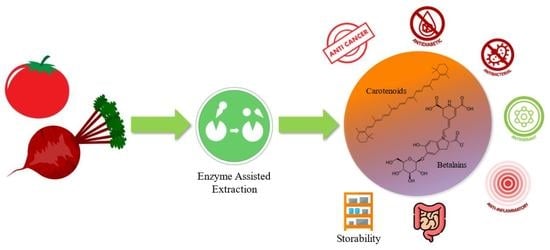
Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits
Abstract
1. Introduction
2. Carotenoids and Betalains: Bioactivity and Health Benefits
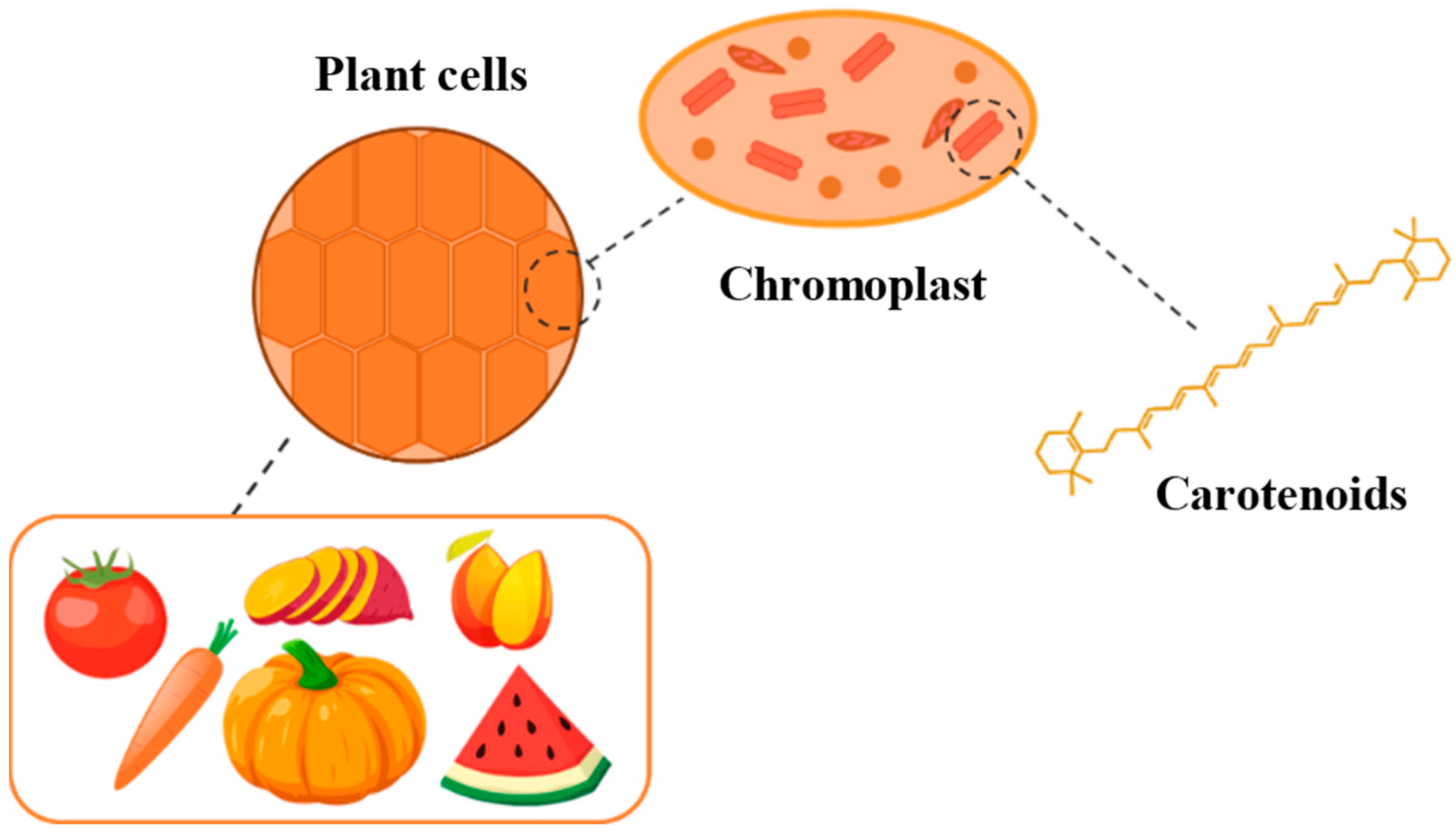
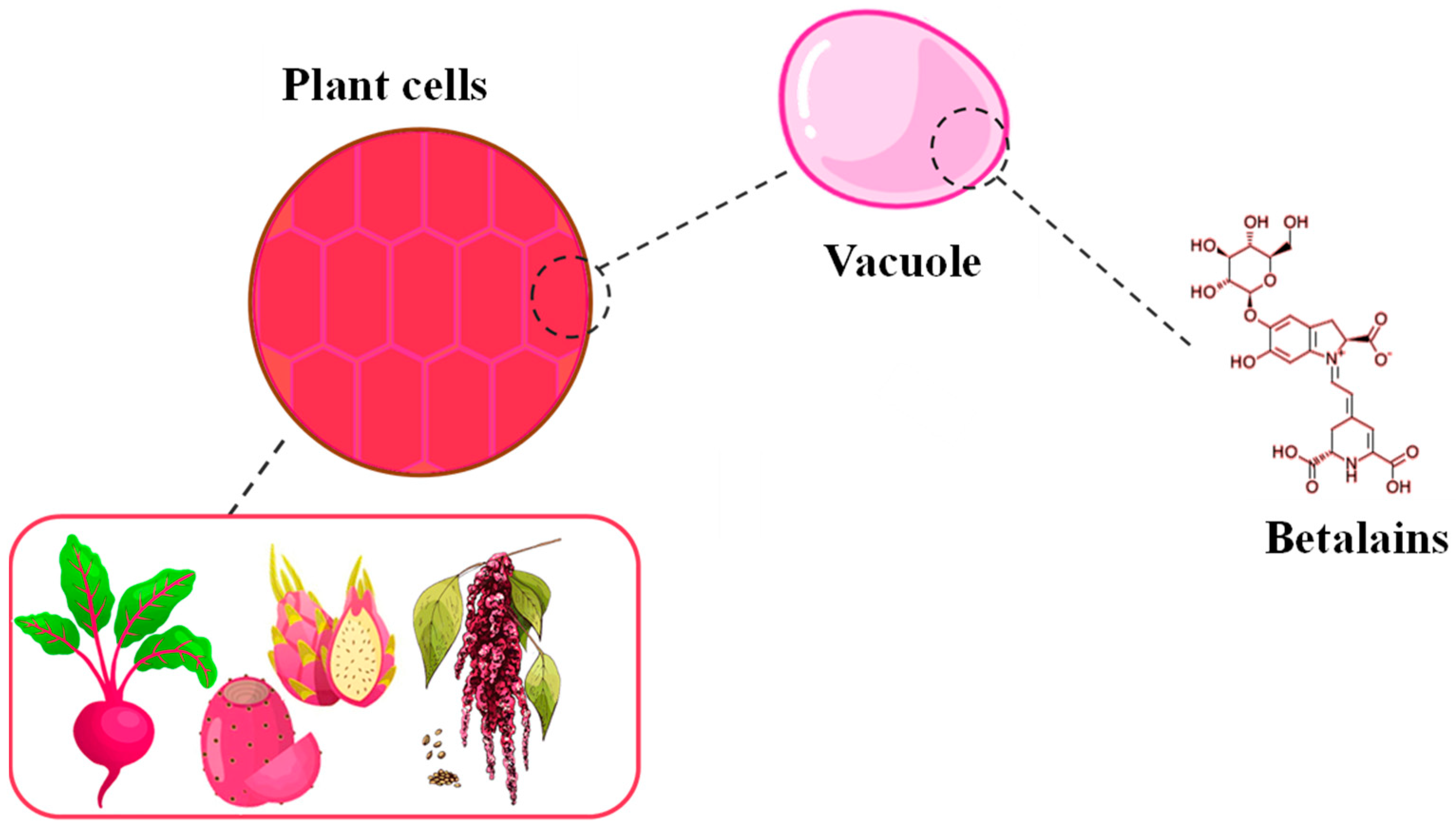
3. Carotenoids and Betalains: Natural Source and Cellular Localization
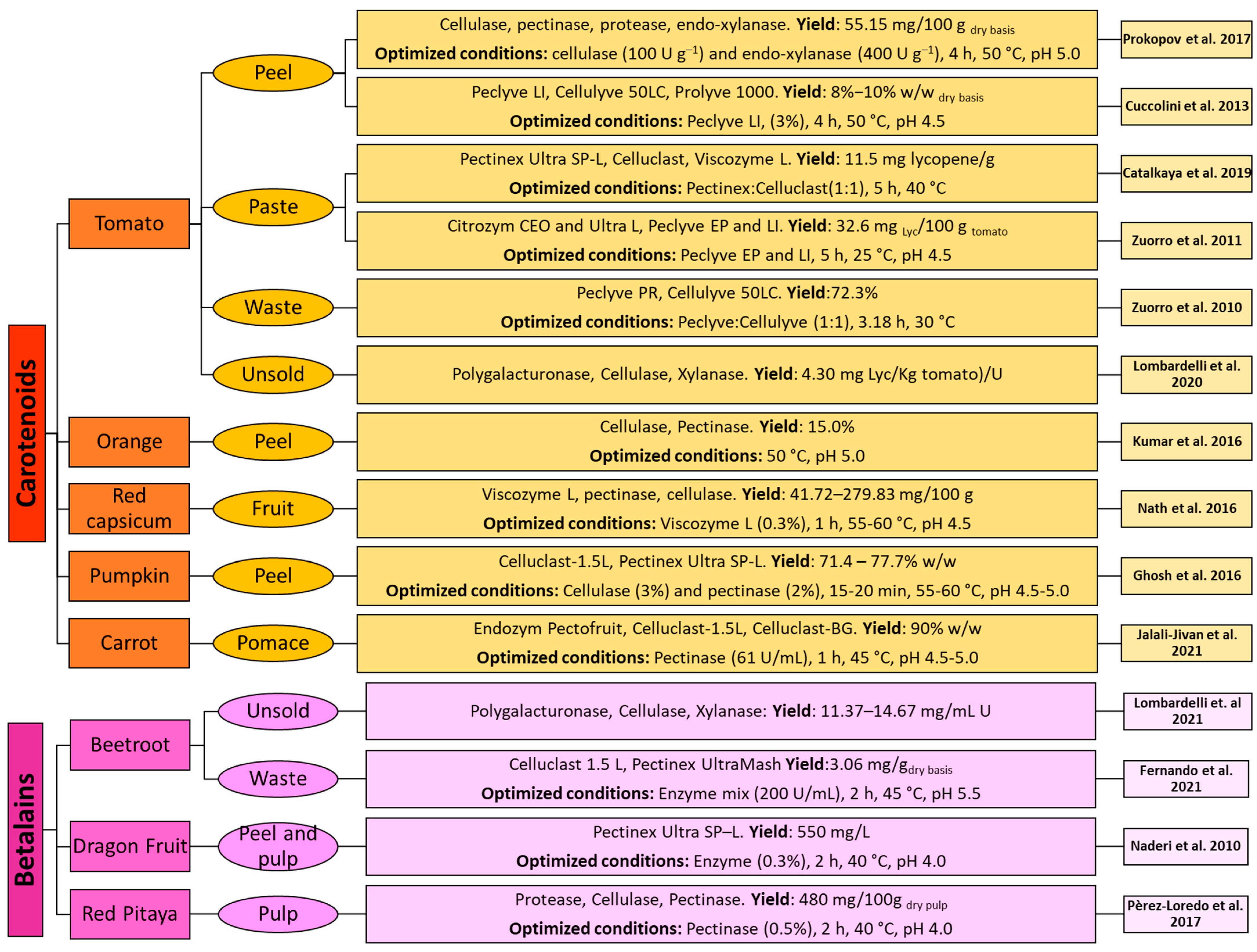
4. Enzyme-Assisted Extraction for the Recovery of Carotenoids and Betalains
5. Stability of Carotenoids and Betalains
| Pigment | Stabilizing Study | Results | Reference | |
|---|---|---|---|---|
| Carotenoid | Stabilizing method | Stabilizing conditions | ||
| Antioxidants | α-tocopherol, tripolyphosphate, EDTA, citric acid, gallic acid, propyl gallate. Storage conditions: 32 °C in the dark | α-tocopherol was the most effective in decreasing lycopene oxidation. | Bou et al. [84] | |
| Mixed tocopherols and sodium ascorbate (250–5000 µg/g). Storage conditions: 35 °C, air exposure (91 days) | Both antioxidants improved carotenoid stability, specifically when used in elevated concentrations (2500–5000 µg/g), but were not able to prevent carotenoid degradation when subjected to oxygen. | Haas et al. [85] | ||
| Encapsulation | α-, β- and γ-cyclodextrins (CDs) Storage conditions: room temperature, light and oxygen exposure (24 h, 1 month and 6 months) | β-CD showed the best complexation yields (93.8%) and was the most favorable to stabilize lycopene. | Blanch et al. [86] | |
| β-cyclodextrins (method A, ultrasonic homogenization; method B, kneading). Storage conditions: irradiance (1400 lx) at temperatures 25–31 °C (21 days) | Complex B offered bigger color stability of the isotonic drink with respect to complex A. | Lobo et al. [87] | ||
| α-, β- and γ-cyclodextrins (CDs) Storage conditions: temperature 4 or 25 °C in the dark (180 days) | β-CDs increased the stability of carotenoids for 90 days at 4 and 25 °C | Durante et al. [88] | ||
| Maltodextrin, Arabic gum (GA) and modified starch. Storage conditions: 40 °C and relative humidity of 75% (20 days). | Degradation of lutein after spray drying diminished from 97.62% to 8.06% when modified starch was replaced by GA. | Álvarez-Henao et al. [89] | ||
| Maltodextrin, GA, whey protein isolate, carboxy-methylcellulose and pectin. Storage conditions: 25 °C (40 days) | Native carbohydrates enhanced the encapsulation efficiency (50–95%) with respect to other encapsulating materials. | Curi-Borda et al. [90] | ||
| Liposomes, chitosomes and TPP-chitosomes. Storage conditions: 8 °C (14 days) and thermal stability at 40 °C and 70 °C (1 h) | TPP-chitosome was more useful in shielding carotenes from degradation during storage. | Esposto et al. [91] | ||
| Bovine gelatin, calcium caseinate, whey proteins Storage conditions: 25 °C in the dark (24 h and 48 h) | All formulations efficiently increased carotenoid dispersibility in water. | Petito et al. [92] | ||
| Nanoencapsulation with zein and ethylcellulose. Conditions: In Vitro Digestion | Both nanoparticles protected the β-carotene in the gastrointestinal phase, but only zein nanoparticles showed great bioaccessibility. | Afonso et al. [93] | ||
| Chromoplast (Chr) | Carotenoids in Chr Storage conditions: 4, 25 and 40 °C in the dark and under UV-light irradiation (30 days) | The lowest pigment degradation rates and better colorimetric parameters were found for Chr at 4 and 25 °C in the dark. | Lombardelli et al. [77] | |
| Betalain | Antioxidants | Ascorbic and isoascorbic acids (40 mM) Storage conditions: 100 °C (3 min) and 10 °C (24 h) | Ascorbic and isoascorbic acids (0.003–1%) allowed the greatest regeneration yield at pH 3.8. | Han et al. [94] |
| Chelating agents | EDTA (10,000 ppm) Storage conditions: 75 °C, pH 5 | Increased t1/2 of betanin by 1.5 times. | Herbach et al. [79] | |
| Encapsulation | Maltodextrin and combination with pectin, GA, guar gum, and xanthan gum (XG) | +21% increased stability of betalain. | Ravichandran et al. [95] | |
| GA, maltodextrin, modified starch (MS), chitosan and their combination Storage conditions: 40 °C (10 weeks) | Extracts encapsulated in GA–MS revealed the best colorimetric parameters. | Chranioti et al. [96] | ||
| Native potato starch and its modification (e.g., phosphorylation and succinylation). Storage conditions: 40 °C, pH 4.6 (39 days) | Succinylated potato starch was the best alternative for stabilizing betalains. | Vargas-Campos et al. [97] | ||
| Maltodextrin and XG by freeze and spray drying Storage conditions: room temperature and pH 3–6 | Microcapsules obtained by freeze-drying were characterized by greater stability in terms of betanin and color parameters. | Antigo et al. [98] | ||
| Pea protein (3.5–7%) as an encapsulating agent using Spray Drying (SD 125–150 °C) | 7% pea protein protected the most content of the studied bioactive compounds. | García-Segovia et al. [99] | ||
| Additives | Catechin (2.5–10 mM), ascorbic acid (0.025–0.1% w/v), EDTA (2–10 mM), β-cyclodextrin (100–250 ppm), maltodextrin (100–250 ppm) and GA (0.5–2.0% w/v) Storage conditions: 40 °C, for 5 days and at 4 °C in the absence of light and oxygen | Maximum stabilizing effect was exhibited by catechin (t1/2 203.9 days), EDTA (t1/2 187.3 days), and β-cyclodextrin (t1/2 144.4 days) compared with control (t1/2 119.5 days). Ascorbic acid behaved as a prooxidant (t1/2 78.8 days). | Karangutkar et al. [100] | |
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riesenegger, L.; Hübner, A. Reducing food waste at retail stores—An explorative study. Sustainability 2022, 14, 2494. [Google Scholar] [CrossRef]
- Cicatiello, C.; Franco, S.; Pancino, B.; Blasi, E. The value of food waste: An exploratory study on retailing. J. Retail. Consum. Serv. 2016, 30, 96–104. [Google Scholar] [CrossRef]
- Klingler, R.; Hübner, A.; Kempcke, T. End-to-End Supply Chain Management in Grocery Retailing; European Retail Institute: Köln, Germany, 2016. [Google Scholar]
- González-Torre, P.L.; Coque, J. From food waste to donations: The case of marketplaces in Northern Spain. Sustainability 2016, 8, 575. [Google Scholar] [CrossRef]
- Piirsalu, E.; Moora, H.; Väli, K.; Värnik, R.; Aro, K.; Lillemets, J. The Generation of Food Waste and Food Loss in the Estonian Food Supply Chain; Stockholm Environment Institute: Stockholm, Sweden, 2022. [Google Scholar]
- Dou, Z. Leveraging livestock to promote a circular food system. Front. Agric. Sci. Eng. 2021, 8, 188–192. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Scarioni, S.; Tava, A.; Panseri, S.; Zuorro, A. Fruit and Vegetable Wholesale Market Waste: Safety and Nutritional Characterisation for Their Potential Re-Use in Livestock Nutrition. Sustainability 2021, 13, 9478. [Google Scholar] [CrossRef]
- Bonadonna, A.; Matozzo, A.; Giachino, C.; Peira, G. Farmer behavior and perception regarding food waste and unsold food. Brit. Food J. 2018, 121, 89–103. [Google Scholar] [CrossRef]
- Katsarova, I. Resource Efficiency: Reducing Food Waste, Improving Food Safety; European Parliamentary Research Service: London, UK, 2017. [Google Scholar]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Fox, E.M.; Cobiac, L.; Cole, M.B. Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci. Technol. 2020, 95, 75–85. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domınguez, C.; Vega-Vega, V. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for integral exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Biolie. Available online: https://www.biolie.fr/ (accessed on 18 November 2022).
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Yawale, P.; Upadhyay, N. Carotenoids extraction strategies and potential applications for valorization of under-utilized waste biomass. Food Biosci. 2022, 48, 101812. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Cassani, L.; Marcovich, N.E.; Gomez-Zavaglia, A. Valorization of fruit and vegetables agro-wastes for the sustainable production of carotenoid-based colorants with enhanced bioavailability. Food Res. Int. 2021, 152, 110924. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. From Diospyros kaki L. (Persimmon) phytochemical profile and health impact to new product perspectives and waste valorization. Nutrients 2021, 13, 3283. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.D.L.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Santos, D.; Campos, D.A.; Ratinho, M.M.; Rodrigues, I.E.; Pintado, M. Development of frozen pulps and powders from carrot and tomato by-products: Impact of processing and storage time on bioactive and biological properties. Horticulturae 2021, 7, 185. [Google Scholar] [CrossRef]
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanovć-Brunet, J.; Šaponjac, V.T.; Stajčić, S. Natural bioactive compounds in carrot waste for food applications and health benefits. Stud. Nat. Prod. Chem. 2020, 67, 307–344. [Google Scholar]
- Gallo, M.; Formato, A.; Ciaravolo, M.; Langella, C.; Cataldo, R.; Naviglio, D. A water extraction process for lycopene from tomato waste using a pressurized method: An application of a numerical simulation. Eur. Food Res. Technol. 2019, 245, 1767–1775. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, G.B.; Castillejo, N.; Artés-Hernández, F. Effect of fresh–cut apples fortification with lycopene microspheres, revalorized from tomato by-products, during shelf life. Postharvest Biol. Technol. 2019, 156, 110925. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zha, X.; Mei, Y.; Xia, J.; Jiao, Z. Supercritical carbon dioxide extraction of β-carotene and α-tocopherol from pumpkin: A Box–Behnken design for extraction variables. Anal. Methods 2017, 9, 294–303. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of phytochemicals via electromagnetic irradiation (microwave-assisted-extraction): Betalain and phenolic compounds in perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juárez-Onofre, J.E.; Carvajal-Millán, E.; López-Ahumada, G.A.; Rodríguez-Félix, F. Effect of ultrafiltration of Pitaya extract (Stenocereus thurberi) on Its phytochemical content, antioxidant capacity, and UPLC-DAD-MS profile. Molecules 2020, 25, 281. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Sawicki, T.; Bartoszek, A. The comparison of betalain composition and chosen biological activities for differently pigmented prickly pear (Opuntia ficus-indica) and beetroot (Beta vulgaris) varieties. Int. J. Food Sci. 2019, 70, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, sources, and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Kovačević, D.B.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Vidallon, M.L.P.; Mendoza, D.J.R.; Reyes, C.T. Health-promoting bioactivities of betalains from red dragon fruit (Hylocereus polyrhizus (Weber) Britton and Rose) peels as affected by carbohydrate encapsulation. J. Sci. Food Agric. 2016, 96, 4679–4689. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Šaponjac, V.T.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Future Market Insights. Betanin Food Color Market. Market Insights on Betanin Food Color Covering Sales Outlook, Demand Forecast & Up-to-Date Key Trends. Betanin Food Color Market by Nature, Product Source, Application, Form & Region-Forecast 2022–2032; Future Market Insights: Valley Cottage, NY, USA, 2022. [Google Scholar]
- Wrischer, M.; Devide, Z.V.O.N.I.M.I.R. Chromoplasts--the last stages in plastid development. Int. J. Dev. Biol. 2002, 35, 251–258. [Google Scholar]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and β-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S. Nondestructive evaluation of internal maturity of tomatoes using spatially offset Raman spectroscopy. Postharvest Biol. Technol. 2012, 71, 21–31. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; D’Amico, L.; Piro, G.; Mita, G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO2 extracted pumpkin (Cucurbita moschata Duch.) oil. Food Chem. 2014, 148, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Veda, S.; Platel, K.; Srinivasan, K. Varietal differences in the bioaccessibility of β-carotene from mango (Mangifera indica) and papaya (Carica papaya) fruits. J. Agric. Food Chem. 2007, 55, 7931–7935. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid content of 50 watermelon cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Bengtsson, A.; Namutebi, A.; Alminger, M.L.; Svanberg, U. Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. J. Food Compos. Anal. 2008, 21, 134–143. [Google Scholar] [CrossRef]
- Marty, F.; Branton, D. Analytical characterization of beetroot vacuole membrane. J. Cell Biol. 1980, 87, 72–83. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). Sci. World J. 2014, 2014, 964731. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Loredo, M.G.; Jesús, H.D.; Barragán-Huerta, B.E. Extraction of red pitaya (Stenocereus stellatus) bioactive compounds applying microwave, ultrasound and enzymatic pretreatments. Agrociencia 2017, 51, 135–151. [Google Scholar]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of agricultural lignocellulosic plant byproducts through enzymatic and enzyme-assisted extraction of high-value-added compounds: A Review. ACS Sustain. Chem. Eng. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A. (Eds.) Alternative Solvents for Natural Products Extraction; Springer: Berlin, Germany, 2014; Volume 381. [Google Scholar]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Prokopov, T.; Nikolova, M.; Dobrev, G.; Taneva, D. Enzyme-assisted extraction of carotenoids from Bulgarian tomato peels. Acta Aliment. 2017, 46, 84–91. [Google Scholar] [CrossRef]
- Cuccolini, S.; Aldini, A.; Visai, L.; Daglia, M.; Ferrari, D. Environmentally friendly lycopene purification from tomato peel waste: Enzymatic assisted aqueous extraction. J. Agric. Food Chem. 2013, 61, 1646–1651. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Mild enzymatic method for the extraction of lycopene from tomato paste. Biotechnol. Biotechnol. Equip. 2010, 24, 1854–1857. [Google Scholar] [CrossRef]
- Lombardelli, C.; Liburdi, K.; Benucci, I.; Esti, M. Tailored and synergistic enzyme-assisted extraction of carotenoid-containing chromoplasts from tomatoes. Food Bioprod. Process. 2020, 121, 43–53. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Ratrey, P.; Datta, B. Reusable nanobiocatalysts for the efficient extraction of pigments from orange peel. J. Food Sci. Technol. 2016, 53, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kaur, C.; Rudra, S.G.; Varghese, E. Enzyme-assisted extraction of carotenoid-rich extract from red capsicum (Capsicum annuum). Agric. Res. 2016, 5, 193–204. [Google Scholar] [CrossRef]
- Ghosh, D.; Biswas, P.K. Enzyme-aided extraction of carotenoids from pumpkin tissues. Indian Chem. Eng. 2016, 58, 1–11. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Fathi-Achachlouei, B.; Ahmadi-Gavlighi, H.; Jafari, S.M. Improving the extraction efficiency and stability of β-carotene from carrot by enzyme-assisted green nanoemulsification. Innov. Food Sci. Emerg. Technol. 2021, 74, 102836. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. A novel process for the recovery of betalains from unsold red beets by low-temperature enzyme-assisted extraction. Foods 2021, 10, 236. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an ultrasound-assisted extraction method to recover betalains and polyphenols from red beetroot waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Naderi, N.; Stintzing, F.C.; Ghazali, H.M.; Manap, Y.A.; Jazayeri, S.D. Betalain extraction from Hylocereus polyrhizus for natural food coloring purposes. Prof. Assoc. Cactus Dev. 2010, 12, 143–154. [Google Scholar]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- De Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Esti, M. Novel food colorants from tomatoes: Stability of carotenoid-containing chromoplasts under different storage conditions. LWT 2021, 140, 110725. [Google Scholar] [CrossRef]
- Azeredo, H. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Perales Romero, E.; Jordán-Núñez, J.; Viqueira, V. Halloysite and Laponite Hybrid Pigments Synthesis with Copper Chlorophyll. Appl. Sci. 2021, 11, 5568. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Herbach, K.M.; Rohe, M.; Stintzing, F.C.; Carle, R. Structural and chromatic stability of purple pitaya (Hylocereus polyrhizus [Weber] Britton & Rose) betacyanins as affected by the juice matrix and selected additives. Food Res. Int. 2006, 39, 667–677. [Google Scholar]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of betalains by encapsulation—A review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. Betalain extracts from beetroot as food colorants: Effect of temperature and UV-light on storability. Plant Foods Hum. Nutr. 2021, 76, 347–353. [Google Scholar] [CrossRef]
- Bou, R.; Boon, C.; Kweku, A.; Hidalgo, D.; Decker, E.A. Effect of different antioxidants on lycopene degradation in oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2011, 113, 724–729. [Google Scholar] [CrossRef]
- Haas, K.; Robben, P.; Kiesslich, A.; Volkert, M.; Jaeger, H. Stabilization of crystalline carotenoids in carrot concentrate powders: Effects of drying technology, carrier Material, and antioxidants. Foods 2019, 8, 285. [Google Scholar] [CrossRef]
- Blanch, G.P.; del Castillo, M.L.R.; del Mar Caja, M.; Pérez-Méndez, M.; Sánchez-Cortés, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341. [Google Scholar] [CrossRef]
- Lobo, F.A.T.; Silva, V.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcão, D.; de Lima Araújo, K.G. Inclusion complexes of yellow bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as food natural colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Milano, F.; Caroli, M.D.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato oil encapsulation by α-, β-, and γ-Cyclodextrins: A comparative study on the formation of supramolecular structures, antioxidant activity, and carotenoid stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Cartagena, C.J.; Alzate, L.M.; Londoño-Londoño, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Curi-Borda, C.K.; Linares-Pastén, J.A.; Tat, T.; Tarqui-Dueñas, R.; Chino-Flores, N.; Alvarado, J.A.; Bergenstahl, B. Multilayer bixin microcapsules: The impact of native carbohydrates on the microencapsulation efficiency and dispersion stability. Foods 2019, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Esposto, B.S.; Pinho, S.G.B.; Thomazini, M.; Ramos, A.P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. TPP-chitosomes as potential encapsulation system to protect carotenoid-rich extract obtained from carrot by-product: A comparison with liposomes and chitosomes. Food Chem. 2022, 397, 133857. [Google Scholar] [CrossRef]
- Petito, N.D.L.; Devens, J.M.; Falcão, D.Q.; Dantas, F.M.L.; Passos, T.S.; Araujo, K.G.D.L. Nanoencapsulation of Red Bell Pepper Carotenoids: Comparison of Encapsulating Agents in an Emulsion Based System. Colorants 2022, 1, 132–148. [Google Scholar] [CrossRef]
- Afonso, B.S.; Azevedo, A.G.; Gonçalves, C.; Amado, I.R.; Ferreira, E.C.; Pastrana, L.M.; Cerqueira, M.A. Bio-based nanoparticles as a carrier of β-carotene: Production, characterisation and in vitro gastrointestinal digestion. Molecules 2020, 25, 4497. [Google Scholar] [CrossRef]
- Han, D.; Kim, S.J.; Kim, S.H.; Kim, D.M. Repeated regeneration of degraded red beet juice pigments in the presence of antioxidants. J. Food Sci. 1998, 63, 69–72. [Google Scholar] [CrossRef]
- Ravichandran, K.; Palaniraj, R.; Saw, N.M.M.T.; Gabr, A.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of different encapsulation agents and drying process on stability of betalains extract. J. Food Sci. Technol. 2014, 51, 2216–2221. [Google Scholar] [CrossRef]
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263. [Google Scholar] [CrossRef]
- Vargas-Campos, L.; Valle-Guadarrama, S.; Martínez-Bustos, F.; Salinas-Moreno, Y.; Lobato-Calleros, C.; Calvo-López, A.D. Encapsulation and pigmenting potential of betalains of pitaya (Stenocereus pruinosus) fruit. J. Food Sci. Technol. 2018, 55, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Antigo, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2017, 38, 72–77. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Beetroot microencapsulation with pea protein using spray drying: Physicochemical, structural and functional properties. Appl. Sci. 2021, 11, 6658. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Evaluating the effect of additives on stability of betacyanin pigments from Basella rubra in a model beverage system during storage. J. Food Sci. Technol. 2021, 58, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
| Pigment | Source | Bioactivity/Health Benefits | Reference |
|---|---|---|---|
| Carotenoids | Waste biomass | Antioxidant, Anti-mutagenic, anti-proliferative, anti-inflammatory, anti-hypertension and anti-atherogenic activities. Radical scavenging activity. | Tiwari et al. [17] |
| Microalgals | Rammuni et al. [18] | ||
| Agro wastes | Cassani et al. [19] | ||
| Persimmon | Direito et al. [20] | ||
| Bell peppers | Anaya-Esparza et al. [21] | ||
| Carrot and tomato by-products | Araújo-Rodrigues et al. [22] | ||
| Carrot waste | Šeregelj et al., [23] | ||
| Tomato waste | Gallo et al. [24] | ||
| Vegetable waste | de Andrade Lima et al. [25] | ||
| Tomato by-products | Martínez-Hernández et al. [26] | ||
| Pomegranate wastes | Goula et al. [27] | ||
| Tomato peel | Kehili et al. [28] | ||
| Pumpkin | Wang et al. [29] | ||
| Tomato and tomato byproducts | Viuda-Martos et al. [30] | ||
| Tomato peel | Rizk et al. [31] | ||
| Tomato | Palozza et al. [32] | ||
| Betalains | Amaranthus, Prickly pear, Red dragon fruit, Red pitaya, Red beetroot | Antioxidant, anticarcinogenic, hepatoprotective, antibacterial, and anti-inflammatory activities. Intestinal and immune regulatory effects and prevent cardiovascular diseases. | Calva-Estrada et al. [33] |
| Agro-industrial wastes | Zin et al. [34] | ||
| Pitaya fruit | Castro-Enríquez et al. [35] | ||
| Red beetroot | Fu et al. [36] | ||
| Prickly pear, beetroot | Koss-Mikołajczyk et al. [37] | ||
| Pitaya peel | Tenore et al. [38] | ||
| Amaranthus, Prickly pear, Red dragon fruit, Red pitaya, Red beetroot | Polturak et al. [39] | ||
| Prickly pear | Barba et al. [40] | ||
| Red dragon fruit peel | Rodriguez et al. [41] | ||
| Red beet, Cacti fruits, Dragon fruits, Swiss chard | Gandía-Herrero et al. [42] | ||
| Beetroot pomace | Vulić et al. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits. Appl. Sci. 2022, 12, 12249. https://doi.org/10.3390/app122312249
Lombardelli C, Benucci I, Mazzocchi C, Esti M. Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits. Applied Sciences. 2022; 12(23):12249. https://doi.org/10.3390/app122312249
Chicago/Turabian StyleLombardelli, Claudio, Ilaria Benucci, Caterina Mazzocchi, and Marco Esti. 2022. "Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits" Applied Sciences 12, no. 23: 12249. https://doi.org/10.3390/app122312249
APA StyleLombardelli, C., Benucci, I., Mazzocchi, C., & Esti, M. (2022). Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits. Applied Sciences, 12(23), 12249. https://doi.org/10.3390/app122312249