1. Introduction
Different levels of pain, with various symptoms, are manifested by patients with rotator cuff tears. The severity of pain may mainly be dependent on the status of the main pathology (which would be the rotator cuff tear), but regarding the fact that many concomitant lesions are often revealed during evaluation, there is a high chance that accompanying but concealed lesions may contribute to the pattern and severity of shoulder pain.
Despite the uncertainty of its role within the shoulder joint, the pathologic long head of the biceps brachii tendon (LHBT) is known to be one of the pain sources of the shoulder, especially when it is involved with rotator cuff problems [
1,
2,
3]. Their relationship is reported to be up to 70% [
1,
4]. A pathologic LHBT is manifested in various forms of degeneration, instability, and partial or complete tears. However, despite the well-known close relationship between rotator cuff lesions and LHBT pathology, the decision to either ignore or treat the LHBT in patients with a rotator cuff tear is often controversial, especially for older patients [
5,
6]. There have also been numerous studies regarding surgical methods and whether it should be tenotomized or tenodesed, as both modalities clearly have their own pros and cons.
Aside from the pain caused by the LHBT, the rate of concomitant stiffness is reported to be high when the LHBT is involved in a rotator cuff tear, due to continuous pain or contracture of the capsule that occurs gradually over time [
7,
8,
9]. This fact clearly indicates that capsules and rotator intervals are under the influence of other pathologic conditions of the shoulder joint, and they contribute to the evocation of the shoulder joint. However, in the case of rotator cuff tears with stiffness, the recovery factors that enable immediate rotator cuff recovery or delayed recovery of the rotator cuff after the recovery of range of motion have not yet been determined when selecting the optimal treatment. Regarding the close geographical proximity of the rotator interval, joint capsule, and LHBT to the rotator cuff, there are chances that these structures are affected by one another and that may manifest in various symptoms and different levels of pain. In addition, the subacromial bursa, which is known to be one of the most common sources of shoulder pain, especially when it is involved with rotator cuff tendinopathy, may also be affected by the condition of the LHBT. If we are able to predict the potential pain source in the shoulder that is coexistent with a rotator cuff tear and LHBT pathology, indications for prophylactic or simultaneous treatment of the lesions can lead to optimal postoperative outcomes along with successful repair of the torn tendon.
The purpose of this study was to elucidate whether adjacent anatomical structures including the anterior capsule, rotator interval, and subacromial bursa are affected by the condition of the LHBT in patients with rotator cuff tears by evaluating the expression of pain-related factors and comparing patients with and without LHBT lesions. These factors were also evaluated among LHBT tissues in order to verify LHBT itself as a pain source in pathologic conditions. The analyzed markers were deliberately chosen among the neuronal and pain-related peptides that have been proven to be involved in different aspects of the pain-generating mechanism with the confirmation of their presence in various bone and joint problems, including shoulder diseases.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
This is a descriptive laboratory study conducted among 40 consecutive patients with isolated supraspinatus tears who were enrolled between January 2018 and March 2020 in a university-affiliated hospital. Full-thickness tears with a tear size of small to medium were included in this study. The size of the tear was classified as small (<1 cm) or medium (1–3 cm) according to the largest dimensions [
10]. Preoperative evaluation of plain radiographs and magnetic resonance images of the shoulder were performed for all patients.
Exclusion criteria were carefully selected to exclude other possible sources of pain that are irrelevant to either rotator cuff tear or LHBT problems. Patients with concomitant glenohumeral lesions, glenohumeral joint arthritis, and large or massive rotator cuff tears were excluded. Patients having symptomatic acromioclavicular joint lesions, including arthritic changes on either plain shoulder radiography or magnetic resonance images, were also excluded. Patients with a history of any kind of intraarticular injection within 3 months, operation, or fracture were not included. Patients with shoulder stiffness were also not included. Forward flexion of less than 100° (maximum: 150°; forward flexion is defined as glenohumeral motion without scapulohumeral rhythm [
11]), external rotation less than 30°, and internal rotation behind the back at a level equal or lower than L5 was defined as shoulder stiffness. In order to obtain tissues from both normal and abnormal LHBTs, the age of the enrolled patients was at least 65 years for the justification of prophylactic LHBT tenotomy. Meanwhile, patients over 75 years old were excluded in order to minimize the risk of obtaining tissues with unrecognizable excessive degenerative change that might affect the results, regardless of the existence of rotator cuff tear or LHBT lesions. Patients who were diagnosed with thyroid diseases, diabetes mellitus, or a history of treatment for shoulder stiffness were not included in order to minimize the intervention of confounding factors related to the pathogenesis of shoulder stiffness, of which the main pathology is within the capsule and rotator interval of the joint, regardless of the condition of the rotator cuff or the LHBT.
When addressing the torn supraspinatus arthroscopically, the patients were divided into two groups depending on the condition of the LHBT. Patients with a healthy-looking LHBT without apparent lesions throughout the visible area of tendon and the adjacent superior labrum were allocated to group 1. Patients with apparent degenerative partial LHBT lesions, including partial tear or instability, were allocated to group 2. Study approval was given by the institutional review board with the confirmation of informed consents from each participant.
2.2. Capsule Tissue Harvest
All surgical procedures were performed by two shoulder surgeons with the patients under general anesthesia. Both surgeons have at least 10 years of experience in arthroscopic shoulder surgery. A standard glenohumeral arthroscopic procedure was performed with the patients in the lateral decubitus position. Tissues were collected during the arthroscopic procedure of the glenohumeral joint and subacromial space using a meniscal basket forceps. A standard posterior portal was used for visualization. An anterior portal in the rotator interval and a lateral portal were used as working portals. Rotator interval tissues were obtained during the procedure to establish an anterior working portal. Anterior joint capsule tissue was collected just above the subscapularis tendon, which would be the closest region to the LHBT. Tenotomy of the LHBT was performed in all patients. For group 1 patients with intact LHBTs, tissues from the LHBT were obtained from the middle substance of the tendon, at least 1 cm above the base. For group 2, tissues were obtained directly from the lesion with partial tear or apparent changes before tenotomy. After obtaining tissues within the glenohumeral joint, the scope was redirected to the subacromial space in order to obtain tissue from the subacromial bursa. Subsequent rotator cuff repair was performed after tissue harvest. The acquired samples were divided in two. One half was stored for ribonucleic acid (RNA) extraction and the other for immunohistochemical analysis.
2.3. Gene Expression Evaluation
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed for the evaluation of the obtained tissue. The samples were stored in RNAlater
® solution (Thermo Fisher, Waltham, MA, USA) at −20 °C. Total RNA was extracted using RNeasy Fibrous Tissue Mini Kit (QIAGEN, Hilden, Germany). Purification of extracted RNA was performed with the 260 nm/280 nm light absorption ratio set at >1.7. The expressions of protein gene product 9.5 (PGP9.5), growth-associated protein 43 (GAP43), calcitonin gene-related peptide (CGRP), substance P, P75, S100, and CD34 were analyzed to assess the involvement of pain-related factors affected by the condition of the LHBT. Quantitative RT-PCR was performed using the iTaq
TM Universal SYBR
® Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) on a CFX96 Real-Time Detection System (Bio-Rad Laboratories, Inc.). The final solution was directly used to achieve PCR amplification. Each cDNA reaction was diluted with a calibrator sample containing the transcript of interest and run in 25 μL of Bio-Rad SYBR Green using 10 pmol of primers. The PCR program consisted of one initial denaturation step (3 min at 94 °C) and 40 cycles of denaturation (10 s at 95 °C), annealing (20 s at a temperature optimal for each gene), and amplification (30 s at 72 °C), followed by melt curve determination consisting of one denaturation step (10 s at 95 °C) and one annealing step (0.5 s at 65 °C). Calculations of relative mRNA levels were performed according to the 2
−ΔΔCT method using CFX manager [
12]. The β2 microglobulin housekeeping gene was used as the reference for normalization of the values obtained for each gene.
2.4. Immunohistochemistry
Immunohistochemical staining was performed for histological evaluation of the tissues. After overnight fixation with 10% buffered formalin, the samples were washed and dehydrated through a series of graded alcohols. The specimen was embedded in paraffin and cut into 4 um thick sections for slide attachment. Fifteen minutes of Dako REALTM Peroxidase-Blocking Solution (DAKO, Agilent, Santa Clara, CA, USA) treatment, 10 min of 0.1% Triton x-100 solution treatment, and 20 min of 10% normal goat serum (Vector Laboratories, Inc., Burlingame, CA, USA) treatment were performed. Primary antibody monoclonal anti-human PGP9.5, GAP43, CGRP, substance P, P75, S100, and CD34 were incubated overnight at 4 °C. After washing with phosphate-buffered saline, biotinylated secondary antibody (Vectastain Elite ABC Kit; Vector, Burlingame, CA, USA) was applied for 20 min. In order to catalyze chromogen development in 3, 3′-diaminobenzidine tetrachloride (DAB), streptavidin-conjugated peroxidase was applied. The stained sections were examined and the distribution of evaluated factors was determined under optical microscopy after counterstaining with hematoxylin. The slides were scanned with a Panoramic MIDI scanner (3DHISTECH Ltd., Budapest, Hungary) and the amounts of stained factors were expressed quantitatively using an automated method. Positively stained pixels were digitally selected on all slides and the total positivity ratio of immunoreaction for the entire slide was calculated, which is the total number of positively stained pixels divided by the total number of pixels.
2.5. Statistical Methods
Statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). p-values less than 0.05 were considered statistically significant. Data are expressed as median and range or as mean and standard error of the mean. Quantitative data were analyzed by un-paired Student t-tests and nonparametric Mann–Whitney tests.
3. Results
The demographic data of the enrolled patients are listed in
Table 1. No significant differences were seen between the groups in terms of participant configuration and shoulder condition. Among the patients of group 2 who had pathologic LHBTs, 12 patients had a degenerative partial tear at the mid stump, 6 patients had instability along with overall degeneration on the tendon, and 2 patients had a dislocated LHBT.
Gene expression analysis revealed increased expression of pain-related factors with the pathologic change of the LHBT itself (group 2). Significant responses were shown in PGP9.5 (0.008 vs. 0.017,
p = 0.02), GAP43 (0.001 vs. 0.006,
p = 0.03), CGRP (
p = 0.003 vs. 0.060,
p = 0.007), and CD34 (0.183 vs. 0.472,
p = 0.03) (
Figure 1).
The majority of the expression of pain-related factors in adjacent structures was higher in group 2 with the abnormal LHBTs when compared to group 1 with the normal LHBTs. In the anterior capsule, the expressions of PGP9.5 (0.014 vs. 0.054, p = 0.004), GAP43 (0.054 vs. 0.110, p = 0.02), CGRP (p = 0.081 vs. 0.252, p = 0.005), P75 (1.199 vs. 2.417, p = 0.02), S100 (0.013 vs. 0.049, p = 0.008), and CD34 (1.035 vs. 1.721, p = 0.005) were significantly higher in group 2. In the rotator interval, PGP9.5 (0.002 vs. 0.027, p = 0.01), GAP43 (0.014 vs. 0.017, p = 0.004), and P75 (0.886 vs. 1.782, p = 0.02) were more highly expressed in group 2. Meanwhile, the expression of pain-related factors from bursal tissue did not show significant differences between the groups, except P75 (0.013 vs. 0.045, p = 0.008).
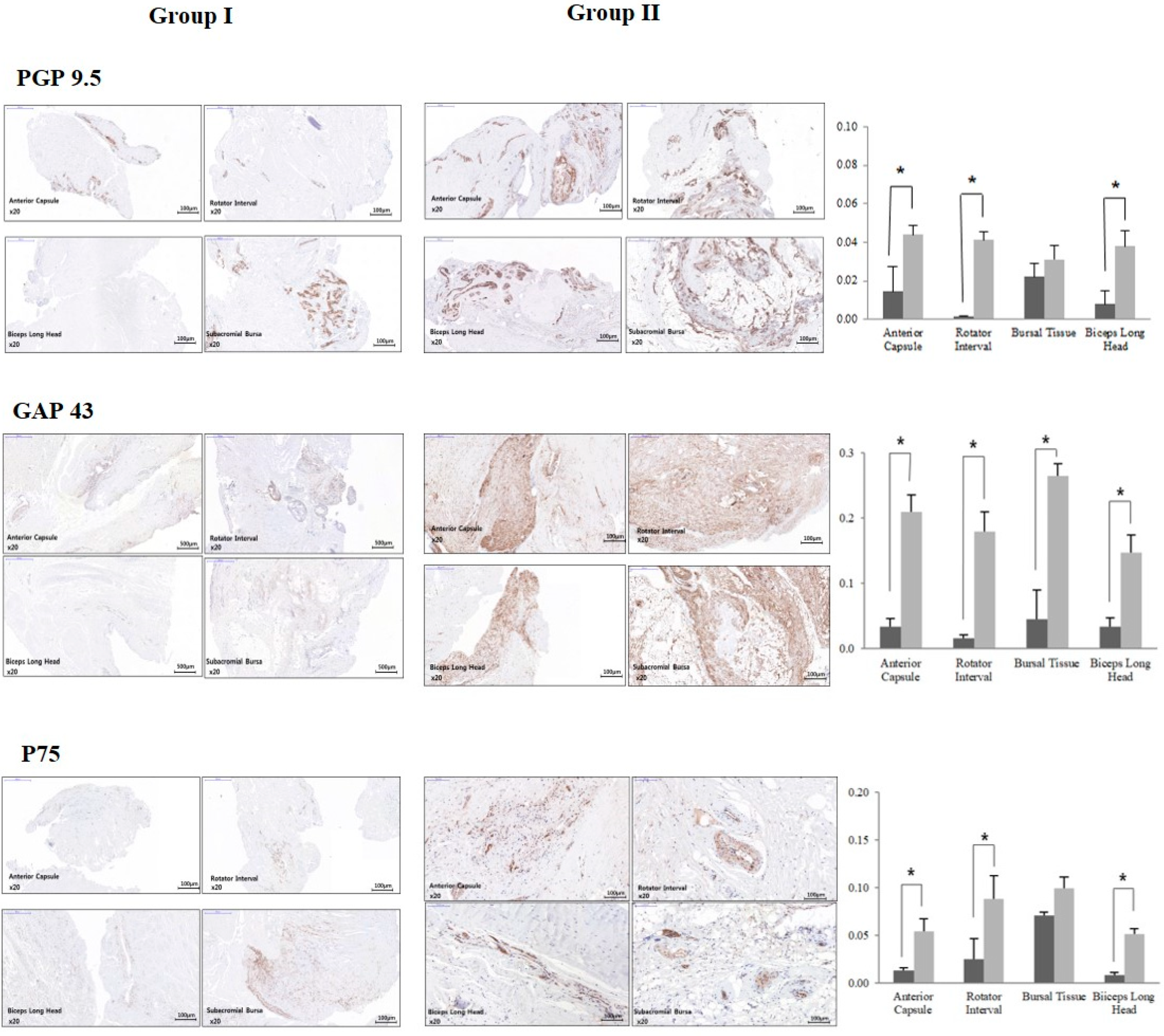
Immunohistochemistry revealed an increased expression of pain-related factors in the anterior capsule, rotator interval, and biceps long head from group 1. The expression of PGP 9.5 and P75 was significantly higher in tissues from the anterior capsule, rotator interval, and LHBT for group 1, but the difference was not significant in subacromial bursal tissue. In the case of GAP43, all the obtained tissues from group 1 showed significantly higher expression when compared to group 2 (
Figure 2).
4. Discussion
In this study, increased expression of pain-related factors was found in pathologic LHBTs, and these expressions also showed an increase in the adjacent structures, including the anterior capsule and rotator interval, in patients with full-thickness rotator cuff tears. These results indicated that a pathologic LHBT acts as a pain generator itself, and adjacent structures reacted differently depending on the status of the LHBT.
The analyzed markers are neuronal peptides involved in different aspects of the pain-generating mechanism, and their appearances have been confirmed in various tendon and joint diseases [
4,
13,
14,
15,
16]. The abnormal increase in neural and pain-related markers demonstrated the presence of pathological neural changes in various tendon diseases [
17,
18]. PGP 9.5 is a general neuronal marker that represents the presence of general nerve fibers [
19,
20]. GAP43 is a neuronal membrane protein involved in axonal growth and regeneration that represents a marker for nerve growth [
19,
21]. The neuropeptide CGRP is widely distributed in nociceptive pathways in both the central and peripheral nervous systems and is involved with the sensation of joint pain [
14,
22]. CD34 is an endothelial cell marker and CD34-positive vessels are related to increased fibroblast cellularity in tendons under pathologic conditions [
13]. Substance P is a neuropeptide acting as both a neurotransmitter and a neuromodulator and is released from the terminals of specific sensory nerves. Painful tendons showed an increased presence of substance P-containing nerves, and neural sprouting of substance P fibers accompanies vascular hyperplasia [
23]. P75 is a nerve growth factor receptor that is expressed in nerve fibers around blood vessels. It also showed increased expression in the capsules of patients with frozen shoulder [
16]. Finally, the expression of the S100 protein is significantly altered in various neurodegenerative diseases, inflammation, and autoimmune diseases [
24,
25,
26,
27].
In the case of the LHBT under pathologic conditions (group 2), the expressions of PGP9.5, GAP43, CGRP, and CD34 were significantly higher than in group 1 with the normal LHBTs. CGRP-positive nerve fibers are significantly increased in chronic tendinopathy with confirmation of CGRP expression in both unmyelinated and myelinated axons of the LHBT [
28]. Along with CGRP, PGP9.5 was detected in nerve fibers of the human patellar tendon in the vicinity of some of the blood vessels and in thin nerve fascicles [
29]. In fact, increased expression of pain-related factors is highly predictable, as the LHBT under pathologic conditions is a well-known pain generator that coexists with rotator cuff tears. However, one marker that showed no significance among the analyzed factors in any obtained tissues was substance P. Considering the finding of no significant difference in the expression of substance P between normal cadaveric samples and pathologic LHBTs in a previous study by Singaraju et al [
30], the reactivity of substance P in the etiology of LHBT pathology can be relatively weak compared to other factors.
The anterior capsule and rotator interval also contribute to the generation of shoulder pain in various ways. In particular, with the clinical manifestation of joint stiffness in rotator cuff tear patients, factors associated with inflammation or fibrosis have shown upregulation in capsules and rotator interval tissues [
31]. In a similar fashion, the expression of the evaluated factors was consistently upregulated with abnormality of the LHBT. Previously, the pathologic connection between the LHBT and the shoulder capsule was revealed by Hashimoto et al., which explained the insertion of relatively large nerve fibers from the capsule to the central portion of the LHBT [
32]. Similar to the reaction shown with the LHBT, the response shown for substance P was lukewarm compared to other factors. There is no doubt that substance P is a crucial neuropeptide for pain transmission, but all we can say was that the reactivity was not that significant in this particular study.
The subacromial bursa has been implicated as the primary pain-producing tissue in the shoulder, whereas it also plays a role in blood supply to the rotator cuff, proprioception, and lubrication [
33]. The fact that downregulation of inflammatory cytokines is expressed in subacromial bursitis by anti-inflammatory agents provides biologic evidence for a pain source that can be controlled clinically [
34]. The fact that the subacromial bursa possesses a high density of free nerve endings comprised of both myelinated and non-myelinated fibers that outnumber the surrounding structures may have resulted in the relatively high expression of every pain-related factor in both groups [
15,
35]. In fact, relatively high expression of pain-related factors from the subacromial bursa can simply result from the rotator cuff tear itself with a high probability. Still, even though the difference was not always statistically significant, the collective response toward a pathologic LHBT implies that the subacromial space may also be affected by the condition of the LHBT.
Although enrollment in this study was confined to patients with rotator cuff tears with or without LHBT lesions, we believe that the strength of this study is in providing basic information that can be applied clinically in a direct manner. To the best of our knowledge, biological evidence that could justify the extent of surgical treatment of rotator cuff tears with an abnormal LHBT has not yet been provided in any published studies. There is no doubt that the treatment of tendons with the apparent pathology should be dealt with as a priority. However, regarding the result that adjacent anatomical structures are affected by the condition of the LHBT, with significantly increased expression of pain-related factors and neuropeptides, the possibility remains of having suboptimal results even after the successful repair of the torn supraspinatus or LHBT with the incubated pathology remaining in the untreated structures. The finding that an abnormal LHBT is a pain source that affects surrounding tissues clinically supports the need for surgical treatment for an abnormal LHBT. For instance, tenotomy not only removes the pain source directly but may also prevent further influence of the LHBT on adjacent structures that may also contribute to pain generation, even after repair of the torn cuff. Furthermore, in cases of patients with concomitant stiffness, release of the rotator interval along with anterior capsulectomy may not only contribute to the improvement of ROM but may also its role on removing the actual pain source. Early postoperative motion was acquired by the preemptive release of the rotator interval in patients with rotator cuff tears [
36]. The benefit of rotator interval release can be one of the clinical backgrounds to our results. In addition, thorough subacromial bursectomy under the circumstances of a pathologic LHBT may contribute to postoperative pain reduction.
The results of this study should be interpreted with some limitations. First, the number of patients was relatively small and power analysis was not performed for this particular study. Second, we were not able to guarantee that all the enrolled patients were in the same pathologic condition of rotator cuff tears or LHBT pathology within the group. We tried our best to keep the LHBT as the only variable, but the enrolled patients may have been in different statuses of evaluated tissues, including the rotator cuff. This might have affected the expression of pain-related factors in the evaluated tissues. Third, analyses were conducted using only two analytical methods, which were RT-PCR and immunohistochemistry. RT-PCR and immunohistochemistry only describe what genes or proteins are being expressed at the time of harvest, so progression toward the problem cannot be clearly revealed. Additional analyses are expected to provide more objective support to the results of this study.