Abstract
Allelopathy, a biological phenomenon, is a valuable tool for weed management and minimization of synthetic pesticide use in sustainable agricultural production. The aim of the study was to evaluate the allelopathic potential of nine sunflower genotypes. Water extracts from dry sunflower leaves collected in two growth stages (butonisation and flowering) were tested in two concentrations (1 and 2.5%) on germination and growth of lettuce under laboratory conditions. The allelopathic effect of extracts was influenced by genotype, growth stage and extract concentration. The majority of extracts exhibited negative allelopathic potential with seed germination being the least affected, and root length reduced up to 85% compared to the control. A higher concentration of water extracts resulted in a greater reduction of lettuce growth parameters. On average, extracts collected in the flowering stage inhibited lettuce shoot length to a greater degree. Several genotypes showed a greater negative impact, especially on shoot length and seedlings’ fresh weight. Further investigations of selected sunflower genotypes with the highest allelopathic potential on weed species and studies on phytochemical analysis are needed.
Keywords:
allelopathy; sunflower; genotypes; growth stage; water extracts; inhibition; seedlings growth 1. Introduction
Modern agricultural production relies heavily on applying synthetic herbicides as a simple and effective weed control measure. However, their excessive and improper use causes a series of negative consequences, such as the occurrence of resistant weed populations and herbicide residues in food, soil and water, that is, environmental pollution with adverse effects on human and animal health [1,2]. Additionally, herbicide application restrictions, a ban on active ingredients and the demand of users for food produced in organic systems [3,4] call for the search for alternative methods in weed control.
Allelopathy represents both harmful and beneficial, direct and indirect effects of one species (donor) on the growth and development of the other (receiver) through the secretion of chemical compounds (allelochemicals) in the environment [5]. Allelochemicals, which are mainly secondary metabolites, are present in almost all plant species and in various concentrations in different plant parts, from roots to flowers and seeds [6,7,8]. The release of allelochemicals by means of root exudation, volatilization, leaching and decomposition of plant material [9] occurs in natural ecosystems and plays an important role in plant diversity and species succession, as well as in agroecosystems in crop productivity [10]. Allelopathic crops can be utilized in plant protection for integrated weed management in different ways, such as water extracts or natural bioherbicides, in crop rotation, as mulches or incorporated residues, as cover crops, or as catch crops [11,12,13,14]. Crops with excellent allelopathic potential include rice (Oryza sativa), sorghum (Sorghum bicolor), rye (Secale cereale) [15], barley (Hordeum vulgare), triticale (× Tritico secale) [16], alfalfa (Medicago sativa) [17], as well as species from the Brassiceae family, such as white mustard (Sinapis alba), radish (Raphanus sativus) and camelina (Camellina sativa) [13,14]. Medicinal and aromatic plants, both wild and cultivated, are also frequently screened due to the wide variety of compounds they possess [18,19,20].
The allelopathic potential of plant species is dependent on multiple factors, with the concentration/dose, plant parts and test species being the subject of the majority of studies [14,17,19,21,22,23,24]. Differences in allelopathic potential were also recorded among fresh or dry plant materials and extraction methods [18,19,22]. Both seasonal variation and species’ growth stages may influence the allelopathic performance of species. However, studies usually focus only on one sampling time. Appiah et al. [25] evaluated the effect of seasonal variation on the phytotoxic potential of rosemary (Salvia rosmarinus). They concluded that the highest inhibitory potential on lettuce (Lactuca sativa) was observed with samples collected from early summer (June), coinciding with the highest average concentration of carnosic acid. Similarly, plant materials collected from vegetative, flowering and/or mature stages significantly vary in their ability to impede the germination and growth of target species [26,27,28]. Variability in the allelopathic potential of genotypes has been previously documented for several plant species, such as rice and wheat (Triticum aestivum) [29]. Scavo et al. [24] pointed out that durum wheat’s local landraces showed higher phytotoxic potential compared to the modern variety. Zubair et al. [30] found a significant difference in 40 alfalfa genotypes for their ability to suppress annual ryegrass (Lolium rigidum) with an allelopathic inhibition index for roots that ranged from 3.5 to 45.5%.
Sunflower (Helianthus annuus L.) is a highly allelopathic plant, and its phytotoxic potential has been demonstrated on crops and weeds in the laboratory, greenhouse and field trials with regard to a variety of factors, such as concentration, test species and genotype [12,23,31,32,33,34,35]. However, studies on the effect of sunflower growth stages are scarce. Additionally, no previous reports exist on the allelopathic potential of Croatian sunflower genotypes. Thus, this study aimed to test the allelopathic potential of water extracts from several Croatian sunflower genotypes. To reach the aim, the following objectives were defined: (I) to compare the allelopathic effect of leaf extracts from different sunflower genotypes with regard to the sunflower growth stage; and (II) to assess seed germination and growth parameters of lettuce seedlings under allelopathic stress. The results are aimed to identify the best sunflower genotypes to be selected for further studies with the purpose of evaluating their bioherbicidal properties.
2. Materials and Methods
2.1. Sunflower Genotypes
Nine sunflower genotypes were included in the study (Table 1), of which three were hybrids and six were parental lines. Sunflower genotypes were cultivated at the experimental field of Agricultural Institute Osijek (AIO) in Croatia. Seeds of each genotype were planted by hand in a randomized block design with three replicates. Each plot consisted of four rows with a length of 4 m spaced 70 cm apart, while interrow spacing was 23 cm. Standard agrotechnical measures were applied for all genotypes.

Table 1.
Sunflower genotypes (AIO—Agricultural Institute Osijek) included in the study.
2.2. Collection of Plant Material and Preparation of Water Extracts
Leaves of each sunflower genotype were collected at two different growth stages, butonisation and flowering, during June 2021 and July 2021, respectively. Ten healthy leaves from the top of plants were collected from each repetition from randomly selected plants and then pooled to form one sample for each genotype and growth stage. In the laboratory, all samples were air-dried for four days, and afterwards, oven-dried at 45 °C for 48 h. Dried leaves were chopped, ground into a fine powder with a mill to pass a 1 mm sieve, and stored in paper bags in a cool place until further use.
According to Norsworthy [21], water extracts were prepared with some modifications. An amount of 2.5 g of each plant material was mixed with 100 mL of distilled water and kept for 24 h at room temperature (22 ± 1 °C). The mixtures were filtered through filter paper and the obtained water extracts were further diluted with distilled water in order to give final concentrations of 1 and 2.5%.
2.3. Bioassay
The allelopathic potential of sunflower water extracts was evaluated in a laboratory bioassay at the Faculty of Agrobiotechnical Sciences Osijek. Commercially purchased lettuce seeds (cv. Majska kraljica) were used as test species. Lettuce seed was chosen as a test species due to its high sensitivity to allelochemicals and rapid germination [36]. The seeds were surface-sterilized with 1% NaOCl for 10 min and rinsed with distilled water.
The experiment was conducted in Petri dishes. Thirty lettuce seeds were placed in 90 mm Petri dishes lined with two layers of filter paper. In each Petri dish, 3 mL of sunflower extract was added, while distilled water was used in control. Petri dishes were incubated at room temperature (22 ± 1 °C) for five days. All treatments had four repetitions.
2.4. Data Collection and Statistical Analysis
The following parameters were measured: germination percentage (%), root and shoot length (cm), and fresh weight (mg) of lettuce seedlings. The seeds were considered germinated when the radicle protruded over 2 mm.
Analysis of variance (ANOVA) was used to determine the statistical differences between growth stages (butonisation and flowering), treatments (control, 1 and 2.5% water extract concentrations) and sunflower genotypes, which were followed by the least significant difference (LSD) post hoc test at p < 0.05.
3. Results
Results of the ANOVA showed that sunflower water extracts had different effects on the germination and growth of lettuce seedlings (Table 2). The concentration of water extracts significantly affected all measured parameters, while only the growth stage had no significant effect on seedlings’ root length. Particular main factors and their interactions influenced seedlings’ shoot length and fresh weight.

Table 2.
Analysis of variance (mean squares) for traits in tested growth stages (butonisation and flowering), treatments (control, 1 and 2.5% water extract concentrations) and sunflower genotypes.
Germination of lettuce was significantly affected by the “growth stage × concentration” interaction (Table 2, Figure 1). For both growth stages, 2.5% concentration showed significantly higher allelopathic potential, with average germination reduction of 6.3% compared to control. The greatest negative effect was recorded in the butonisation stage with 2.5% concentration.
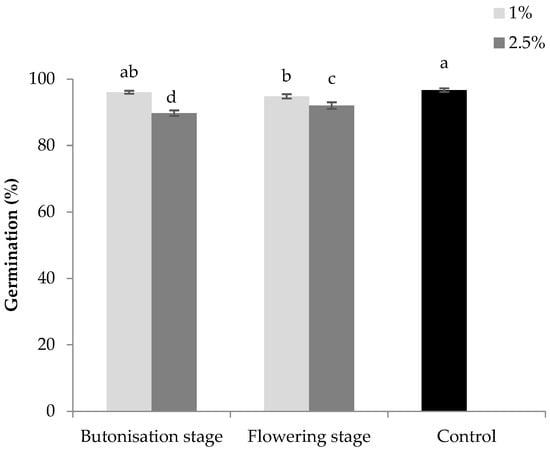
Figure 1.
Allelopathic effect of water extracts from sunflower leaves collected in the butonisation and flowering stage on the germination of lettuce. Columns with the same letters are not significantly different at p < 0.05.
A significant decrease in lettuce seedlings’ root length was recorded in all treatments with sunflower water extracts (Figure 2a,b). The lowest inhibition was with Genotype 2 (11.3%) and the highest with Genotypes 3, 4, 5 and 7 (74.1–80.6%) for a lower concentration of extracts from leaves collected in the butonisation stage. An increase in concentration resulted in a root length decrease of over 80% in all treatments, except Genotypes 1 and 2. Similarly, with lower concentrations of extracts from the flowering stage, root length was markedly inhibited in the majority of treatments for over 70%. Only Genotype 3 had slightly lower allelopathic potential with a root length reduction of 56.1%. Extracts in higher concentrations obtained in the flowering stage induced substantial inhibition, which was highest with Genotypes 1, 2, 4, 5, 6 and 7, and ranged from 80.1% to 84.7%.
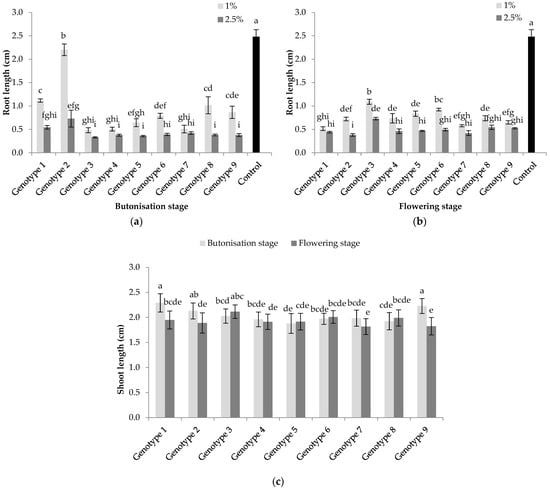
Figure 2.
Allelopathic effect of water extracts from sunflower leaves collected in the butonisation and flowering stage on the root (a,b) and shoot (c) length of lettuce seedlings. Columns with the same letters are not significantly different at p < 0.05.
The allelopathic effect of sunflower extracts on the shoot length of lettuce seedlings is presented in Figure 2c with significant interaction for “growth stage × genotype”. Significantly higher reduction of lettuce shoot length was observed in treatments with Genotypes 1, 2 and 9 from the flowering stage compared to the butonisation stage. For the butonisation stage, the greatest inhibition was recorded with Genotypes 5 and 8.
The fresh weight of lettuce seedlings was significantly affected by the “concentration × genotype” and “growth stage × genotype” interactions (Table 2, Figure 3a,b). Regarding the former (Figure 3a), for all the sunflower genotypes, 2.5% concentration showed significantly higher inhibitory potential compared to 1% concentration and control. Genotypes 4, 5 and 7 at 2.5% concentration showed the highest allelopathic potential and reduced the fresh weight of seedlings for 51.9%, 56.5% and 54.2%. A positive effect was recorded with Genotypes 1 and 9 at lower concentration. For the “growth stage × genotype” interaction (Figure 3b), results revealed a significantly greater reduction of fresh weight with Genotype 9 from the flowering stage compared to the butonisation stage. The greatest inhibition for the butonisation stage was recorded with Genotypes 5 and 7, and with Genotypes 4 and 5 for the flowering stage.
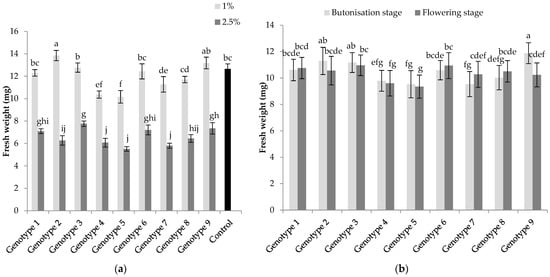
Figure 3.
Allelopathic effect of water extracts from sunflower leaves on the fresh weight of lettuce seedlings: (a) “concentration × genotype” and (b) “growth stage × genotype” interactions. Columns with the same letters are not significantly different at p < 0.05.
4. Discussion
Results of the experiment showed that sunflower water extracts possess significant allelopathic potential for lettuce germination and growth, thereby confirming similar findings in previous studies. Laboratory bioassays confirmed sunflower leaf water extract in 10% concentration significantly delayed and reduced mustard and oilseed rape germination but promoted seedlings’ dry weight [23]. Greenhouse experiments showed that incorporated sunflower residues significantly delayed and inhibited germination of jungle rice (Echinochloa colona) by 22.5% [31], while sunflower leaf extract in combination with herbicide pretilachlor reduced germination of barnyard grass (E. crus-galli) [35]. Crop density, weight of seed/grain and total yield of guar (Cyamopsis tetragonoloba), maize (Zea mays), sorghum (Sorghum vulgare) and pearl millet (Pennisetum americanum) were significantly reduced in fields with sunflower residues [37].
When comparing the response of seedlings to the effect of extracts, germination was the least affected, followed by shoot length and fresh weight, while the greatest negative potential was observed for root length. Tian et al. [38] also concluded that root elongation of maize, soybean (Glycine max) and wheat (Triticum aestivum) seedlings were more sensitive to the effect of extracts compared to seed germination. The presence of sunflower water extracts caused morphological abnormalities in seedlings in the form of root thickening with brown colour [33]. Besides germination and growth, the application of sunflower extracts affects the contents of gibberellic (GA), indole-3-acetic (IAA) and abscisic (ABA) acids in leaves and roots, as well as proline, chlorophyll, sugar, protein, superoxide dismutase (SOD) and peroxidase (POD) contents in leaves of wheat [32]. Various allelopathic compounds, such as phenolics (syringic, vanillic, sinapic and p-coumaric acids) and sesquiterpene lactones, have been identified in sunflower residues and leaves [12,39].
Negative allelopathic potential significantly increased with the increase of sunflower water extracts concentration, which was especially pronounced in the growth of seedlings. Higher concentrations usually have a greater detrimental effect, and studies sometimes report germination and growth inhibition up to 100% [19,22,38,40]. On the contrary, positive effects can occur with extracts in lower concentrations promoting seedlings’ growth and accumulation of biomass [14,23], which in our study was recorded with Genotypes 1 and 2. Such extracts could be used as biostimulators for crop growth promotion [13]. Pannacci et al. [40] stated that, in addition to the stimulative effect on crops, ideal extracts should simultaneously have herbicidal activity on weeds. This emphasizes the need for assessing allelopathic potential on both weeds and crops as test species.
The results of this experiment indicated that although all genotypes showed a certain degree of allelopathic activity, depending on both growth stage and extract concentration, several genotypes proved to possess greater overall inhibitory potential. Among these were Genotypes 4 and 7, which were hybrids, and Genotypes 5 and 8, which were used as their maternal lines. The reduction of seedlings’ root length and fresh weight amounted to 80.6% and 38.1%, respectively. Alsaadawi et al. [12] demonstrated significant differences among eight sunflower genotypes in their suppressive abilities towards weeds by root exudation and residue incorporation. They marked a higher total content of phenolic compounds in genotypes with high inhibitory potential. Using the relay seeding method, Silva et al. [34] studied 23 sunflower genotypes in order to evaluate the allelopathic potential of root exudates against the weed species Bidens pilosa. Great variability among genotypes was observed, and although the decline in B. pilosa germination was not recorded, the reduction in root and shoot length amounted to 51.2% and 27.9%, respectively, suggesting the possibility of including genotypes with high suppressive abilities in breeding programs [15,41].
The diversity and concentration of allelochemicals and allelopathic potential vary with the phenological stage of the donor plant [26,27,42]. This was also confirmed in this study for the shoot length of seedlings, which was, on average, more inhibited with leaves collected in the flowering stage than in the butonisation stage (Table 2 and Figure 2c). Đikić et al. [43] found that extracts from buckwheat collected in the flowering stage suppressed weed germination and growth to a greater extent compared to buckwheat in the ripening stage. Higher phytotoxicity of extract from the full flowering stage compared to straw was also recorded for Brassica species [44]. In several studies, plant material from the vegetative stage was more allelopathic than in the fruiting stage [27,28]. In addition, this research showed a significant interaction of growth stage and genotype for all growth parameters of lettuce (Table 2). For example, Genotypes 1 and 9 exhibited a higher negative effect on shoot length in the flowering stage, while Genotype 3 significantly reduced root length in the butonisation compared to the flowering stage. Screening 26 sunflower genotypes collected in four developmental stages, Macías et al. [45] determined that 1 m tall plants (one month before harvest) showed better allelopathic potential. Zribi et al. [26] also reported differences in Nigella sativa varieties harvested at the different developmental stages, with the Indian variety being the most toxic at the vegetative and Tunisian at the flowering stage. Identification of the growth stage with the highest allelopathic activity enables more efficient utilization of allelopathic plants [26,46].
5. Conclusions
Inhibitory allelopathic effects of sunflower water extracts were determined on the germination and growth of lettuce seedlings. Several genotypes (4, 5, 7 and 8) exhibited higher phytotoxic potential. Both the genotype and growth stage should be considered when evaluating the allelopathic potential of plant species. Further studies of the phytotoxic potential of sunflower genotypes on different test species and phytochemical analysis are required.
Author Contributions
Conceptualization, M.R. and A.M.K.; methodology, M.R., A.M.K. and M.V.V.; validation, A.M.K. and A.S.; formal analysis, A.M.K. and D.K.; investigation, M.R., A.M.K. and M.M.; resources, R.B.; writing—original draft preparation, M.R.; writing—review and editing, M.R., A.M.K., M.M., A.S., M.V.V. and D.K.; visualization, M.R. and D.K.; supervision, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M.G. Allelopathy as new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, P.B.S. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 400. [Google Scholar]
- Swain, T. Secondary compounds as protective agents. Annu. Rev. Plant Physiol. 1977, 28, 479–501. [Google Scholar] [CrossRef]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Review: Allelochemicals as multi-kingdom plant defense compounds: Towards an integrated approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef]
- Alam, S.M.; Ala, S.A.; Azmi, A.R.; Khan, M.A.; Ansari, R. Allelopathy and its Role in Agriculture. J. Biol. Sci. 2001, 1, 308–315. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2002, 26, 2079–2094. [Google Scholar] [CrossRef]
- Chou, C.H. Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. Plant Sci. 1999, 18, 609–636. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathy in Agroecosystems. J. Crop Prod. 2001, 4, 1–41. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Sarbout, A.K.; Al-Shamma, L.M. Differential allelopathic potential of sunflower (Helianthus annuus L.) genotypes on weeds and wheat (Triticum aestivum L.) crop. Arch. Agron. Soil Sci. 2012, 58, 1139–1148. [Google Scholar] [CrossRef]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth. Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Šćepanović, M.; Sarić-Krsmanović, M.; Šoštarčić, V.; Brijačak, E.; Lakić, J.; Špirović Trifunović, B.; Gajić Umiljendić, J.; Radivojević, L. Inhibitory Effects of Brassicaceae Cover Crop on Ambrosia artemisiifolia Germination and Early Growth. Plants 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Eleftherohorinos, I.G.; Lithourgidis, A.S. Allelopathic potential of winter cereals and their cover crop mulch effect on grass weed suppression and corn development. Crop Sci. 2006, 46, 345–352. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Tucak, M.; Mijić, M.; Stanić, L.; Stojanović, N.; Skokić, V. Allelopathic potential of alfalfa (Medicago sativa L.) on seed germination and seedling growth of vegetables. Glas. Zaštite Bilja 2021, 44, 17–22. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Nikolić, M.; Sarajlić, A. Assessment of allelopathic potential of fennel, rue and sage on weed species hoary cress (Lepidium draba). Not. Bot. Horti Agrobot. Cluj Napoca 2016, 44, 48–52. [Google Scholar] [CrossRef]
- Qasim, M.; Fujii, Y.; Ahmed, M.Z.; Aziz, I.; Watanabe, K.N.; Khan, M.A. Phytotoxic analysis of coastal medicinal plants and quantification of phenolic compounds using HPLC. Plant Biosyst. 2019, 153, 767–774. [Google Scholar] [CrossRef]
- Norsworthy, J.K. Allelopathic potential of wild radish (Raphanus raphanistrum). Weed Technol. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Golubinova, I.; Marinov-Serafimov, P. Allelopathic potential of dodder (Cuscuta epithymum L.) on different genotypes bird’s-foot trefoil (Lotus corniculatus L.). Bulg. J. Agric. Sci. 2019, 25, 1198–1204. [Google Scholar]
- Rys, M.; Saja-Garbarz, D.; Skoczowski, A. Phytotoxic Effects of Selected Herbal Extracts on the Germination, Growth and Metabolism of Mustard and Oilseed Rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Caruso, P.; Lombardo, S.; Mauromicale, G. Allelopathy in Durum Wheat Landraces as Affected by Genotype and Plant Part. Plants 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Appiah, K.S.; Omari, R.A.; Onwona-Agyeman, S.; Amoatey, C.A.; Ofosu-Anim, J.; Smaoui, A.; Arfa, A.B.; Suzuki, Y.; Oikawa, Y.; Okazaki, S.; et al. Seasonal Changes in the Plant Growth-Inhibitory Effects of Rosemary Leaves on Lettuce Seedlings. Plants 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Omezzine, F.; Haouala, R. Variation in phytochemical constituents and allelopathic potential of Nigella sativa with developmental stages. S. Afr. J. Bot. 2014, 94, 255–262. [Google Scholar] [CrossRef]
- de Sousa, M.V.; de Farias, S.G.G.; de Castro, D.P.; e Silva, R.B.; de Oliveira Silva, D.Y.B.O.; Souto Dias, B.A.S.; da Silva, A.F.; dos Santos, G.N.L.; de Matos, D.C.P.; de Almada Oliveira, C.V.A. Allelopathy of the Leaf Extract of Eucalyptus Genetic Material on the Physiological Performance of Millet Seeds. Am. J. Plant Sci. 2018, 9, 34–45. [Google Scholar] [CrossRef][Green Version]
- Omezzine, F.; Haouala, R. Effect of Trigonella foenum-graecum L. development stages on some phytochemicals content and allelopathic potential. Sci. Hortic. 2013, 160, 335–344. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Crop cultivars with allelopathic capability. Weed Res. 1999, 39, 171–180. [Google Scholar] [CrossRef]
- Zubair, H.M.; Pratley, J.E.; Sandral, G.A.; Humphries, A. Allelopathic interference of alfalfa (Medicago sativa L.) genotypes to annual ryegrass (Lolium rigidum). J. Plant Res. 2017, 130, 647–658. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Cheema, Z.A.; Farooq, M. Allelopathic activity of crop residue incorporation alone or mixed against rice and its associated grass weed jungle rice (Echinochloa colona (L.) Link). Chil. J. Agric. Res. 2011, 71, 418–423. [Google Scholar] [CrossRef]
- Kamal, J. Impact of allelopathy of sunflower (Helianthus annuus L.) roots extract on physiology of wheat (Triticum aestivum L.). Afr. J. Biotechnol. 2011, 10, 14465–14477. [Google Scholar] [CrossRef][Green Version]
- Bogatek, R.; Gniazdowska, A.; Zakrzewska, W.; Oracz, K.; Gawroński, S.W. Allelopathic effect of sunflower extracts on mustard seed germination and seedling growth. Biol. Plant. 2006, 50, 156–158. [Google Scholar] [CrossRef]
- Silva, H.L.; Trezzi, M.M.; Marchese, J.A.; Buzzello, G.; Miotto, E., Jr.; Patel, F.; Debastiani, F.; e Fiorese, J. Determination of Indicative Species and Comparison of Sunflower Genotypes as to their Allelopathic Potential. Planta Daninha 2009, 27, 655–663. [Google Scholar] [CrossRef][Green Version]
- Dilipkumar, M.; Adzemi, M.A.; Chuah, T.S. Effects of Soil Types on Phytotoxic Activity of Pretilachlor in Combination with Sunflower Leaf Extracts on Barnyardgrass (Echinochloa crus-galli). Weed Sci. 2012, 60, 126–132. [Google Scholar] [CrossRef]
- Díaz de Villegas, M.E.; Delgado, G.; Rivas, M.; Torres, E.; Saura, M. Implementation of an in vitro bioassay as an indicator of the bionutrient FitoMas E. Cienc. Investig. Agrar. 2011, 38, 205–210. [Google Scholar] [CrossRef]
- Batish, D.R.; Tung, P.; Singh, H.P.; Kohli, R.K. Phytotoxicity of Sunflower Residues against Some Summer Season Crops. J. Agron. Crop Sci. 2002, 188, 19–24. [Google Scholar] [CrossRef]
- Tian, M.; Li, Q.; Zhao, W.; Qiao, B.; Shi, S.; Yu, M.; Li, X.; Li, C.; Zhao, C. Potential Allelopathic Interference of Abutilon theophrasti Medik. Powder/Extract on Seed Germination, Seedling Growth and Root System Activity of Maize, Wheat and Soybean. Agronomy 2022, 12, 844. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliva, R.M.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Allelochemicals from sunflower leaves cv. Peredovick. Phytochemisty 1999, 52, 613–621. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Ebana, K.; Yan, W.; Dilday, R.H.; Namai, H.; Okuno, K. Variation in Allelopathic Effect of Rice with Water Soluble Extracts. Agron. J. 2001, 93, 12–16. [Google Scholar] [CrossRef]
- Fernandez, C.; Monnier, Y.; Ormeño, E.; Baldy, V.; Greff, S.; Pasqualini, V.; Mévy, J.P.; Bousquet-Mélou, A. Variations in Allelochemical Composition of Leachates of Different Organs and Maturity Stages of Pinus halepensis. J. Chem. Ecol. 2009, 35, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Đikić, M.; Gadžo, D.; Šarić, T.; Gavrić, T.; Muminović, Š. Investigation of allelopathic potential of buckwheat. Herbologia 2008, 9, 59–71. [Google Scholar]
- Jafariehyazdi, E.; Javidfar, F. Comparison of allelopathic effects of some brassica species in two growth stages on germination and growth of sunflower. Plant Soil Environ. 2011, 57, 52–56. [Google Scholar] [CrossRef]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Potential of Cultivar Sunflowers (Helianthus annuus L.) as a Source of Natural Herbicide Template. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 531–550. [Google Scholar]
- Zucareli, V.; Coelho, E.M.P.; Fernandes, W.V.; Peres, E.M.; Stracieri, J. Allelopathic potential of Sorghum bicolor at different phenological stages. Planta Daninha 2019, 37, e019184017. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).