Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Culture Conditions
2.3. Wastes
2.4. Analytical Methods
2.5. Fatty Acids Profile and Sterols Determination
2.6. PDSC Analysis of Extracted Oils
2.7. Statistical Analyses
3. Results
3.1. Batch and Fed-Batch Yarrowia lipolytica Cultures
3.2. Effect of the Culture Mode on Lipid Accumulation
3.3. Fatty Acids Composition of Cellular Lipids from Y. lipolytica Cultures
3.4. Effect of the Culture Mode on the Sterol Content and the Elemental Composition of the Biomass
3.5. PDSC of Cellular Oil Samples
4. Discussion
4.1. Effect of Culture Mode on Lipid Accumulation in Fed-Batch Culture with Post-Frying Rapeseed Oil
4.2. Comparison of Batch and Fed-Batch Cultures in Molasses Media
4.3. Effect of Culture Conditions on Microbial Sterols Content
4.4. Analysis of Fatty Acid Composition and Oxidative Stability
4.5. Elemental Composition of Y. lipolytica Dry Biomass from Waste Media
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, M.I.; Shahzad, K. Food waste recycling for compost production and its economic and environmental assessment as circular economy indicators of solid waste management. J. Clean. Prod. 2021, 317, 128467. [Google Scholar] [CrossRef]
- FAO. Moving Forward on Food Loss and Waste Reduction. In The State of Food and Agriculture 2019; Licence: CC BY-NC-SA 3.0 IGO; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Wierzchowska, K.; Nowak, D.; Wołoszynowska, M.; Zieniuk, B. Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture. Processes 2022, 10, 294. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass- Valorization 2021, 13, 757–779. [Google Scholar] [CrossRef]
- Liu, N.; Soong, Y.V.; Mirzaee, I.; Olsen, A.; Yu, P.; Wong, H.; Xie, D. Biomanufacturing of value-added products from oils or fats: A case study on cellular and fermentation engineering of Yarrowia lipolytica. Biotechnol. Bioeng. 2021, 118, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P.; Wang, Q.-Q.; Liu, S.; Liu, X.-F.; Yu, X.-J.; Jiang, Y.-L. Efficient Conversion of Cane Molasses Towards High-Purity Isomaltulose and Cellular Lipid Using an Engineered Yarrowia lipolytica Strain in Fed-Batch Fermentation. Molecules 2019, 24, 1228. [Google Scholar] [CrossRef]
- Bao, W.; Li, Z.; Wang, X.; Gao, R.; Zhou, X.; Cheng, S.; Men, Y.; Zheng, L. Approaches to improve the lipid synthesis of oleaginous yeast Yarrowia lipolytica: A review. Renew. Sustain. Energy Rev. 2021, 149, 111386. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Phosphorus and Nitrogen Limitation as a Part of the Strategy to Stimulate Microbial Lipid Biosynthesis. Appl. Sci. 2021, 11, 11819. [Google Scholar] [CrossRef]
- Chauhan, M.K.; Varun, S.C.; Kumar, S.; Samar. Life cycle assessment of sugar industry: A review. Renew. Sustain. Energy Rev. 2011, 15, 3445–3453. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2020, 346, 128860. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production—A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2583–2616. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y.; Liu, Y.; Wang, Q. Waste cooking oil used as carbon source for microbial lipid production: Promoter or inhibitor. Environ. Res. 2021, 203, 111881. [Google Scholar] [CrossRef]
- Rattray, J.B.M. Yeasts. In Microbial Lipids; Ratledge, C., Wilkinson, S.G., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 1, p. 555. [Google Scholar]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2021, 119, 593–607. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Misiukiewicz-Stępień, P.; Paplińska-Goryca, M.; Zieniuk, B.; Białecka-Florjańczyk, E. An Insight into Storage Lipid Synthesis by Yarrowia lipolytica Yeast Relating to Lipid and Sugar Substrates Metabolism. Biomolecules 2019, 9, 685. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. Quantitative Analysis of Reducing Sugars by 3, 5-Dinitrosalicylic Acid (DNSA Method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Humana: New York, NY, USA, 2020; pp. 181–183. [Google Scholar] [CrossRef]
- Derewiaka, D.; Stepnowska, N.; Bryś, J.; Ziarno, M.; Ciecierska, M.; Kowalska, J. Chia seed oil as an additive to yogurt. Grasas Y Aceites 2019, 70, 302. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Ostrowska-Ligęza, E.; Kalisz, S. The Study of Thermal Properties of Blackberry, Chokeberry and Raspberry Seeds and Oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by Differential Scanning Calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Imatoukene, N.; Back, A.; Nonus, M.; Thomasset, B.; Rossignol, T.; Nicaud, J.-M. Fermentation process for producing CFAs using Yarrowia lipolytica. J. Ind. Microbiol. Biotechnol. 2020, 47, 403–412. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.; Pietrzak, W.; Dobrowolski, A. Rye and Oat Agricultural Wastes as Substrate Candidates for Biomass Production of the Non-Conventional Yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Ryu, S.; Trinh, C.T. Exceptional Solvent Tolerance in Yarrowia lipolytica Is Enhanced by Sterols. bioRxiv 2018, 54, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kodedová, M.; Sychrová, H. Changes in the Sterol Composition of the Plasma Membrane Affect Membrane Potential, Salt Tolerance and the Activity of Multidrug Resistance Pumps in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0139306. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, P.; Nicaud, J.-M.; Rossignol, T.; Čertík, M. Single cell oil production on molasses by Yarrowia lipolytica strains overexpressing DGA2 in multicopy. Appl. Microbiol. Biotechnol. 2015, 99, 8065–8074. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Methods 2017, 11, 1095–1104. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of cold-pressed rapeseed oil using Pressure Differential Scanning Calorimetry and Rancimat methods. Eur. J. Lipid Sci. Technol. 2016, 119, 1600182. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J. Therm. Anal. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef]
- Groombridge, A.S.; Miyashita, S.-I.; Fujii, S.-I.; Nagasawa, K.; Okahashi, T.; Ohata, M.; Umemura, T.; Takatsu, A.; Inagaki, K.; Chiba, K. High Sensitive Elemental Analysis of Single Yeast Cells (Saccharomyces cerevisiae) by Time-Resolved Inductively-Coupled Plasma Mass Spectrometry Using a High Efficiency Cell Introduction System. Anal. Sci. 2013, 29, 597–603. [Google Scholar] [CrossRef]
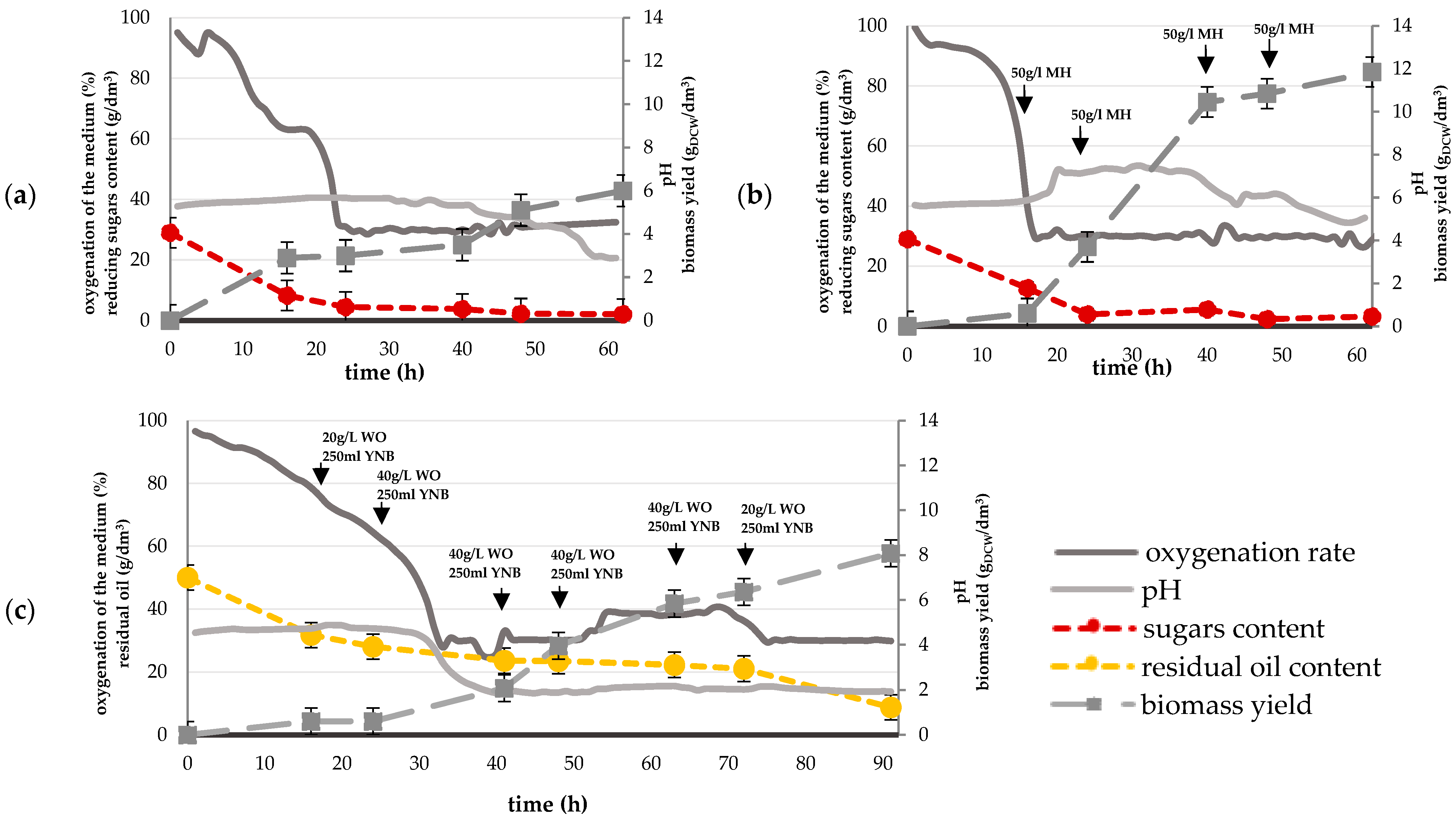
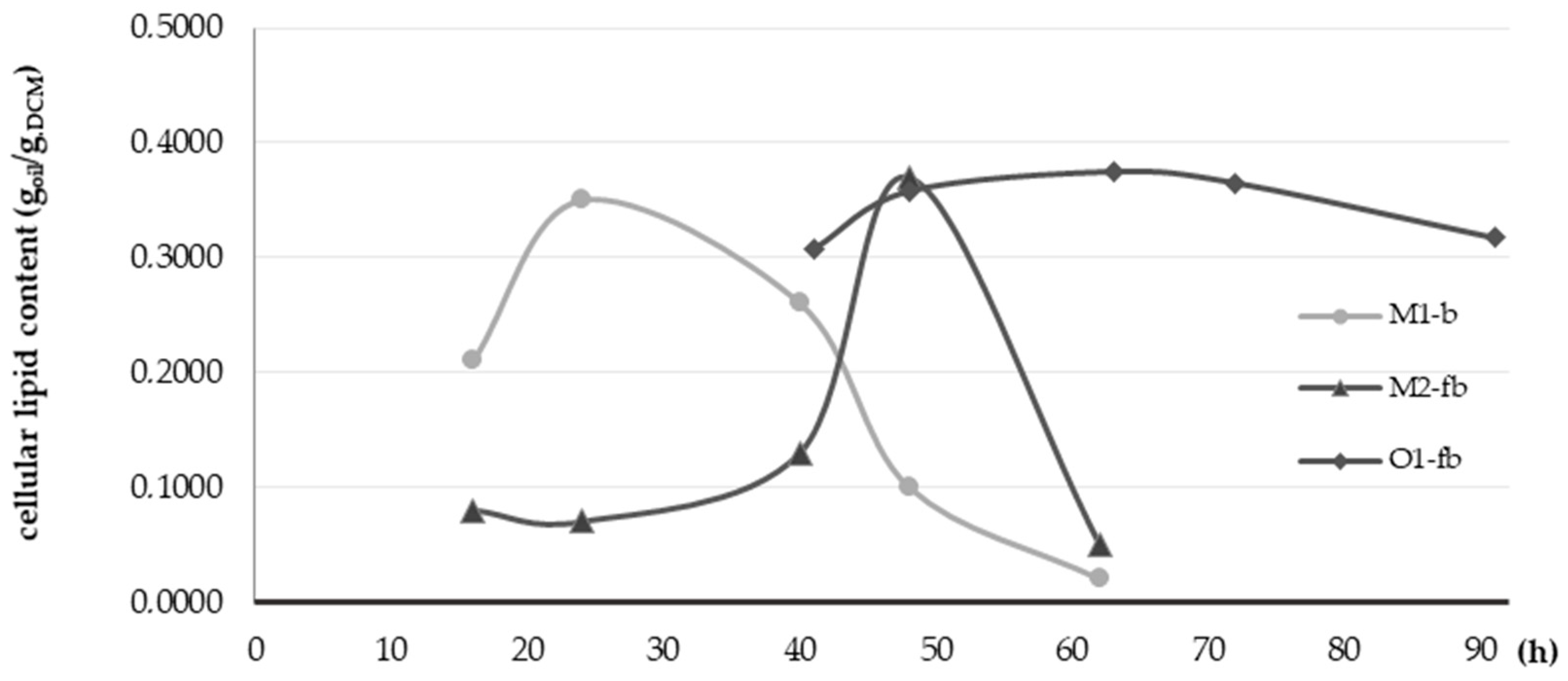
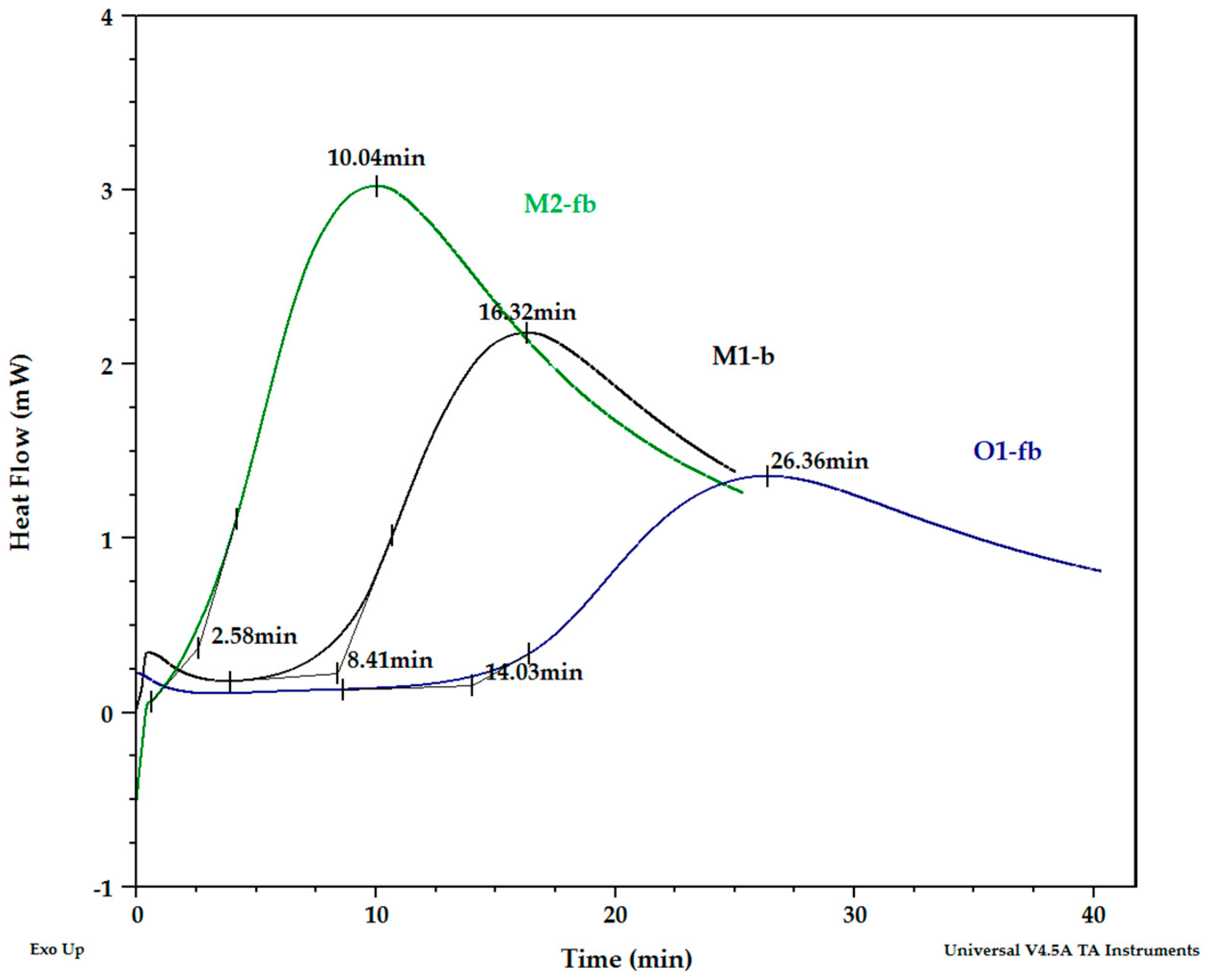
| Parameter | Unit | M1-b | M2-fb | O1-fb | |
|---|---|---|---|---|---|
| Initial concentration of carbon source | g/L | 50 | 50 | 50 | |
| Time (t) | h | 62 | 62 | 91 | |
| Biomass yield (X) | gDCW/L | 6.00 | 11.82 | 8.08 | |
| Conversion biomass yield per carbon substrate (YX/S) | gDCW/g | 0.1200 | 0.2364 | 0.1616 | |
| Concentration of lipids produced (L) | g/L | 16 h | 0.6090 | 0.0480 | - |
| 24 h | 1.0500 | 0.2590 | - | ||
| 40 h | 0.9100 | 1.3585 | 0.6600 | ||
| 48 h | 0.5100 | 4.0108 | 1.4454 | ||
| 62 h | 0.1200 | 0.5925 | 2.1900 | ||
| 72 h | - | - | 2.2762 | ||
| 90 h | - | - | 2.4846 |
| Culture Variant | Time | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 |
|---|---|---|---|---|---|---|---|---|
| M1-b | 62 h | 11.67 | 5.51 | 52.66 | 22.49 | 7.68 | n.d. | n.d. |
| M2-fb | 62 h | 13.69 | 4.65 | 44.43 | 30.75 | 6.48 | n.d. | n.d. |
| O1-fb | 40 h | 3.01 | 9.25 | 60.36 | 18.23 | 4.20 | 4.09 | 1.06 |
| 48 h | 4.09 | 11.08 | 58.43 | 18.88 | 2.94 | 3.75 | 0.92 | |
| 62 h | 4.21 | 10.86 | 61.81 | 16.41 | 1.76 | 4.15 | 1.05 | |
| 72 h | 5.20 | 10.13 | 63.17 | 15.26 | 2.23 | 3.61 | 0.68 | |
| 90 h | 4.09 | 8.49 | 66.94 | 14.41 | 0.72 | 4.93 | 0.51 |
| Oil Samples | M1-b | M2-fb | O1-fb | |||
|---|---|---|---|---|---|---|
| % | mg/goil | % | mg/goil | % | mg/goil | |
| Cholesterol | 5.34 ± 0.50 | 0.45 ± 0.13 b | 0.32 ± 0.05 | 0.22 ± 0.04 a | 1.02 ± 0.26 | 0.04 ± 0.02 a |
| Dehydroergosterol | 6.51 ± 0.31 | 0.54 ± 0.08 a | 1.85 ± 0.24 | 1.26 ± 0.11 b | 12.67 ± 1.28 | 0.42 ± 0.23 a |
| Ergosterol | 57.95 ± 2.58 | 5.00 ± 1.33 a | 87.94 ± 0.91 | 60.16 ± 3.33 b | 13.86 ± 1.74 | 0.40 ± 0.00 c |
| Campesterol | 10.24 ± 0.91 | 0.90 ± 0.10 a | 2.15 ± 0.17 | 1.47 ± 0.05 b | 25.22 ± 3.22 | 0.84 ± 0.49 a |
| Stigmasterol | 2.67 ± 0.53 | 0.22 ± 0.01 a | 2.90 ± 0.38 | 1.98 ± 0.17 b | 2.96 ± 0.57 | 0.12 ± 0.09 a |
| β-sitosterol | 18.25 ± 3.81 | 1.50 ± 0.02 a | 4.86 ± 0.16 | 3.32 ± 0.04 b | 44.29 ± 0.38 | 1.44 ± 0.69 a |
| ∑ | 100 | 8.34 ± 1.66 | 100 | 68.40 ± 3.75 | 100 | 3.25 ± 1.55 |
| Biomass Samples | C | N | K | Na | P | S | Ca | Mg | Fe | Al | Ti | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | mg/kg | |||
| M1-b | 447.87 | 65.56 | 25.63 | 18.47 | 9.84 | 13.61 | 0.87 | 0.69 | 0.46 | 19.25 | 3.41 | ||
| M2-fb | 376.26 | 43.21 | 47.54 | 16.89 | 11.96 | 8.76 | 2.13 | 0.65 | 0.20 | 7.98 | 1.09 | ||
| O1-fb | 601.12 | 20.78 | 16.48 | 0.46 | 10.05 | 2.04 | 0.08 | 0.84 | 0.59 | 55.47 | 2.03 | ||
| Mn | Cu | Zn | Cr | Ni | Pb | V | Zr | Co | Sr | Ba | Li | ||
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | ||
| M1-b | 136.05 | 6.90 | 168.64 | 10.71 | 15.08 | 1.13 | 0.62 | 3.94 | 2.43 | 2.77 | 0.54 | 0.18 | |
| M2-fb | 197.35 | 5.77 | 209.80 | 3.12 | 2.54 | 0.90 | 0.53 | 1.27 | 2.23 | 6.45 | 0.77 | 0.29 | |
| O1-fb | 50.79 | 0.79 | 93.34 | 2.29 | 1.08 | 1.20 | 0.14 | 1.30 | 0.01 | 0.40 | 0.66 | 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzchowska, K.; Pakulska, A.; Derewiaka, D.; Piasecka, I.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil. Appl. Sci. 2022, 12, 12877. https://doi.org/10.3390/app122412877
Wierzchowska K, Pakulska A, Derewiaka D, Piasecka I, Zieniuk B, Nowak D, Fabiszewska A. Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil. Applied Sciences. 2022; 12(24):12877. https://doi.org/10.3390/app122412877
Chicago/Turabian StyleWierzchowska, Katarzyna, Anna Pakulska, Dorota Derewiaka, Iga Piasecka, Bartłomiej Zieniuk, Dorota Nowak, and Agata Fabiszewska. 2022. "Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil" Applied Sciences 12, no. 24: 12877. https://doi.org/10.3390/app122412877
APA StyleWierzchowska, K., Pakulska, A., Derewiaka, D., Piasecka, I., Zieniuk, B., Nowak, D., & Fabiszewska, A. (2022). Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil. Applied Sciences, 12(24), 12877. https://doi.org/10.3390/app122412877









