.; Martí-De Olives, A.; Fernández, N.; Rodríguez, M.; Peris, C. Effect of stress on somatic cell count and milk yield and composition in goats. Res. Vet. Sci. 2019, 125, 61–70. [Google Scholar] [CrossRef]
- Persson, Y.; Olofsson, I. Direct and indirect measurement of somatic cell count as indicator of intramammary infection in dairy goats. Acta Vet. Scand. 2011, 53, 15. [Google Scholar] [CrossRef]
- Min, B.R.; Tomita, G.; Hart, S.P. Effect of subclinical intramammary infection on somatic cell counts and chemical composition of goats’ milk. J. Dairy Res. 2007, 74, 204–210. [Google Scholar] [CrossRef]
- Hussein, H.A.; Fouad, M.T.; Abd El-Razik, K.A.; Abo El-Maaty, A.M.; D’Ambrosio, C.; Scaloni, A.; Gomaa, A.M. Study on prevalence and bacterial etiology of mastitis, and effects of subclinical mastitis and stage of lactation on SCC in dairy goats in Egypt. Trop. Anim. Health Prod. 2020, 52, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, C.; Carriedo, J.A.; García-Jimeno, M.C.; Pérez-Bilbao, M.; de la Fuente, L.F. Factors influencing variation of bulk milk antibiotic residue occurrence, somatic cell count, and total bacterial count in dairy sheep flocks. J. Dairy Sci. 2010, 93, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Raynal-Ljutovac, K.; Pirisi, A.; de Crémoux, R.; Gonzalo, C. Somatic cells and goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Andrade, N.C.; Laranjo, M.; Costa, M.M.; Queiroga, M.C. Virulence factors in Staphylococcus associated with small ruminant mastitis: Biofilm production and antimicrobial resistance genes. Antibiotics 2021, 10, 633. [Google Scholar] [CrossRef]
- Romanò, A.; Gazzola, A.; Bianchini, V.; Cortimiglia, C.; Maisano, A.M.; Cremonesi, P.; Graber, H.U.; Vezzoli, F.; Luini, M. Staphylococcus aureus from goats are genetically heterogeneous and distinct to bovine ones. Front. Vet. Sci. 2020, 7, 628. [Google Scholar] [CrossRef]
- Skoufos, I.; Giannenas, I.; Karamoutsios, A.; Tsinas, A.; Papadopoulos, G.K.; Tzora, A. Milk quality characteristics of indigenous sheep breeds Boutsko, Frisarta and Karagouniko. J. Hellenic Vet. Med. Soc. 2017, 68, 59–66. [Google Scholar] [CrossRef][Green Version]
- Fotou, K.; Tzora, A.; Voidarou, C.; Alexopoulos, A.; Plessas, S.; Avgeris, I.; Demertzis, P.G.; Bezirtzoglou, E.; Demertzi-Akrida, K. Isolation of microbial pathogens of Subclinical Mastitis from raw sheep’s milk of Epirous (Greek) and their role in its hygiene. Anaerobe 2011, 17, 315–319. [Google Scholar] [CrossRef]
- Condoleo, R.; Giangolini, G.; Chiaverini, A.; Patriarca, D.; Scaramozzino, P.; Mezher, Z. Occurrence of Listeria monocytogenes and Escherichia coli in raw sheep’s milk from farm bulk tanks in central Italy. J. Food Prot. 2020, 83, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; ElMougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; et al. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: A risk to public health and food safety. Sci. Rep. 2018, 11, 5859. [Google Scholar] [CrossRef] [PubMed]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and antimicrobial resistance profiles of foodborne pathogens isolated from dairy cattle and poultry manure amended farms in Northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Sarrou, S.; Fragkou, I.A.; Katsafadou, A.I.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Role of staphylococci in mastitis in sheep. J. Dairy Res. 2019, 86, 254–266. [Google Scholar] [CrossRef]
- Tonamo, A.; Komlósi, I.; Varga, L.; Kačániová, M.; Peles, F. Identification of ovine-associated staphylococci by MALDI-TOF mass spectrometry. Acta Aliment. 2021, 50, 210–218. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C. Prevalence and aetiology of subclinical mastitis in ewes of Southern Greece. Small Rumin. Res. 1994, 13, 293–300. [Google Scholar] [CrossRef]
- Pyörälä, S.; Taponen, S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol. 2009, 134, 3–8. [Google Scholar] [CrossRef]
- Virdis, S.; Scarano, C.; Cossu, F.; Spanu, V.; Spanu, C.; De Santis, E.P. Antibiotic resistance in Staphylococcus aureus and coagulase negative staphylococci isolated from goats with subclinical mastitis. Vet. Med. Int. 2010, 2010, 517060. [Google Scholar] [CrossRef]
- Zigo, F.; Sasáková, N.; Gregová, G.; Výrostková, J.; Ondrašovicová, S. Effects of using an alternative bedding composition on the levels of indicator microorganisms and mammary health in dairy farm conditions. Agriculture 2020, 10, 245. [Google Scholar] [CrossRef]
- Zigo, F.; Vasil, M.; Ondrašovičová, S.; Výrostková, J.; Bujok, J.; Pecka-Kielb, E. Maintaining optimal mammary gland health and prevention of mastitis. Front. Vet. Sci. 2021, 8, 607311. [Google Scholar] [CrossRef]
- Martins, K.B.; Faccioli, P.Y.; Bonesso, M.F.; Fernandes, S.; Oliveira, A.A.; Dantas, A.; Zafalon, F.; Cunha, M.L.; Maria de Lourdes, R.S. Characteristics of resistance and virulence factors in different species of coagulase-negative staphylococci isolated from milk of healthy sheep and animals with subclinical mastitis. J. Dairy Sci. 2017, 100, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.; Lavon, Y.; Merin, U.; Jacoby, S.; Blum, S.E.; Krifucks, O.; Silanikove, N. Milk quality and milk transformation parameters from infected mammary glands depends on the infecting bacteria species. PLoS ONE 2019, 14, e0213817. [Google Scholar] [CrossRef] [PubMed]
- Turchi, B.; Bertelloni, F.; Marzoli, F.; Cerri, D.; Tola, S.; Azara, E.; Longheu, C.M.; Tassi, R.; Schiavo, M.; Cilia, G.; et al. Coagulase negative staphylococci from ovine milk: Genotypic and phenotypic characterization of susceptibility to antibiotics, disinfectants and biofilm production. Small Ruminant. Res. 2020, 183, 106030. [Google Scholar] [CrossRef]
- Libera, K.; Konieczny, K.; Grabska, J.; Smulski, S.; Szczerbal, I.; Szumacher-Strabel, M.; Pomorska-Mól, M. Potential novel biomarkers for mastitis diagnosis in sheep. Animals 2021, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Dore, S.; Liciardi, M.; Amatiste, S.; Bergagna, S.; Bolzoni, G.; Caligiuri, V.; Cerrone, A.; Montagna, C.O.; Saletti, M.A.; Scatassa, M.L.; et al. Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rumin. Res. 2016, 141, 91–93. [Google Scholar] [CrossRef][Green Version]
- Pikhtirova, A.; Bujok, J.; Pecka-Kiełb, E.; Zachwieja, A.; Vasil, M.; Elečko, J.; Zigo, F. Fatty acid profile of ewe’s milk infected with Staphylococcus spp. Iran. J. Vet. Res. 2020, 21, 216–220. [Google Scholar] [PubMed]
- Pizauro, L.J.L.; de Almeida, C.C.; Soltes, G.A.; Slavic, D.; de Ávila, F.A.; Zafalon, L.F.; MacInnes, J.I. Short communication: Detection of antibiotic resistance, mecA, and virulence genes in coagulase-negative Staphylococcus spp. from buffalo milk and the milking environment. J. Dairy Sci. 2019, 102, 11459–11464. [Google Scholar] [CrossRef]
- Vasiľ, M.; Farkašová, Z.; Elečko, J.; Zigo, F. Occurrence of resistance to antibiotics therapy in coagulase-positive and coagulase-negative Staphylococci isolated from sheep’s milk in holding in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 781–787. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Politis, A.P.; Mavrogianni, V.S.; Barbagianni, M.S.; Vasileiou, N.G.; Fthenakis, G.C.; Fragkou, I.A. Mammary defenses and immunity against mastitis in sheep. Animals 2019, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.Z.; Kay, J.G.; Sangermani, D.G.; Stow, J.L. A role for the phagosome in cytokine secretion. Science 2005, 310, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Achek, R.; Hotzel, H.; Nabi, I.; Kechida, S.; Mami, D.; Didouh, N.; Tomaso, H.; Neubauer, H.; Ehricht, R.; Monecke, S.; et al. Phenotypic and molecular detection of biofilm formation in Staphylococcus aureus isolated from different sources in Algeria. Pathogens 2020, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Thorberg, B.M.; Kühn, I.; Aarestrup, F.M.; Brändström, B.; Jonsson, P.; Danielsson-Tham, M.L. Pheno- and genotyping of Staphylococcus epidermidis isolated from bovine milk and human skin. Vet. Microbiol. 2006, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Abbondio, M.; Fois, I.; Longheu, C.; Azara, E.; Tola, S. Biofilm production, quorum sensing system and analysis of virulence factors of Staphylococcus epidermidis collected from sheep milk samples. Small Rumin. Res. 2019, 174, 83–87. [Google Scholar] [CrossRef]
- Pilipčincová, I.; Bhide, M.; Dudriková, E.; Trávniček, M. Genotypic characterization of coagulase-negative staphylococci isolated from sheep milk in Slovakia. Acta Vet. Brno 2010, 79, 269–275. [Google Scholar] [CrossRef]
- Giadinis, D.; Arsenos, G.; Tsakos, P.; Psychas, V.; Dovas, C.I.; Papadopoulos, E.; Karatzias, H.; Fthenakis, G.C. “Milk-drop syndrome of ewes”: Investigation of the causes in dairy sheep in Greece. Small Rumin. Res. 2012, 106, 33–35. [Google Scholar] [CrossRef]
- Martí-De Olives, A.; Le Roux, Y.; Rubert-Alemán, J.; Peris, C.; Molina, M.P. Short communication: Effect of subclinical mastitis on proteolysis in ovine milk. J. Dairy Sci. 2011, 94, 5369–5374. [Google Scholar] [CrossRef]
- Martí-De Olives, A.; Díaz, J.R.; Molina, M.P.; Peris, C. Quantification of milk yield and composition changes as affected by subclinical mastitis during the current lactation in sheep. J. Dairy Sci. 2013, 96, 7698–7708. [Google Scholar] [CrossRef]
- Revilla, I.; Lurueña-Martínez, M.A.; Vivar-Quintana, A.M. Influence of somatic cell counts and breed on physico-chemical and sensory characteristics of hard ewes’-milk cheeses. J. Dairy Res. 2009, 76, 283–289. [Google Scholar] [CrossRef]
- Revilla, I.; Rodríguez-Nogales, J.M.; Vivar-Quintana, A.M. Effect of somatic cell counts on ewes’ milk protein profile and cheese-making properties in different sheep breeds reared in Spain. J. Dairy Res. 2009, 76, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Rovai, M.; Caja, G.; Salama, A.A.; Jubert, A.; Lázaro, B.; Lázaro, M.; Leitner, G. Identifying the major bacteria causing intramammary infections in individual milk samples of sheep and goats using traditional bacteria culturing and real-time polymerase chain reaction. J. Dairy Sci. 2014, 97, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Merin, U.; Shapiro, F.; Leitner, G. Subclinical mastitis in goats is associated with upregulation of nitric oxide-derived oxidative stress that causes reduction of milk antioxidative properties and impairment of its quality. J. Dairy Sci. 2014, 97, 3449–3455. [Google Scholar] [CrossRef]
- Leitner, G.; Lavi, Y.; Merin, U.; Lemberskiy-Kuzin, L.; Katz, G. Online evaluation of milk quality according to coagulation properties for its optimal distribution for industrial applications. J. Dairy Sci. 2011, 94, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.A.; Deiana, P.; Nudda, A.; Correddu, F.; Montanari, L.; Mangia, N.P. Assessment of microbiological quality and physicochemical parameters of fruhe made by ovine and goat milk: A Sardinian (Italy) cheese. Fermentation 2020, 6, 119. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef]
- Gosselin, V.B.; Dufour, S.; Middleton, J.R. Association between species-specific staphylococcal intramammary infections and milk somatic cell score over time in dairy goats. Prev. Vet. Med. 2020, 174, 104815. [Google Scholar] [CrossRef] [PubMed]
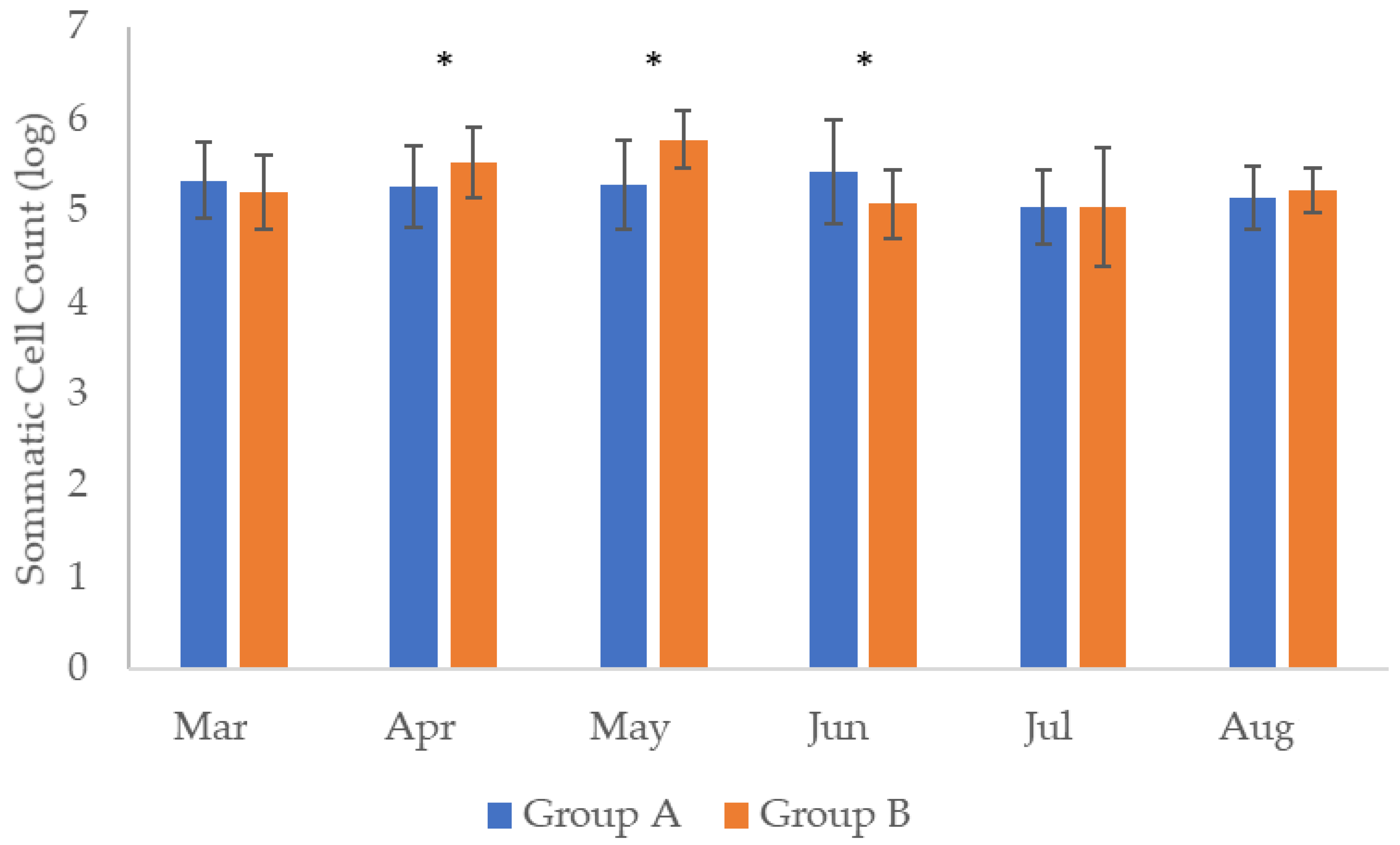
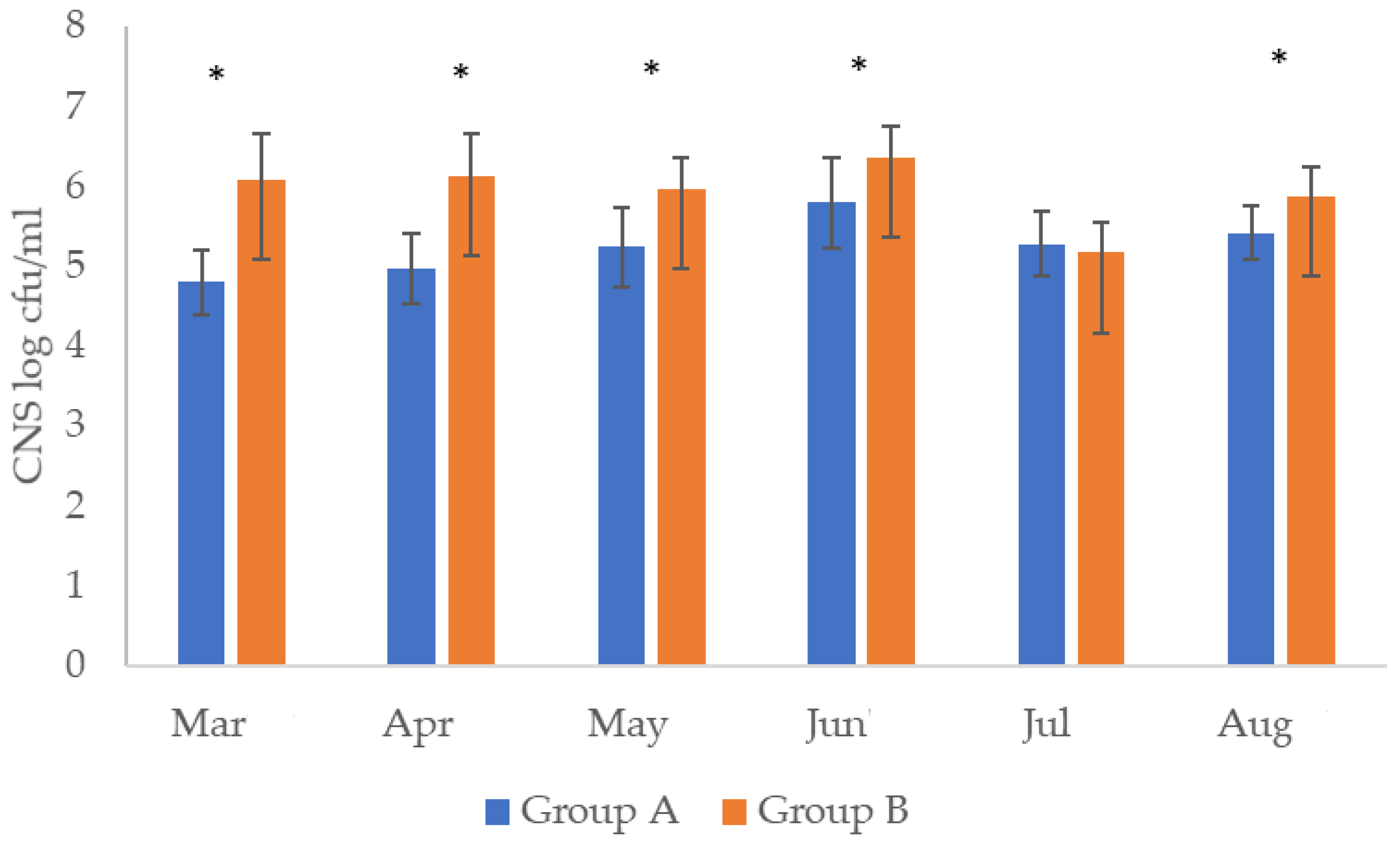
| Month | Group A | Group B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCC | CNS | Res. | S. aureus | E. coli | SCC | CNS | Res. | S. aureus | E. coli | |
| March | 5.33 ± 0.42 ab | 4.8 ± 1.1 c | 6 | 1.61 ± 0.2 | 1.62 ± 0.38 | 5.20 ± 0.39 d | 6.08 ± 0.57 ab | 1 | 1.20 ± 0.21 | - |
| April | 5.25 ± 0.43 cab | 4.9 ± 0.9 da | 2 | 1.34 ± 0.2 | - | 5.52 ± 0.37 a | 6.12 ± 0.53 b | 0 | 1.20 ± 0.21 | - |
| May | 5.28 ± 0.49 cb | 5.2 ± 0.9 ca | 7 | - | 2.41 ± 0.3 | 5.77 ± 0.31 b | 5.59 ± 0.39 ab | 3 | 1.36 ± 0.20 | - |
| June | 5.42 ± 0.36 b | 5.8 ± 1.0 b | 6 | - | - | 5.07 ± 0.37 c | 6.35 ± 0.39 d | 0 | - | - |
| July | 5.05 ± 0.40 c | 5.3 ± 0.7 ca | 0 | - | 1.01 ± 0.3 | 5.04 ± 0.64 c | 5.16 ± 0.37 c | 3 | 1.32 ± 0.37 | 1.81 ± 0.2 |
| August | 5.1 4± 0.47 ca | 5.4 ± 0.8 ab | 1 | - | 1.89 ± 0.49 | 5.22 ± 0.24 c | 5.87 ± 0.35 a | 0 | - | - |
| Group A | |||||||
|---|---|---|---|---|---|---|---|
| March | April | May | June | July | August | Total | |
| BH (+) | 9 | 7 | 15 | 11 | 4 | 8 | 54 |
| AH (+) | 6 | 2 | 7 | 6 | 0 | 1 | 22 |
| FP | 3 | 5 | 8 | 5 | 4 | 7 | 32 |
| Group B | |||||||
| March | April | May | June | July | August | Total | |
| BH (+) | 5 | 5 | 5 | 5 | 9 | 13 | 42 |
| AH (+) | 1 | 0 | 3 | 0 | 3 | 0 | 7 |
| FP | 4 | 5 | 2 | 5 | 6 | 13 | 35 |
| Microorganism | Antibiotic | % Resistance |
|---|---|---|
| S. aureus(N = 8) | amoxicillin-clavulanate (30 μg) | 12.5 |
| ampicillin (20 μg) | 87.5 | |
| ceftriaxone (25 μg) | 0 | |
| cefuroxime (20 μg) | 0 | |
| ceftiofur (30 μg, 3rd generation cephalosporin) | 0 | |
| ciprofloxacin (10 μg) | 25 | |
| clindamycin (2 μg) | 0 | |
| chloramphenicol (30 μg) | 0 | |
| cefoxitin (30 μg) | 0 | |
| erythromycin (15 μg) | 0 | |
| gentamicin (10 μg) | 0 | |
| nalidixic acid (30 μg) | 0 | |
| norfloxacin (10 μg) | 25 | |
| pefloxacin (10 μg) | 0 | |
| rifampicin (5 μg) | 0 | |
| streptomycin (30 μg) | 12.5 | |
| trimethoprim sulfamethoxazole (23.75–1.25 μg) | 0 | |
| teicoplanin (30 μg) | 0 | |
| tetracycline (30 μg) | 62.5 | |
| vancomycin (30 μg) | 25 | |
| Morphological—phenotypic identification of methicillin-resistant S. aureus (MRSA) | 37.5 | |
| E. coli(N = 8) | amoxicillin-clavulanic acid (20–10 μg) | 75 |
| ampicillin (10 μg) | 87.5 | |
| aztreonam (30 μg) | 0 | |
| cefotaxime (5 μg) | 0 | |
| ceftiofur (30 μg) | 0 | |
| ceftazidime (10 μg) | 0 | |
| chloramphenicol (30 μg) | 12.5 | |
| enrofloxacin (5 μg) | 0 | |
| imipenem (10 μg) | 12.5 | |
| kanamycin (30 μg) | 12.5 | |
| oxytetracycline (30 μg) | 50 | |
| streptomycin (30 μg) | 37.5 | |
| sulfamethoxazole-trimethoprim (23.75–1.25 μg) | 37.5 | |
| tetracycline (30 μg) | 75 | |
| 3rd generation cephalosporins and clavulanate | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozos, G.; Skoufos, I.; Fotou, K.; Alexopoulos, A.; Tsinas, A.; Bezirtzoglou, E.; Tzora, A.; Voidarou, C. Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems. Appl. Sci. 2022, 12, 1009. https://doi.org/10.3390/app12031009
Rozos G, Skoufos I, Fotou K, Alexopoulos A, Tsinas A, Bezirtzoglou E, Tzora A, Voidarou C. Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems. Applied Sciences. 2022; 12(3):1009. https://doi.org/10.3390/app12031009
Chicago/Turabian StyleRozos, Georgios, Ioannis Skoufos, Konstantina Fotou, Athanasios Alexopoulos, Anastasios Tsinas, Eugenia Bezirtzoglou, Athina Tzora, and Chrysoula (Chrysa) Voidarou. 2022. "Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems" Applied Sciences 12, no. 3: 1009. https://doi.org/10.3390/app12031009
APA StyleRozos, G., Skoufos, I., Fotou, K., Alexopoulos, A., Tsinas, A., Bezirtzoglou, E., Tzora, A., & Voidarou, C. (2022). Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems. Applied Sciences, 12(3), 1009. https://doi.org/10.3390/app12031009








