Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sage Ethanol Extracts and Essential Oil
2.2. Production of Chicken Meatballs
2.3. Assessment of Quality Features of Chicken Meatballs
2.3.1. Determination of Thermal Treatment Yield of Chicken Meatballs
2.3.2. Determination of Basic Chemical Components in Chicken Meatballs
2.3.3. Determination of TBARS Content in Chicken Meatballs
2.3.4. Assessment of Microbiological Quality of Chicken Meatballs
2.3.5. Measurement of Color Parameters of Chicken Meatballs
2.3.6. Evaluation of Selected Organoleptic Attributes of Chicken Meatballs
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Thermal Treatment Yield and Chemical Composition of Chicken Meatballs
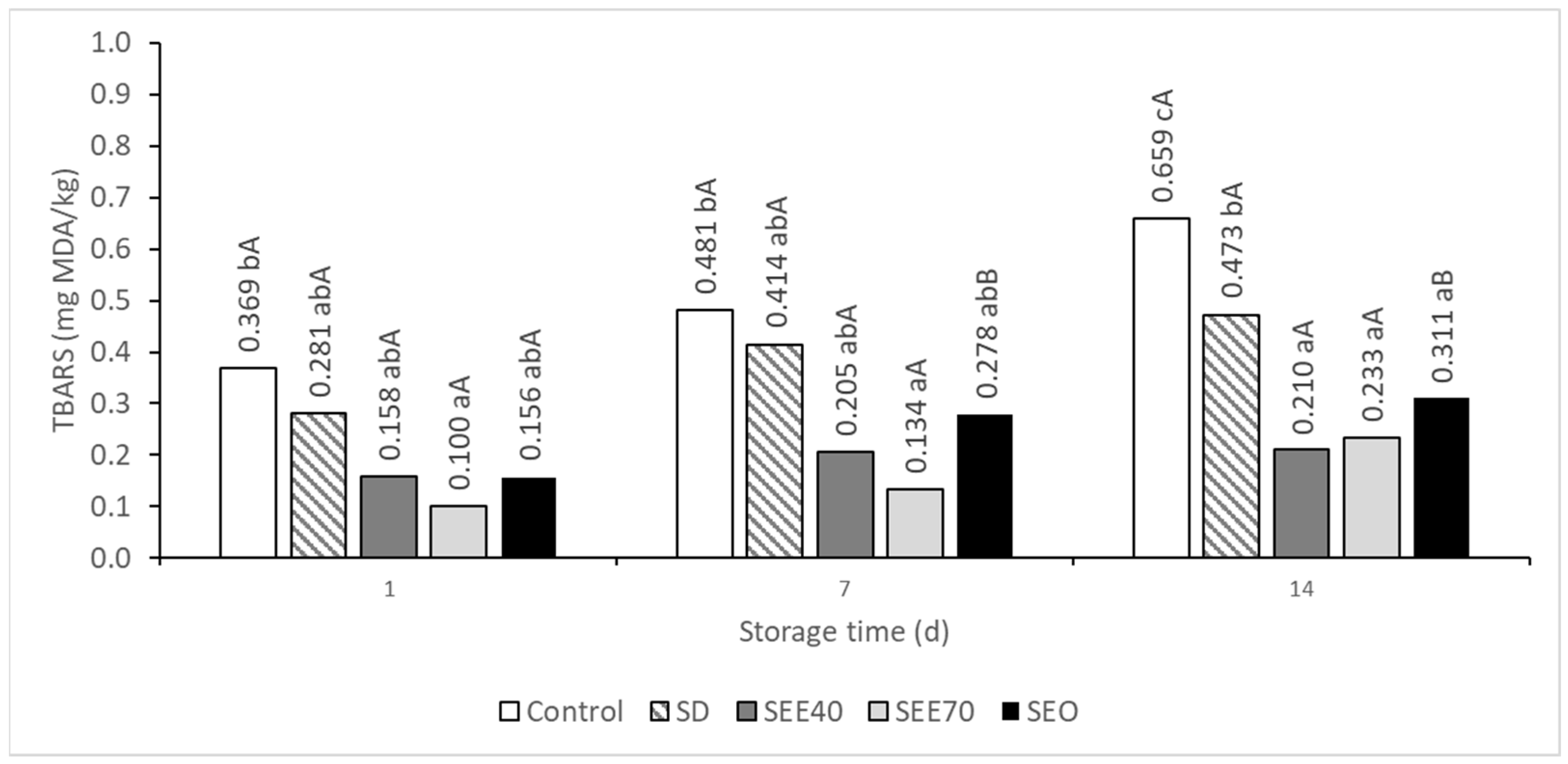
3.2. Lipid Oxidation in Chicken Meatballs
3.3. Microbiological Quality of Chicken Meatballs
3.4. Color Parameters of Chicken Meatballs
3.5. Organoleptic Quality of Chicken Meatballs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abay, S.; Irkin, R.; Aydin, F.; Müştak, H.K.; Diker, K.S. The prevalence of major foodborne pathogens in ready-to-eat chicken meat samples sold in retail markets in Turkey and the molecular characterization of the recovered isolates. LWT Food Sci. Technol. 2017, 81, 202–209. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Grashorn, M.A. Functionality of poultry meat. J. Appl. Poult. Res. 2011, 16, 99–106. [Google Scholar] [CrossRef]
- Püssa, T.; Raudsepp, P.; Toomik, P.; Pällin, R.; Mäeorg, U.; Kuusik, S.; Soidla, R.; Rei, M. A study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J. Food Compos. Anal. 2009, 22, 307–314. [Google Scholar] [CrossRef]
- Bigolin, J.; Weber, C.I.; Alfaro, A. Lipid oxidation in mechanically deboned chicken meat: Effect of the addition of different agents. Food Nutr. Sci. 2013, 4, 219–223. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on the public health risks related to mechanically separated meat (MSM) derived from poultry and swine. EFSA J. 2013, 11, 3137. [Google Scholar] [CrossRef]
- Łaszkiewicz, B.; Szymański, P.; Kołożyn-Krajewska, D. Quality problems in mechanically separated meat. Med. Weter. 2019, 75, 131–137. [Google Scholar] [CrossRef]
- Dawson, P.L. Novel methods to improve the safety and quality of in-pack processed ready-to-eat meat and poultry products. In In-Pack Processed Foods: Improving Quality; Richardson, P., Ed.; Cambridge: Woodhead, UK, 2008; pp. 358–381. [Google Scholar] [CrossRef]
- Shao, L.; Chen, S.; Wang, H.; Zhang, J.; Xu, X.; Wang, H. Advances in understanding the predominance, phenotypes, and mechanisms of bacteria related to meat spoilage. Trends Food Sci. Technol. 2021, 118, 822–832. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Pejčić, M.; Joković, N.; Jokanović, M.; Ivić, M.; Šojić, B.; Škaljac, S.; Stojanović, P.; Mihajilov-Krstev, T. Inhibition of Salmonella Enteritidis growth and storage stability in chicken meat treated with basil and rosemary essential oils alone or in combination. Food Control 2018, 90, 332–343. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Kumar, S.; Soni, A. Evaluation of anti-oxidant and anti-microbial activity of various essential oils in fresh chicken sausages. J. Food Sci. Technol. 2017, 54, 279–292. [Google Scholar] [CrossRef]
- Can, Ö.P. The effect of thyme oil on the shelf life of chicken balls during storage period. Slov. Vet. Res. 2012, 49, 19–26. Available online: https://www.slovetres.si/index.php/SVR/issue/view/14/14 (accessed on 29 October 2022).
- Can, Ö.P.; Şahin, S. Effect of rosemary essential oil coated vacuum packaging on the quality of chicken meatballs at +4 °C. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 2165–2169. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Karami, N.; Shavisi, N. Effect of Ziziphora clinopodioides essential oil on shelf life and fate of Listeria monocytogenes and Staphylococcus aureus in refrigerated chicken meatballs. J. Food Saf. 2018, 38, e12394. [Google Scholar] [CrossRef]
- Sorour, M.A.E.; Abd El-Hamied, A.; Mahmoud, A.; Mahmoud, E. Impact of liquid smoke and thyme oil on quality of chicken and turkey chilled meatballs. J. Sohag Agrisci. 2021, 6, 137–150. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—a review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT Food Sci. Technol. 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Hać-Szymańczuk, E.; Cegiełka, A.; Lipińska, E.; Ilczuk, P. Effect of sage on the microbial quality and TBARS value of mechanically deboned poultry meat. Med. Weter. 2014, 70, 704–708. (In Polish) [Google Scholar]
- Hać-Szymańczuk, E.; Cegiełka, A.; Lipińska, E.; Czapska, S. Evaluation of chemical composition and antibacterial activity of water extracts from selected spices. Zesz. Probl. Post. Nauk Rol. 2015, 582, 3–11. (In Polish) [Google Scholar]
- Estévez, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.H.; Leng, X.J.; Huang, M.; Zhou, G.H. Effect of sage (Salvia officinalis) on the oxidative stability of Chinese-style sausage during refrigerated storage. Meat Sci. 2013, 95, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hać-Szymańczuk, E.; Cegiełka, A. Evaluation of antimicrobial and antioxidant activity of sage in meat product. Zywnosc Nauka Technol. Jakosc 2015, 22, 84–94. [Google Scholar] [CrossRef]
- Mizi, L.; Cofrades, S.; Bou, R.; Pintado, T.; López-Caballero, M.E.; Zaidi, F.; Jiménez-Colmenero, F. Antimicrobial and antioxidant effects of combined high pressure processing and sage in beef burgers during prolonged chilled storage. Innov. Food Sci. Emerg. Technol. 2019, 51, 32–40. [Google Scholar] [CrossRef]
- Karpińska, M.; Borowski, J.; Danowska-Oziewicz, M. The use of natural antioxidants in ready-to-serve food. Food Chem. 2001, 72, 5–9. [Google Scholar] [CrossRef]
- Cegiełka, A.; Hać-Szymańczuk, E.; Frączkiewicz, K.; Chmiel, M. Influence of antioxidative and antibacterial activity of sage aqueous extract and chitosan fromulation on chicken burger quality. Med. Weter. 2021, 77, 284–290. [Google Scholar] [CrossRef]
- Cegiełka, A.; Hać-Szymańczuk, E.; Piwowarek, K.; Dasiewicz, K.; Słowiński, M.; Wrońska, K. The use of bioactive properties of sage preparations to improve the storage stability of low-pressure mechanically separated meat from chickens. Poult. Sci. 2019, 98, 5045–5053. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Włostowska, J. Laboratory Experiments in Organic Chemistry, 7th ed; SGGW Publishing: Warsaw, Poland, 2007; pp. 38–44. (In Polish) [Google Scholar]
- Georgantelis, D.; Ambrosiadis, I.; Katikou, P.; Blekas, G.; Georgakis, S.A. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci. 2007, 76, 172–181. [Google Scholar] [CrossRef] [PubMed]
- PN-A-82109:2010; Meat and Meat Products. Determination of Fat, Protein and Water Content—Near Infrared Transmission Spectrometry (NIT) Using Artificial Neural Network (ANN), Calibration. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Shahidi, F. The 2-tiobarbituric Acid (TBA) Methodology for the Evaluation of Warmed-Over Flavor and Oxidative Rancidity in Meat Products. In Proceedings of the 36th International Congress of Meat Science and Technology, Havana, Cuba, 27 August–1 September 1990; pp. 1008–1014. Available online: http://icomst-proceedings.helsinki.fi/papers/1990_09_11.pdf (accessed on 1 September 2022).
- PN-EN ISO 6887-2:2017; Microbiology of the Food Chain. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 2: Specific Rules for the Preparation of Meat and Meat Products. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 4833-1:2013-12; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-ISO 17410:2004; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Detection of Psychrotrophic Microorganisms. Polish Committee for Standardization: Warsaw, Poland, 2004.
- PN-ISO 4832:2007; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Coliform Bacteria. Plate Method. Polish Committee for Standardization: Warsaw, Poland, 2007.
- PN-EN ISO 21528-1:2017; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 1: Detection of Enterobacteriaceae. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-ISO 15214:2002; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Plate Method at 30 °C. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Baryłko-Pikielna, N.; Matuszewska, I. Sensoryczne Badania Żywności. Podstawy—Metody—Zastosowania; Wydawnictwo Naukowe PTTŻ: Cracow, Poland, 2009; pp. 89–108, 163–179. (In Polish) [Google Scholar]
- Zwolan, A.; Kudlik, M.; Adamczak, L.; Pietrzak, D. Effect of black cumin (Nigella sativa) additive on selected properties of poultry meatballs. Zywnosc Nauk Technol. Jakosc 2019, 26, 43–54. [Google Scholar] [CrossRef]
- Keşkekoğlu, H.; Üren, A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 2014, 96, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, P.D.; Araújo, W.M.C.; Patarata, L.; Fraqueza, M.J. Understanding the main factors that influence consumer quality perception and attitude towards meat and processed meat products. Meat Sci. 2022, 193, 108952. [Google Scholar] [CrossRef]
- Bełkot, Z.; Ziomek, M.; Gondek, M. Nutritional value of mechanically recovered goose and chicken meat. Med. Weter. 2013, 69, 499–504. (In Polish) [Google Scholar]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalisz, S.; Wirkowska-Wojdyła, M.; Florowski, T.; Oszmiański, J. Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage. LWT Food Sci. Technol. 2020, 130, 109718. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. 2022, 13, 100213. [Google Scholar] [CrossRef]
- Danyluk, B.; Bilska, A.; Kirklo, P. Assessment of the microbiological quality of the poultry products from convenience food group. Nauka Przyr. Technol. 2015, 9, 31. [Google Scholar] [CrossRef]
- Li, Y.-X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Hać-Szymańczuk, E.; Lipińska, E.; Chlebowska-Śmigiel, A. Comparision of antimicrobial activity of sage (Salvia officinalis L.) and oregano (Origanum vulgare L.) essential oils. Zesz. Probl. Post. Nauk Rol. 2014, 577, 53–62. Available online: http://www.zppnr.sggw.pl/577-06.pdf (accessed on 6 September 2022). (In Polish).
- Aziman, N.; Jawaid, M.; Mutalib, N.A.A.; Yusof, N.L.; Nadrah, A.H.; Nazatul, U.K.; Tverezovskiy, V.V.; Tverezovskaya, O.A.; Fouad, H.; Braganca, R.M.; et al. Antimicrobial potential of plastic films incorporated with sage extract on chicken meat. Foods 2021, 10, 2812. [Google Scholar] [CrossRef] [PubMed]
- Gál, J.; Kameník, J.; Salek, R.N.; Polášek, Z.; Macharáčková, B.; Valenta, T.; Haruštiaková, D.; Vinter, S. Research Note: Impact of applied thermal treatment on textural, and sensory properties and cooking loss of selected chicken and turkey cuts as affected by cooking technique. Poult. Sci. 2022, 101, 101923. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Anjum, F.M.; Arshad, S.M.; Imran, M.; Imran, A.; Hussain, S. Oxidative stability and lipid oxidation flavoring volatiles in antioxidants treated chicken meat patties during storage. Lipids Health Dis. 2017, 16, 27. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, S.; Yousuf, B. Effect of bioactive-rich mango peel extract on physicochemical, antioxidant and functional characteristics of chicken sausage. Appl. Food Res. 2022, 2, 100183. [Google Scholar] [CrossRef]
- Hać-Szymańczuk, E.; Cegiełka, A.; Chmiel, M.; Piwowarek, K.; Tarnowska, K. Addition of different rosemary preparations (Rosmarinus officinalis L.) to chicken meatballs improves their quality profile. Int. J. Food Sci. Technol. 2021, 56, 6236–6245. [Google Scholar] [CrossRef]
| Quality Feature | Treatment 1 | ||||
|---|---|---|---|---|---|
| Control | SD | SEE40 | SEE70 | SEO | |
| Thermal treatment yield (%) | 80.5 a ± 1.16 | 79.1 a ± 3.11 | 79.0 a ± 0.51 | 78.8 a ± 1.00 | 79.0 a ± 2.47 |
| Moisture content (%) | 62.4 a ± 2.57 | 62.3 a ± 1.83 | 62.7 a ± 0.68 | 63.3 a ± 1.70 | 62.7 a ± 0.32 |
| Protein content (%) | 18.3 a ± 0.79 | 18.5 a ± 0.25 | 18.4 a ± 0.30 | 18.4 a ± 0.35 | 18.3 a ± 0.35 |
| Fat content (%) | 18.0 a ± 3.38 | 17.9 a ± 0.56 | 17.8 a ± 2.41 | 17.2 a ± 0.10 | 17.8 a ± 1.93 |
| Time (d) | Treatment 1 | ||||
|---|---|---|---|---|---|
| Control | SD | SEE40 | SEE70 | SEO | |
| Mesophilic Aerobic Microorganisms (CFU/g) | |||||
| 1 | 8.2 × 104 bA ± 1.7 × 104 | 1.6 × 105 cAB ± 6.7 × 104 | 4.5 × 103 aA ± 2.3 × 103 | 2.2 × 104 aB ± 7.1× 103 | 2.1 × 103 aA ± 8.0 × 102 |
| 7 | 3.0 × 105 bAB ± 1.1 × 105 | 3.0 × 103 aA ± 2.0 × 102 | 8.2 × 102 aA ± 5.1 × 102 | 1.0 × 103 aA ± 4.0 × 102 | 6.3 × 103 aA ± 4.3 × 103 |
| 14 | 8.4 × 105 bB ± 6.2 × 105 | 2.6 × 105 aB ± 1.7 × 105 | 4.9 × 104 aB ± 2.2 × 104 | 1.1 × 105 aC ± 1.0 × 104 | 1.4 × 105 aB ± 5.2 × 104 |
| Psychrotrophic bacteria (CFU/g) | |||||
| 1 | 1.0 × 103 aA ± 2.6 × 102 | 1.9 × 103 aA ± 1.4 × 103 | 2.1 × 103 aA ± 1.9 × 103 | 8.5 × 102 aA ± 7.0 × 101 | 5.6 × 102 aA ± 4.0 × 102 |
| 7 | 4.9 × 103 aA ± 2.9 × 103 | 1.7 × 105 aA ± 2.9 × 105 | 2.3 × 104 aA ± 3.6 × 104 | 1.0 × 104 aAB ± 9.4 × 103 | 2.5 × 103 aA ± 1.6 × 103 |
| 14 | 1.0 × 105 aA ± 1.0 × 105 | 3.5 × 105 bA ± 1.7 × 105 | 3.1 × 104 aA ± 1.6 × 104 | 1.6 × 104 aB ± 6.0 × 103 | 2.7 × 104 aB ± 1.6 × 104 |
| Coliform bacteria (CFU/g) | |||||
| 1 | 9.6 × 101 bA ± 8.4 × 101 | 4.0 × 100 aA ± 5.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 |
| 7 | 1.6 × 102 bA ± 1.1 × 102 | 1.0 × 100 aA ± 0.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 | 4.4 × 101 aA ± 7.4 × 101 | 1.1 × 101 aA ± 1.7 × 101 |
| 14 | 1.7 × 103 bA ± 1.7 × 103 | 3.0 × 100 aA ± 2.0 × 100 | 4.0 × 100 aA ± 3.0 × 100 | 4.0 × 100 aA ± 5.0 × 100 | 4.0 × 100 aA ± 3.0 × 100 |
| Enterobacteriaceae (CFU/g) | |||||
| 1 | 2.9 × 101 abA ± 2.3 × 101 | 4.6 × 101 bA ± 3.5 × 101 | 1.0 × 101 abA ± 1.6 × 101 | 5.0 × 100 aA ± 6.0 × 100 | 1.0 × 101 abA ± 1.0 × 100 |
| 7 | 3.1 × 101 aA ± 8.0 × 101 | 1.4 × 102 bA ± 9.7 × 101 | 1.6 × 101 aA ± 1.7 × 101 | 1.0 × 101 aA ± 8.0 × 100 | 4.8 × 101 abA ± 6.2 × 101 |
| 14 | 1.1 × 102 bB ± 4.7 × 101 | 3.8 × 101 aA ± 4.8 × 101 | 4.0 × 100 aA ± 3.0 × 100 | 8.0 × 100 aA ± 6.0 × 100 | 4.0 × 100 aA ± 3.0 × 100 |
| Enterococci (CFU/g) | |||||
| 1 | 2.3 × 101 bA ± 7.0 × 100 | 2.1 × 101 bA ± 2.0 × 101 | 2.0 × 100 aA ± 2.0 × 100 | 1.0 × 100 aA ± 0.00 × 100 | 1.0 × 100 aA ± 0.0 × 100 |
| 7 | 5.8 × 101 bA ± 3.1 × 101 | 4.5 × 101 bA ± 2.7 × 101 | 1.0 × 100 aA ± 0.0 × 100 | 2.0 × 100 aA ± 2.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 |
| 14 | 4.8 × 101 cA ± 2.3 × 101 | 3.9 × 101 bcA ± 3.9 × 101 | 3.0 × 100 abA ± 3.0 × 100 | 3.0 × 100 abA ± 4.0 × 100 | 1.0 × 100 aA ± 0.0 × 100 |
| LAB (CFU/g) | |||||
| 1 | 2.9 × 103 bA ± 9.5 × 102 | 2.4 × 103 bA ± 2.5 ×103 | 4.1× 102 aA ± 3.4 × 102 | 4.9 × 102 aA ± 3.4 × 102 | 3.0 × 102 aA ± 1.4 × 102 |
| 7 | 1.0 × 104 bAB ± 4.8 × 103 | 5.1 × 103 abAB ± 3.4 ×103 | 6.6 × 102 aAB ± 3.4 × 102 | 2.0 × 103 aA ± 2.4 × 103 | 4.6 × 102 aA ± 1.1 × 102 |
| 14 | 3.9 × 104 bB ± 2.5 × 104 | 2.2 × 104 abB ± 1.4 ×104 | 1.5 × 103 aB ± 6.4 × 102 | 4.2 × 103 aA ± 3.3 × 103 | 4.7 × 103 aB ± 3.3 × 103 |
| Time (d) | Treatment 1 | ||||
|---|---|---|---|---|---|
| Control | SD | SEE40 | SEE70 | SEO | |
| L* | |||||
| 1 | 66.78 cC ± 0.53 | 63.14 aB ± 0.75 | 65.00 abB ± 0.12 | 62.31 aA ± 0.08 | 65.08 bA ± 0.62 |
| 7 | 66.02 dB ± 0.18 | 61.90 aA ± 0.18 | 65.52 cC ± 0.28 | 63.20 bB ± 0.20 | 66.10 dB ± 0.33 |
| 14 | 64.24 dA ± 0.15 | 61.65 aA ± 0.27 | 63.53 cA ± 0.21 | 63.04 bB ± 0.17 | 64.97 eA ± 0.06 |
| a* | |||||
| 1 | 5.80 cB ± 0.14 | 4.68 bB ± 0.13 | 4.17 aA ± 0.21 | 4.16 aA ± 0.29 | 5.53 cB ± 0.26 |
| 7 | 4.79 bA ± 0.08 | 4.07 aA ± 0.12 | 3.90 aA ± 0.14 | 3.78 aA ± 0.26 | 4.78 bA ± 0.26 |
| 14 | 5.74 cB ± 0.17 | 4.38 aAB ± 0.31 | 4.16 aA ± 0.19 | 4.78 bB ± 0.06 | 5.13 bAB ± 0.27 |
| b* | |||||
| 1 | 8.30 aA ± 0.06 | 9.25 bC ± 0.62 | 8.29 aA ± 0.15 | 7.99 aB ± 0.16 | 8.48 aB ± 0.29 |
| 7 | 8.24 bA ± 0.10 | 7.47 aA ± 0.33 | 8.17 bA ± 0.28 | 7.26 aA ± 0.42 | 8.00 abA ± 0.08 |
| 14 | 8.81 cB ± 0.17 | 8.51 abcB ± 0.18 | 8.45 abA ± 0.31 | 8.72 bcC ± 0.16 | 8.23 aAB ± 0.10 |
| Time (d) | Treatment 1 | ||||
|---|---|---|---|---|---|
| Control | SD | SEE40 | SEE70 | SEO | |
| Appearance and Color (Points) | |||||
| 1 | 4.6 aA ± 0.21 | 4.5 aA ± 0.26 | 4.5 aC ± 0.06 | 4.5 aB ± 0.17 | 4.5 aB ± 0.12 |
| 7 | 4.4 aA ± 0.21 | 4.3 aA ± 0.21 | 4.2 aB ± 0.06 | 4.4 aAB ± 0.15 | 4.3 aB ± 0.06 |
| 14 | 4.3 bA ± 0.12 | 4.2 abA ± 0.06 | 4.1 abA ± 0.00 | 4.1 abA ± 0.17 | 4.0 aA ± 0.06 |
| Aroma (points) | |||||
| 1 | 4.7 bB ± 0.17 | 4.6 abB ± 0.20 | 4.6 abA ± 0.21 | 4.4 abA ± 0.17 | 4.3 aA ± 0.10 |
| 7 | 4.6 bAB ± 0.06 | 4.4 abAB ± 0.06 | 4.5 bA ± 0.15 | 4.3 aA ± 0.12 | 4.2 aA ± 0.15 |
| 14 | 4.4 aA ± 0.12 | 4.2 aA ± 0.20 | 4.3 aA ± 0.12 | 4.3 aA ± 0.12 | 4.1 aA ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegiełka, A.; Chmiel, M.; Hać-Szymańczuk, E.; Pietrzak, D. Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat. Appl. Sci. 2022, 12, 12890. https://doi.org/10.3390/app122412890
Cegiełka A, Chmiel M, Hać-Szymańczuk E, Pietrzak D. Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat. Applied Sciences. 2022; 12(24):12890. https://doi.org/10.3390/app122412890
Chicago/Turabian StyleCegiełka, Aneta, Marta Chmiel, Elżbieta Hać-Szymańczuk, and Dorota Pietrzak. 2022. "Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat" Applied Sciences 12, no. 24: 12890. https://doi.org/10.3390/app122412890
APA StyleCegiełka, A., Chmiel, M., Hać-Szymańczuk, E., & Pietrzak, D. (2022). Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat. Applied Sciences, 12(24), 12890. https://doi.org/10.3390/app122412890






