Limiting Conditions for Droplet Fragmentation of Stabilized Suspension Fuels
Abstract
1. Introduction
2. Materials
3. Experimental Technique
4. Results and Discussion
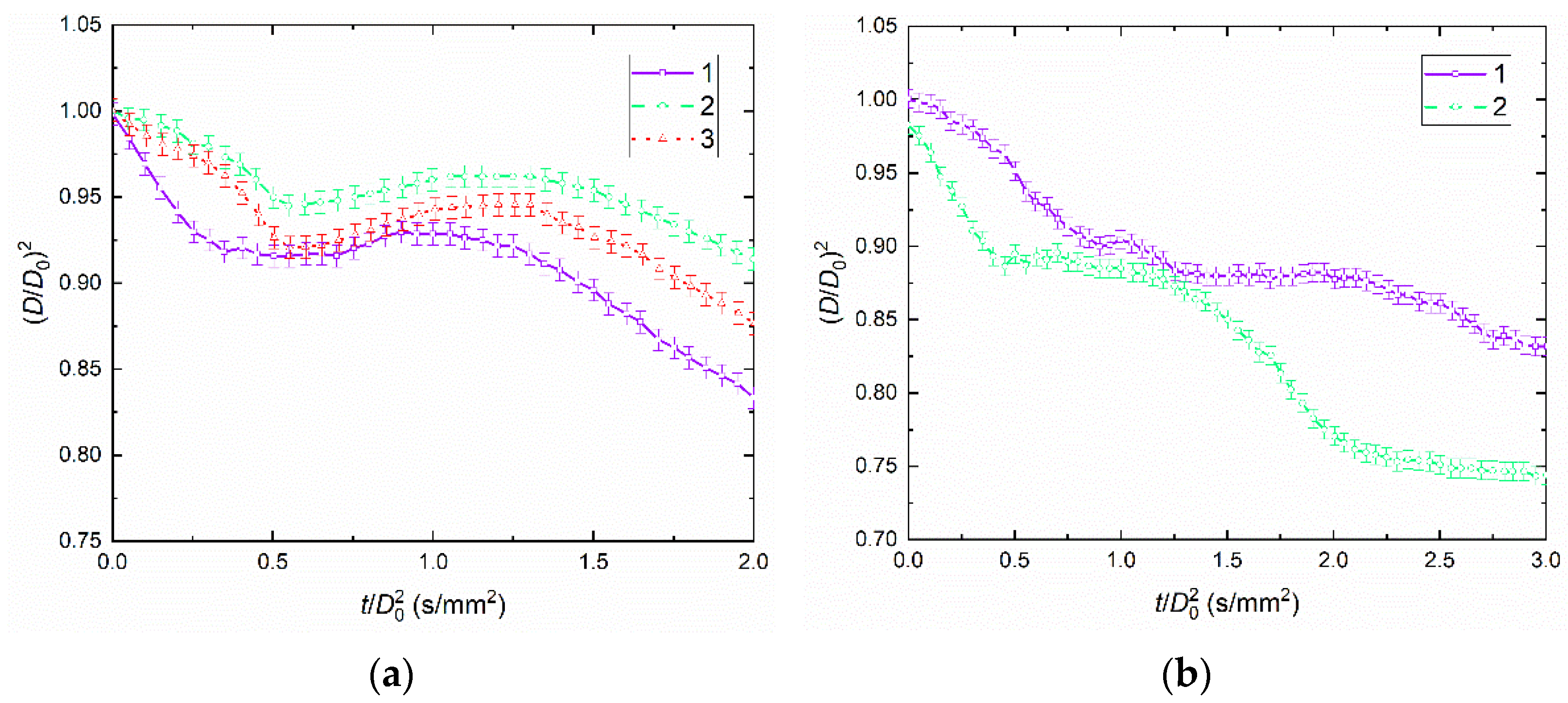
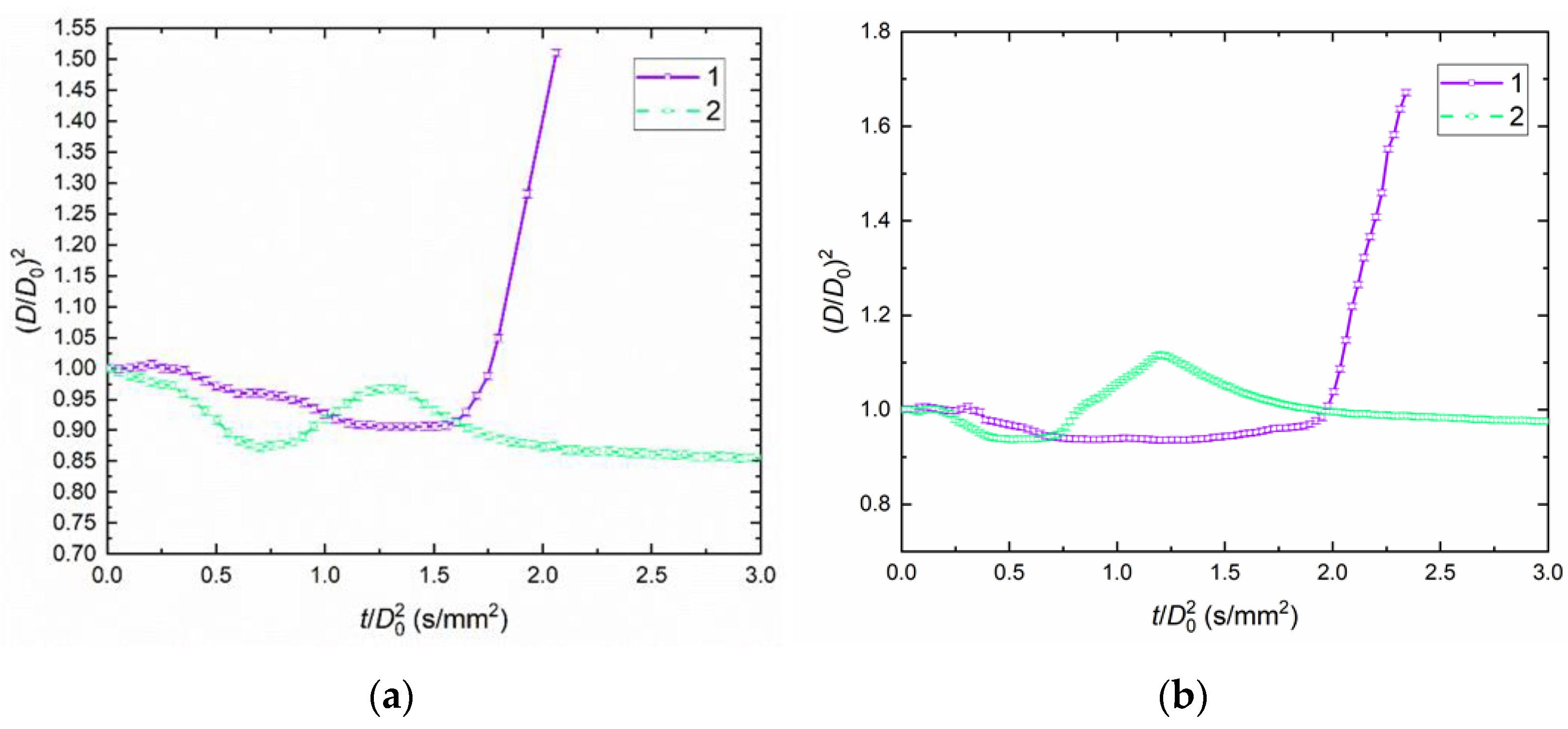
4.1. Droplet Behaviors
4.2. Temporal Characteristics of Droplet Evaporation, Drying, and Breakup
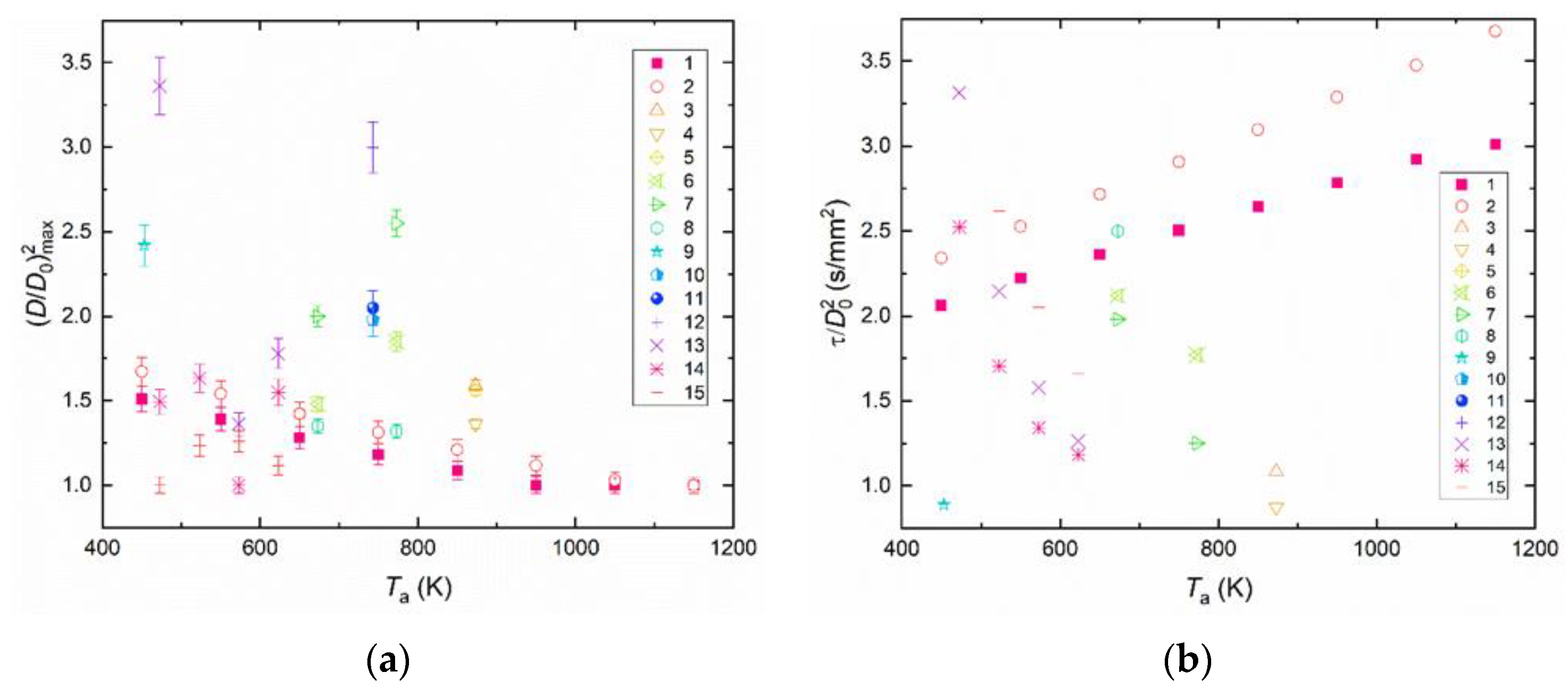
4.3. Generalization of Experimental Data
5. Conclusions
- (i)
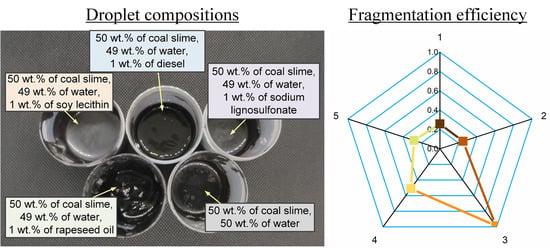
- Soy lecithin and sodium lignosulfonate are the most promising stabilizing additives to slurry fuels. The conditions for the intense droplet breakup were not observed when analyzing the parameter (D/D0)2. The value of this parameter did not exceed 1. The transition between slurry droplet breakup regimes depends most heavily on the addition of a flammable liquid (diesel fuel or rapeseed oil) as well as replacement of filter cake with coal. The parameter (D/D0) reached 1.7 during CWSP droplet breakup.
- (ii)
- The slurry droplet breakup delay time was most significantly affected by the addition of a flammable liquid: diesel fuel or rapeseed oil. The shortest breakup delay times were recorded for the compositions containing diesel fuel as the flammable liquid. In the initial droplet size range from 0.95 to 1.15 mm, the breakup delay times did not exceed 10 s. The properties of child droplets were most significantly influenced by the addition of a flammable liquid: diesel fuel or rapeseed oil. CWSP droplets based on diesel exhibit higher child-droplet velocities and smaller size than droplets based on rapeseed oil. The sizes of child droplets fell into a range of 10 to 140 μm, and their velocities ranged from 0 to 50 m/s.
- (iii)
- A comparative analysis of slurry fuel droplet stabilization and breakup conditions revealed the most promising conditions of their preparation for storage, transportation, and primary and secondary atomization. The efficiency factor values were calculated for the secondary atomization of promising fuel droplets induced by microexplosive breakup. These values ranged from 0.25 to 1. The maximum values (0.95–1) corresponded to water/kerosene droplets.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Xue, Y.; Tian, H.; Yan, J.; Zhou, Z.; Wang, J.; Nie, L.; Pan, T.; Zhou, J.; Hua, S.; Wang, Y.; et al. Temporal Trends and Spatial Variation Characteristics of Primary Air Pollutants Emissions from Coal-Fired Industrial Boilers in Beijing, China. Environ. Pollut. 2016, 213, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, V.; Tunca, S. Experimental Investigation on Using Emulsified Fuels with Different Biofuel Additives in a DI Diesel Engine for Performance and Emissions. Appl. Therm. Eng. 2018, 129, 841–854. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.; Shao, Y.; Zhou, G. Simulation of Co-Gasification of Coal and Wood in a Dual Fluidized Bed System. Adv. Powder Technol. 2021, 32, 52–71. [Google Scholar] [CrossRef]
- Glushkov, D.; Nyashina, G.; Medvedev, V.; Vershinina, K. Relative Environmental, Economic, and Energy Performance Indicators of Fuel Compositions with Biomass. Appl. Sci. 2020, 10, 2092. [Google Scholar] [CrossRef]
- Nyashina, G.; Legros, J.C.; Strizhak, P. Environmental Potential of Using Coal-Processing Waste as the Primary and Secondary Fuel for Energy Providers. Energies 2017, 10, 405. [Google Scholar] [CrossRef]
- Vershinina, K.; Dorokhov, V.; Romanov, D.; Nyashina, G.; Kuznetsov, G. Multi-Criteria Efficiency Analysis of Using Waste-Based Fuel Mixtures in the Power Industries of China, Japan, and Russia. Appl. Sci. 2020, 10, 2460. [Google Scholar] [CrossRef]
- Akhmetshin, M.R.; Nyashina, G.S.; Strizhak, P.A. Comparative Analysis of Factors Affecting Differences in the Concentrations of Gaseous Anthropogenic Emissions from Coal and Slurry Fuel Combustion. Fuel 2020, 270, 117581. [Google Scholar] [CrossRef]
- Staroń, A.; Kowalski, Z.; Staroń, P.; Banach, M. Studies on CWL with Glycerol for Combustion Process. Environ. Sci. Pollut. Res. 2019, 26, 2835–2844. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Lakshman, S.V.; Leong, A.F.T.; Kinsey, A.H.; Gibbins, J.D.; Arlington, S.Q.; Sun, T.; Fezzaa, K.; Hufnagel, T.C.; Weihs, T.P. Viewing Internal Bubbling and Microexplosions in Combusting Metal Particles via X-Ray Phase Contrast Imaging. Combust. Flame 2019, 199, 194–203. [Google Scholar] [CrossRef]
- Alekseenko, S.V.; Anufriev, I.S.; Dekterev, A.A.; Kuznetsov, V.A.; Maltsev, L.I.; Minakov, A.V.; Chernetskiy, M.Y.; Shadrin, E.Y.; Sharypov, O.V. Experimental and Numerical Investigation of Aerodynamics of a Pneumatic Nozzle for Suspension Fuel. Int. J. Heat Fluid Flow 2019, 77, 288–298. [Google Scholar] [CrossRef]
- Tavangar, S.; Hashemabadi, S.H.; Saberimoghadam, A. CFD Simulation for Secondary Breakup of Coal-Water Slurry Drops Using OpenFOAM. Fuel Process. Technol. 2015, 132, 153–163. [Google Scholar] [CrossRef]
- Li, P.W.; Yang, D.J.; Lou, H.M.; Qiu, X.Q. Study on the Stability of Coal Water Slurry Using Dispersion-Stability Analyzer. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2008, 36, 524–529. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Lyrshchikov, S.Y.; Shevyrev, S.A.; Strizhak, P.A. Burning Properties of Slurry Based on Coal and Oil Processing Waste. Energy Fuels 2016, 30, 3441–3450. [Google Scholar] [CrossRef]
- Kumar, A.; Sah, B.; Singh, A.R.; Deng, Y.; He, X.; Kumar, P.; Bansal, R.C. A Review of Multi Criteria Decision Making (MCDM) towards Sustainable Renewable Energy Development. Renew. Sustain. Energy Rev. 2017, 69, 596–609. [Google Scholar] [CrossRef]
- Gaber, C.; Wachter, P.; Demuth, M.; Hochenauer, C. Experimental Investigation and Demonstration of Pilot-Scale Combustion of Oil-Water Emulsions and Coal-Water Slurry with Pronounced Water Contents at Elevated Temperatures with the Use of Pure Oxygen. Fuel 2020, 282, 118692. [Google Scholar] [CrossRef]
- Hammerton, J.M.; Li, H.; Ross, A.B. Char-Diesel Slurry Fuels for Microgeneration: Emission Characteristics and Engine Performance. Energy 2020, 207, 118225. [Google Scholar] [CrossRef]
- Staroń, A.; Kowalski, Z.; Staroń, P.; Banach, M. Analysis of the Useable Properties of Coal-Water Fuel Modified with Chemical Compounds. Fuel Process. Technol. 2016, 152, 183–191. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Strizhak, P.A.; Chernetskii, M.Y. Organic Coal-Water Fuel: Problems and Advances (Review). Therm. Eng. 2016, 63, 707–717. [Google Scholar] [CrossRef]
- Antonov, D.V.; Kuznetsov, G.V.; Strizhak, P.A. Differences of Two-Component Droplets Breakup at the High Temperatures. J. Energy Inst. 2019, 93, 351–366. [Google Scholar] [CrossRef]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Investigation on the Conditions Leading to the Micro-Explosion of Emulsified Fuel Droplet Using Two Colors LIF Method. Exp. Therm. Fluid Sci. 2020, 116, 110106. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Bar-Kohany, T.; Nissar, Z.; Antonov, D.; Strizhak, P.A.; Rybdylova, O.D. A New Approach to Modelling Micro-Explosions in Composite Droplets. Int. J. Heat Mass Transf. 2020, 161, 120238. [Google Scholar] [CrossRef]
- Antonov, D.V.; Nyashina, G.S.; Strizhak, P.A.; Romanov, D.S. Micro-Explosive Droplet Fragmentation of Environmentally Promising Coal-Water Slurries Containing Petrochemicals. Fuel 2021, 283, 118949. [Google Scholar] [CrossRef]
- Piskunov, M.; Romanov, D.; Strizhak, P.; Yanovsky, V. Individual and Synergistic Effects of Modifications of the Carrier Medium of Carbon-Containing Slurries on the Viscosity and Sedimentation Stability. Chem. Eng. Res. Des. 2022, 184, 191–206. [Google Scholar] [CrossRef]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: New York, NY, USA, 2003. [Google Scholar]
- Zhang, Y.; Hu, S.; Yang, X.; Jiang, F.; Wu, C.; Li, J.; Liu, K. Performance and Mechanism of Polyacrylamide Stabilizers in Coal Water Slurry. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 630, 127544. [Google Scholar] [CrossRef]
- ISO 1928:2009; TAPPI Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. TAPPI Test Methods. ISO: Geneva, Switzerland, 2009; Volum 1995.
- ASTM International. Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Ibraeva, K.T.; Manaev, Y.O.; Tabakaev, R.B.; Yazykov, N.A.; Zavorin, A.S. Research of Characteristics and Mineral Composition of Peat of the Tomsk Region Relating to Energy Use. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2019, 330. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke 1; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Antonov, D.; Bellettre, J.; Tarlet, D.; Massoli, P.; Vysokomornaya, O.; Piskunov, M. Impact of Holder Materials on the Heating and Explosive Breakup of Two-Component Droplets. Energies 2018, 11, 3307. [Google Scholar] [CrossRef]
- Vysokomornaya, O.V.; Piskunov, M.V.; Strizhak, P.A. Breakup of Heterogeneous Water Drop Immersed in High-Temperature Air. Appl. Therm. Eng. 2017, 127, 1340–1345. [Google Scholar] [CrossRef]
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A.; Nissar, Z.; Sazhin, S.S. Puffing/Micro-Explosion in Composite Fuel/Water Droplets Heated in Flames. Combust. Flame 2021, 233, 111599. [Google Scholar] [CrossRef]
- Tarlet, D.; Allouis, C.; Bellettre, J. The Balance between Surface and Kinetic Energies within an Optimal Micro-Explosion. Int. J. Therm. Sci. 2016, 107, 179–183. [Google Scholar] [CrossRef]
- Khan, M.Y.; Abdul Karim, Z.A.; Aziz, A.R.A.; Tan, I.M. Experimental Investigation of Microexplosion Occurrence in Water in Diesel Emulsion Droplets during the Leidenfrost Effect. Energy Fuels 2014, 28, 7079–7084. [Google Scholar] [CrossRef]
- Ismael, M.A.; Heikal, M.R.; Aziz, A.R.A.; Syah, F.; Zainal A., E.Z.; Crua, C. The Effect of Fuel Injection Equipment on the Dispersed Phase of Water-in-Diesel Emulsions. Appl. Energy 2018, 222, 762–771. [Google Scholar] [CrossRef]
- Antonov, D.V.; Kuznetsov, G.V.; Strizhak, P.A.; Rybdylova, O.; Sazhin, S.S. Micro-Explosion and Autoignition of Composite Fuel/Water Droplets. Combust. Flame 2019, 210, 479–489. [Google Scholar] [CrossRef]
- Antonov, D.V.; Piskunov, M.V.; Strizhak, P.A. Explosive Disintegration of Two-Component Drops under Intense Conductive, Convective, and Radiant Heating. Appl. Therm. Eng. 2019, 152, 409–419. [Google Scholar] [CrossRef]
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A. Child Droplets Produced by Micro-Explosion and Puffing of Two-Component Droplets. Appl. Therm. Eng. 2020, 164, 114501. [Google Scholar] [CrossRef]
- Tarlet, D.; Mura, E.; Josset, C.; Bellettre, J.; Allouis, C.; Massoli, P. Distribution of Thermal Energy of Child-Droplets Issued from an Optimal Micro-Explosion. Int. J. Heat Mass Transf. 2014, 77, 1043–1054. [Google Scholar] [CrossRef]
- Antonov, D.; Piskunov, M.; Strizhak, P.; Tarlet, D.; Bellettre, J. Dispersed Phase Structure and Micro-Explosion Behavior under Different Schemes of Water-Fuel Droplets Heating. Fuel 2020, 259, 116241. [Google Scholar] [CrossRef]
- Antonov, D.V.; Piskunov, M.V.; Strizhak, P.A. Breakup and Explosion of Droplets of Two Immiscible Fluids and Emulsions. Int. J. Therm. Sci. 2019, 142, 30–41. [Google Scholar] [CrossRef]
- Rosli, M.A.F.; Aziz, A.R.A.; Ismael, M.A.; Elbashir, N.O.; Zainal A., E.Z.; Baharom, M.; Mohammed, S.E. Experimental Study of Micro-Explosion and Puffing of Gas-to-Liquid (GTL) Fuel Blends by Suspended Droplet Method. Energy 2021, 218, 119462. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental Understanding on the Dynamics of Micro-Explosion and Puffing in Ternary Emulsion Droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Antonov, D.; Vysokomornaya, O.; Piskunov, M.; Shlegel, N. Analysis of Statistical Data on Drop Collisions in an Aerosol Flow during Experiments. EPJ Web Conf. 2019, 196, 00013. [Google Scholar] [CrossRef]
- Volkov, R.S.; Kuznetsov, G.V.; Strizhak, P.A. Water Droplet Deformation in Gas Stream: Impact of Temperature Difference between Liquid and Gas. Int. J. Heat Mass Transf. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Yoon, I.; Shin, S. Maximal Spreading of Droplet during Collision on Particle: Effects of Liquid Viscosity and Surface Curvature. Phys. Fluids 2021, 33, 083310. [Google Scholar] [CrossRef]
- Komrakova, A.E.; Shardt, O.; Eskin, D.; Derksen, J.J. Effects of Dispersed Phase Viscosity on Drop Deformation and Breakup in Inertial Shear Flow. Chem. Eng. Sci. 2015, 126, 150–159. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Strizhak, P.A.; Feoktistov, D.V. Physicochemical Features of the Effect of Special Water-Based Fire Retardants on Forest Materials. Fire Saf. J. 2021, 123, 103371. [Google Scholar] [CrossRef]
- Antonov, D.V.; Strizhak, P.A. Intensification of Vaporization and Secondary Atomization of Droplets of Fire-Extinguishing Liquid Composition. Tech. Phys. Lett. 2020, 46, 122–125. [Google Scholar] [CrossRef]
- Kerimbekova, S.A.; Kuznetsov, G.V.; Volkov, R.S.; Strizhak, P.A. Identification of Slurry Fuel Components in a Spray Flow. Fuel. 2022, 323, 124353. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, X.; Ju, D.; Wang, L.; Sun, C. Experimental Study on the Evaporation and Micro-Explosion Characteristics of Nanofuel Droplet at Dilute Concentrations. Energy 2019, 183, 149–159. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Wang, Y.; Qiao, X.; Ju, D.; Sun, C.; Zhang, Q. Experimental Study on Evaporation and Micro-Explosion Characteristics of Biodiesel/n-Propanol Blended Droplet. Energy 2020, 205, 118031. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Wang, B.; Wei, Y. Superheat Limit and Micro-Explosion in Droplets of Hydrous Ethanol-Diesel Emulsions at Atmospheric Pressure and Diesel-like Conditions. Energy 2018, 154, 535–543. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Syam, S.; Karmakar, S.; Joarder, R. Experimental Investigations on Nucleation, Bubble Growth, and Micro-Explosion Characteristics during the Combustion of Ethanol/Jet A-1 Fuel Droplets. Exp. Therm. Fluid Sci. 2017, 89, 284–294. [Google Scholar] [CrossRef]
- Fedorenko, R.M.; Antonov, D.V.; Strizhak, P.A.; Sazhin, S.S. Time Evolution of Composite Fuel/Water Droplet Radii before the Start of Puffing/Micro-Explosion. Int. J. Heat Mass Transf. 2022, 191, 122838. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. The Efficacy of Multiple-Criteria Design Matrix for Biodiesel Feedstock Selection. Energy Convers. Manag. 2019, 198, 111790. [Google Scholar] [CrossRef]







| Material | Properties |
|---|---|
| Water | ρ = 1010.7 kg/m3; C = 4244.5 J/(kg·K); k = 0.61 W/(m·K); µ = 0.0011 Pa·s; L = 2.44 MJ/kg; Psat = 3324.6 Pa |
| Rapeseed oil | ρ = 870.6 kg/m3; C = 2352.1 J/(kg·K); k = 0.17 W/(m·K); µ = 0.0056 Pa·s; L = 0.34 MJ/kg; Psat = 0.0008 Pa |
| Diesel fuel | ρ = 743.7 kg/m3; C = 2215.0 J/(kg·K); k = 0.14 W/(m·K); µ = 0.0013 Pa·s; L = 0.35 MJ/kg; Psat = 21.03 Pa |
| Bituminous coal | ρ = 1367.1 kg/m3; C = 1293.4 J/(kg·K); k = 0.26 W/(m·K) |
| Sample | Wa, % | Ad, % | Vdaf, % | Q, MJ/kg | Cdaf, % | Hdaf, % | Ndaf, % | Std, % | Odaf, % |
|---|---|---|---|---|---|---|---|---|---|
| filter cake | – | 26.46 | 23.08 | 24.83 | 87.20 | 5.090 | 2.05 | 1.022 | 4.46 |
| bituminous coal | 2.05 | 14.65 | 40.19 | 29.76 | 79.79 | 4.486 | 1.84 | 0.868 | 13.016 |
| Short Name | Components | ||||||
|---|---|---|---|---|---|---|---|
| Filter Cake | Bituminous Coal | Water | Sodium Lignosulfonate | Diesel | Rapeseed Oil | Soy Lecithin | |
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | |
| K50W49LI1 | 50 | – | 49 | 1 | – | – | – |
| K50W49D1 | 50 | – | 49 | – | 1 | – | – |
| K50W49R1 | 50 | – | 49 | – | – | 1 | – |
| K50W49LE1 | 50 | – | 49 | – | – | – | 1 |
| K50W50 | 50 | – | 50 | – | – | – | – |
| C50W49LI1 | – | 50 | 49 | 1 | – | – | – |
| C50W49D1 | – | 50 | 49 | – | 1 | – | – |
| C50W49R1 | – | 50 | 49 | – | – | 1 | – |
| C50W49LE1 | – | 50 | 49 | – | – | – | 1 |
| C50W50 | – | 50 | 50 | – | – | – | – |
| Short Name | Viscosity at γ = 100 s−1 (mPa·s) |
|---|---|
| K50W49LI1 | 337.4 |
| K50W49D1 | 640.8 |
| K50W49R1 | 735.4 |
| K50W49LE1 | 431 |
| K50W50 | 324.1 |
| C50W49LI1 | 285.6 |
| C50W49D1 | 277.4 |
| C50W49R1 | 421.9 |
| C50W49LE1 | 589.3 |
| C50W50 | 236.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonov, D.V.; Romanov, D.S.; Kuznetsov, G.V. Limiting Conditions for Droplet Fragmentation of Stabilized Suspension Fuels. Appl. Sci. 2022, 12, 12271. https://doi.org/10.3390/app122312271
Antonov DV, Romanov DS, Kuznetsov GV. Limiting Conditions for Droplet Fragmentation of Stabilized Suspension Fuels. Applied Sciences. 2022; 12(23):12271. https://doi.org/10.3390/app122312271
Chicago/Turabian StyleAntonov, Dmitrii V., Daniil S. Romanov, and Genii V. Kuznetsov. 2022. "Limiting Conditions for Droplet Fragmentation of Stabilized Suspension Fuels" Applied Sciences 12, no. 23: 12271. https://doi.org/10.3390/app122312271
APA StyleAntonov, D. V., Romanov, D. S., & Kuznetsov, G. V. (2022). Limiting Conditions for Droplet Fragmentation of Stabilized Suspension Fuels. Applied Sciences, 12(23), 12271. https://doi.org/10.3390/app122312271