Solid-Phase Extraction of Organic Dyes on Mixed-Ligand Zr(IV) Metal–Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sorbent
2.3. Characterization
2.4. Solid-Phase Extraction Procedures
3. Results and Discussion
3.1. Synthesis and Characterization of the Sorbent
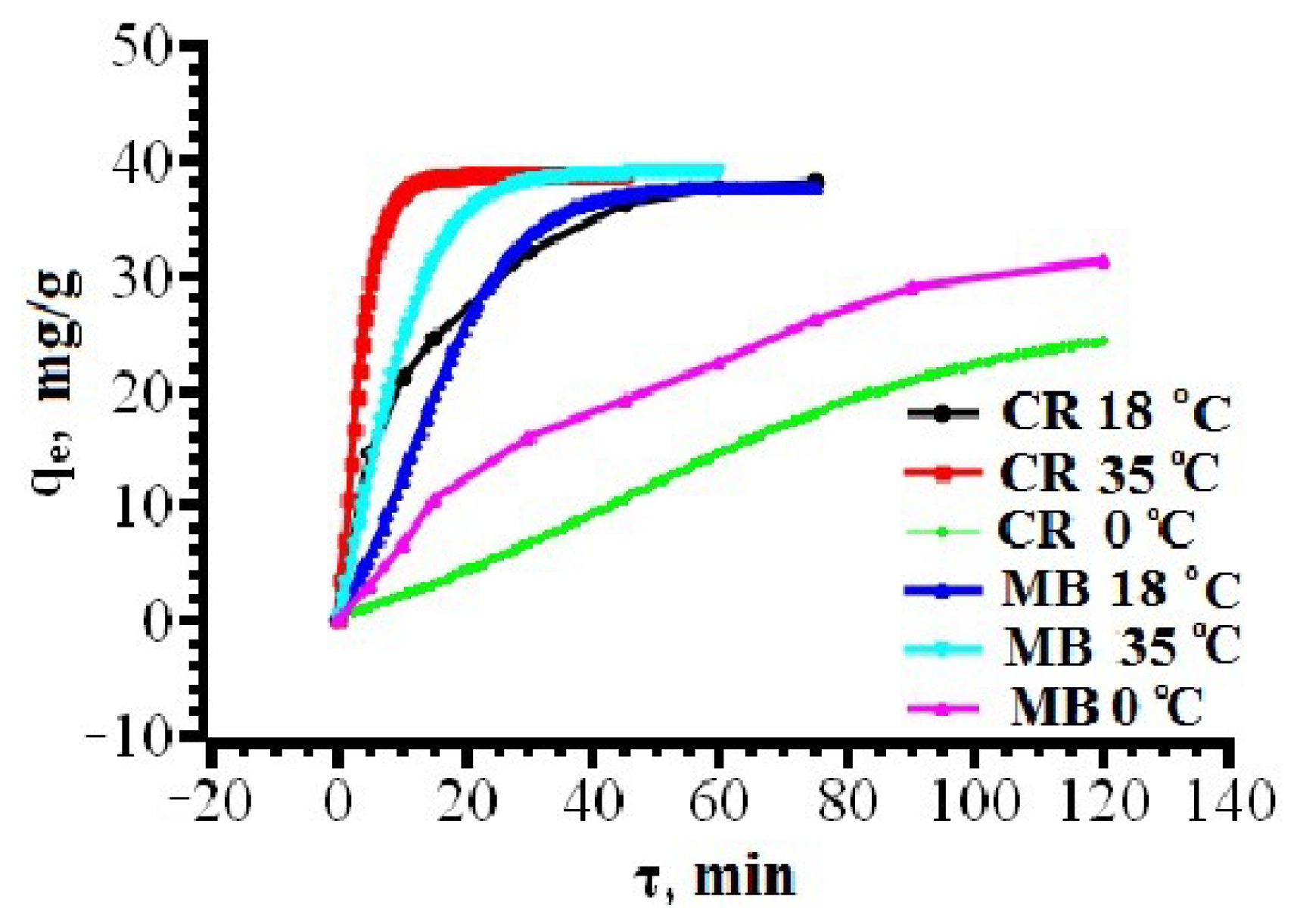
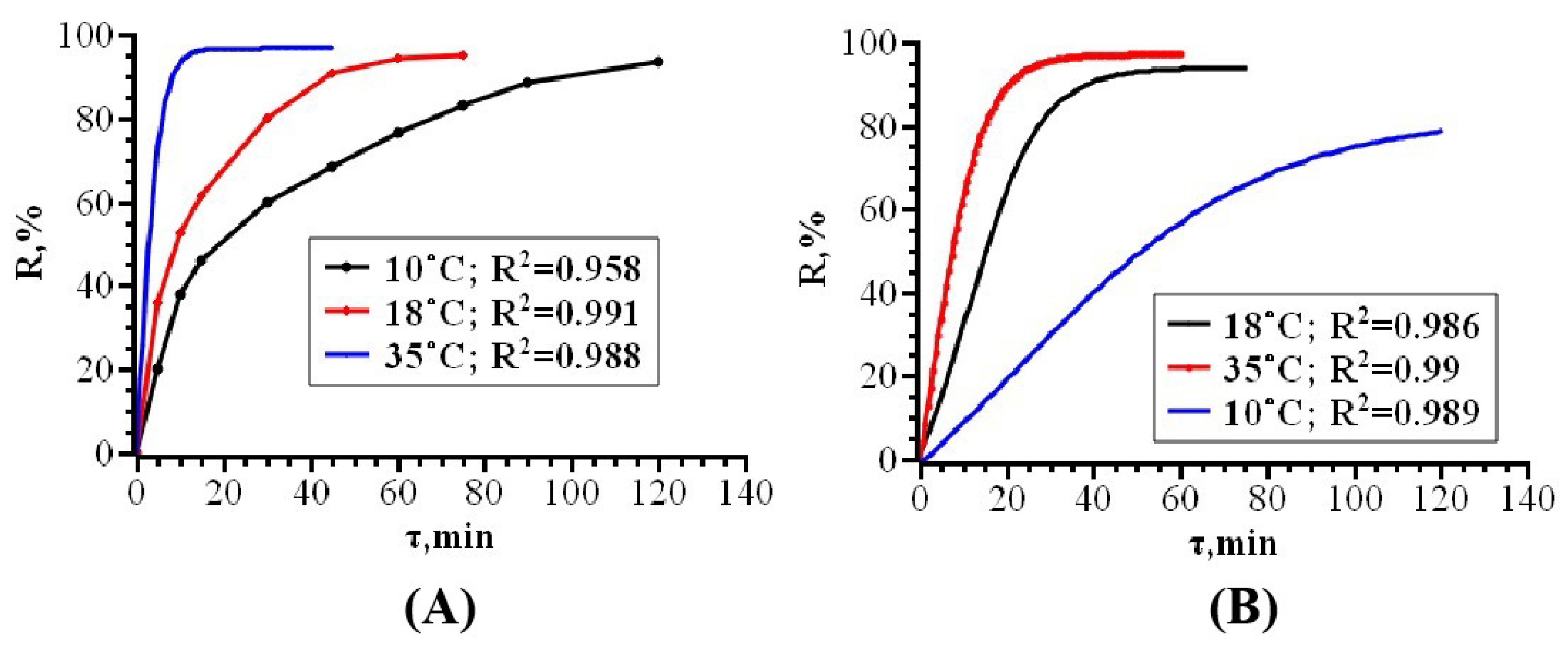
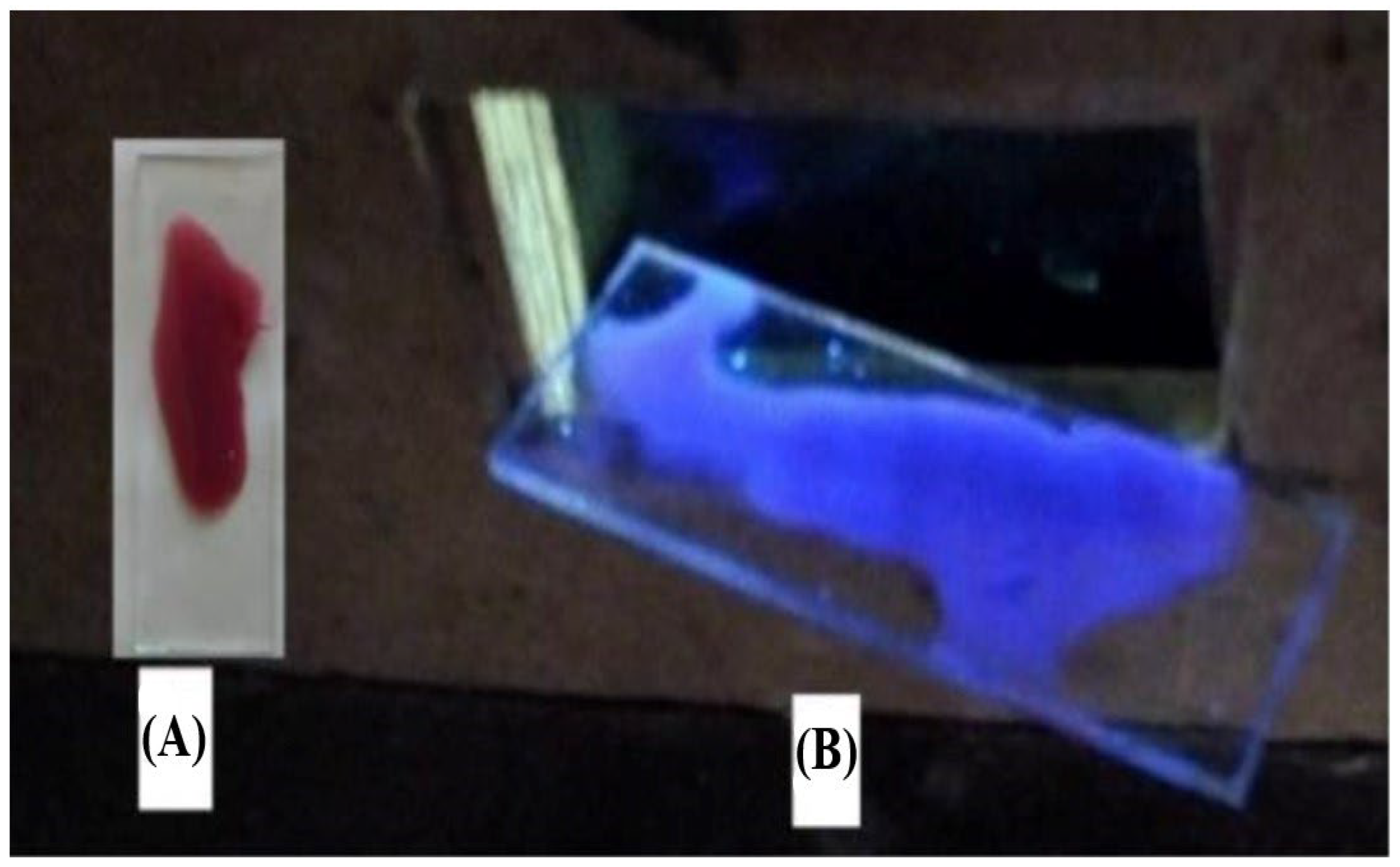
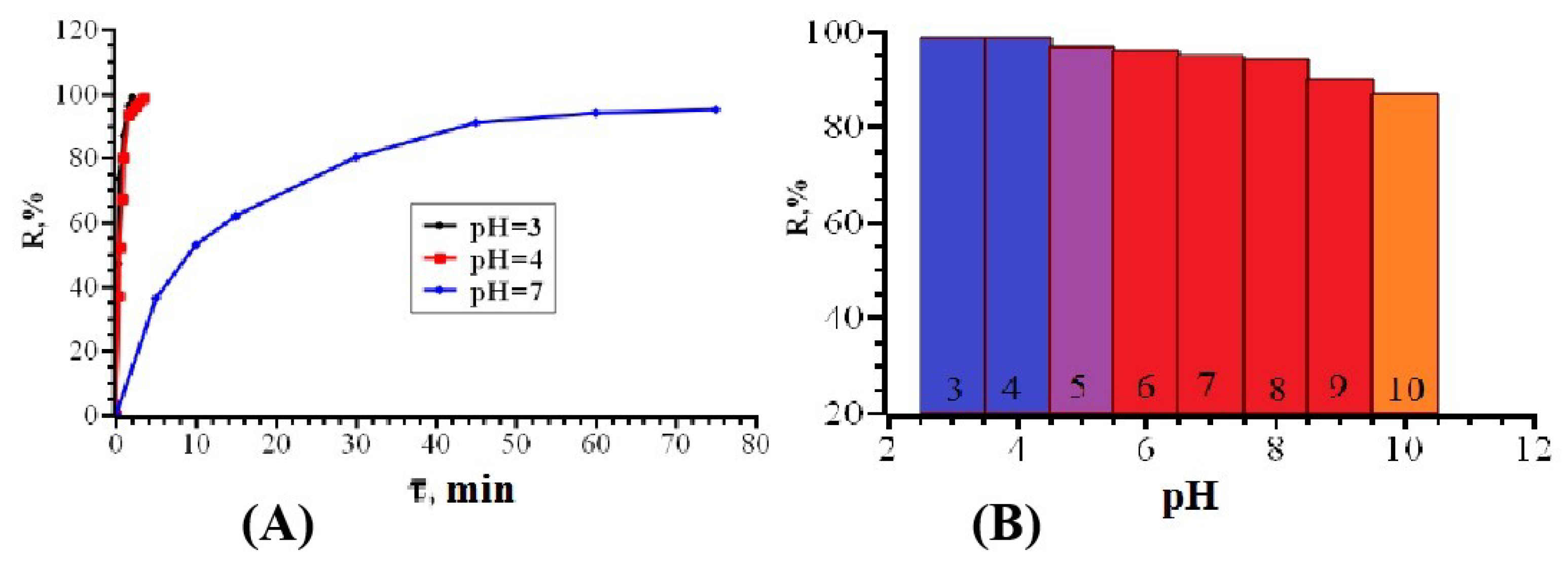
3.2. Solid-Phase Extraction of Organic Dyes
3.3. Solid-Phase Extraction of Dyes in Real Samples
3.4. Solid-Phase Extraction of Dyes in Artificial Seawater
3.5. Sorbent as a Filler for Column Chromatography for Dye Separation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Knyazeva, M.K.; Solovtsova, O.V.; Men’Shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A.; Aksyutin, O.E.; Ishkov, A.G. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Solovtsova, O.V.; Pulin, A.L.; Men’shchikov, I.E.; Platonova, N.P.; Knyazeva, M.K.; Chugaev, S.S.; Shkolin, A.V.; Fomkin, A.A. Zr-based metal-organic nanoporous adsorbents of high density for methane storage. Prot. Met. Phys. Chem. Surf. 2020, 56, 1114–1121. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Murdmaa, K.O.; Solovtsova, O.V. Methane adsorption on the Zr-BDC metal-organic framework structure at supercritical temperatures and pressures. Russ. Chem. Bull. 2021, 70, 665–671. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Nikolaevskaya, V.O.; Kharisov, B.I.; Oliva González, C.M.; Kharissova, O.V. Recent Strategies to Improve MOF Performance in Solid Phase Extraction of Organic Dyes. Microchem. J. 2021, 168, 106387. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional Mechanical Stability of Highly Porous Zirconium Metal-Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chem.–Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective dye adsorption by highly water stable metal-organic framework: Long term stability analysis in aqueous media. Appl. Surf. Sci. 2018, 445, 424–436. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal-organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, Y.-Z.; Kong, X.-J.; Yu, J.; Lv, X.-L.; Wu, Y.; Guo, Z.-J.; Li, J.-R. Zr(IV)-based metal-organic framework with T-shaped ligand: Unique structure, high stability, selective detection, and rapid adsorption of Cr2O72– in water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Wißmann, G.; Schaate, A.; Lilienthal, S.; Bremer, I.; Schneider, A.M.; Behrens, P. Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater. 2012, 152, 64–70. [Google Scholar] [CrossRef]
- Gutov, O.V.; Molina, S.; Escudero-Adan, E.C.; Shafir, A. Modulation by Amino Acids: Toward Superior Control in the Synthesis of Zirconium Metal-Organic Frameworks. Chem.–Eur. J. 2016, 22, 13582–13587. [Google Scholar] [CrossRef]
- Marshall, R.J.; Hobday, C.L.; Murphie, C.F.; Griffin, S.L.; Morrison, C.A.; Moggach, S.A.; Forgan, R.S. Amino acids as highly efficient modulators for single crystals of zirconium and hafnium metal-organic frameworks. J. Mater. Chem. A 2016, 4, 6955–6963. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect Engineering: Tuning the Porosity and Composition of the Metal-Organic Framework UiO-66 via Modulated Synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colon, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design and synthesis of coordination polymers with chelated units and their application in nanomaterials science. RSC Adv. 2017, 7, 42242–42288. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, G.; Li, W.; Gu, D.; Liang, Z.; Liu, Y.; Pan, Q. Three coordination complexes based on mixed ligand strategy: Coordination diversities and nitrobenzene detections. Polyhedron 2020, 185, 114599. [Google Scholar] [CrossRef]
- Feng, X.; Ren, Y.; Jiang, H. Metal-bipyridine/phenanthroline-functionalized porous crystalline materials: Synthesis and catalysis. Coord. Chem. Rev. 2021, 438, 213907. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Dzhardimalieva, G.I.; Knerelman, E.I.; Davydova, G.I.; Shunina, I.G. Synthesis and Properties of Copper Trimesinate Complexes with Polypyridine Ligands. Russ. J. Gen. Chem. 2020, 90, 1884–1891. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Gu, L.-N.; Hu, G.; Zhang, L.-D. Synthesis, structural characterization and selectively catalytic properties of metal–organic frameworks with nano-sized channels: A modular design strategy. J. Solid State Chem. 2009, 182, 502–508. [Google Scholar] [CrossRef]
- Dun, L.; Zhang, B.; Wang, J.; Wang, H.; Chen, X.; Li, C. Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2’-Bipyridine as Ligands. Crystals 2020, 10, 1105. [Google Scholar] [CrossRef]
- Yang, B.-P.; Zeng, H.-Y.; Mao, J.-G.; Guo, G.-C.; Huang, J.-S.; Dong, Z.-C. Solvothermal synthesis and crystal structures of two new copper(II) terephthalates. Transition Met. Chem. 2003, 28, 600–605. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.; Chen, Y.; Li, X.; Xiong, W.; Zhou, Y.; Zhou, C.; Xu, R.; Zhang, Y. Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr(VI) from aqueous solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]
- Xu, H.; Liang, Y.; Su, Z.; Zhao, Y.; Shao, K.; Zhang, H.; Yue, S. Tetraaqua(2,2’-bipyridine)zinc(II) terephthalate. Acta Cryst. 2004, E60, m142–m144. [Google Scholar] [CrossRef]
- Go, Y.; Wang, X.; Anokhina, E.V.; Jacobson, A.J. A Chain of Changes: Influence of Noncovalent Interactions on the One-Dimensional Structures of Nickel(II) Dicarboxylate Coordination Polymers with Chelating Aromatic Amine Ligands. Inorg. Chem. 2004, 43, 5360–5367. [Google Scholar] [CrossRef]
- Go, Y.B.; Wang, X.; Anokhina, E.V.; Jacobson, A.J. Influence of the Reaction Temperature and pH on the Coordination Modes of the 1,4-Benzenedicarboxylate (BDC) Ligand: A Case Study of the NiII(BDC)/2,2’-Bipyridine System. Inorg. Chem. 2005, 44, 8265–8271. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Zhinzhilo, V.A.; Bryantseva, J.D.; Uflyand, I.E.; Kharisov, B.I. ZrIV metal-organic framework based on terephthalic acid and 1,10-phenanthroline as adsorbent for solid-phase extraction of tetracycline antibiotics. Mendeleev Commun. 2022, 32, 661–663. [Google Scholar] [CrossRef]
- Abo-State, M.A.M.; Saleh, Y.E.; Hazaa, H.A. Decolorization of Congo Red dye by bacterial isolates. J. Ecol. Health Environ. 2017, 5, 41–48. [Google Scholar]
- Hu, Z.; Tong, C. Synchronous fluorescence determination of DNA-based on the interaction between methylene blue and DNA. Anal. Chim. Acta 2007, 587, 187–193. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Sheng, G.D.; Shao, D.D.; Ren, X.M.; Wang, X.Q.; Li, J.X.; Chen, Y.X.; Wang, X.K. Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2010, 178, 505–516. [Google Scholar] [CrossRef]
- Wang, W.; He, R.; Yang, T.; Hu, Y.; Zhang, N.; Yang, C. Three-dimensional mesoporous calcium carbonate–silica frameworks thermally activated from porous fossil bryophyte: Adsorption studies for heavy metal uptake. RSC Adv. 2018, 8, 25754–25766. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.; Baimuratova, R.; Knerelman, E.; Davydova, G.; Kudaibergenov, S.; Kharissova, O.; Zhinzhilo, V.; Uflyand, I. Synthesis of Copper(II) Trimesinate Coordination Polymer and Its Use as a Sorbent for Organic Dyes and a Precursor for Nanostructured Material. Polymers 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Mikhalyova, E.A.; Yakovenko, A.V.; Zeller, M.; Kiskin, M.A.; Kolomzarov, Y.V.; Eremenko, I.L.; Addison, A.W.; Pavlishchuk, V.V. Manifestation of π−π Stacking Interactions in Luminescence Properties and Energy Transfer in Aromatically-Derived Tb, Eu and Gd Tris(pyrazolyl)borate Complexes. Inorg. Chem. 2015, 54, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Wang, J.; Liu, F.; Hu, N.; Pei, H.; Yang, W.; Li, Z.; Suo, Y.; Wang, J. High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOFs (UiO-67). Food Chem. 2018, 254, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal-Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef]
- Wood, R.; Foster, L.; Damant, A.; Key, P. Analytical Methods for Food Additives; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and Health: A Review. Crit. Rev. Food Sci. Nutr. 2010, 51, 1. [Google Scholar] [CrossRef]
- Hanaa, A.-R.; Abdalsamad, K.M.E. Passivation characterization of nickel-based glassy alloys in artificial sea water. Met. Mater. Int. 2020, 26, 1688–1696. [Google Scholar]
- Asgher, M.; Bhatti, H.N. Mechanistic and kinetic evaluation of biosorption of reactive azo dyes by free, immobilized and chemically treated Citrus sinensis waste biomass. Ecol. Eng. 2010, 36, 1660–1665. [Google Scholar] [CrossRef]
- O’mahony, T.; Guibal, E.; Tobin, J. Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb. Technol. 2002, 31, 456–463. [Google Scholar] [CrossRef]
- Qin, Q.; Ma, J.; Liu, K. Adsorption of anionic dyes on ammonium-functionalized MCM-41. J. Hazard. Mater. 2009, 162, 133–139. [Google Scholar] [CrossRef]
- Liu, H.; Gao, G.; Liu, J.; Bao, F.; Wei, Y.; Wang, H. Amide-functionalized ionic indium–organic frameworks for efficient separation of organic dyes based on diverse adsorption interactions. CrystEngComm 2019, 21, 2576–2584. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.-Y.; Hou, J.-X.; Liu, J.-M.; Jing, X.; Li, L.-J.; Du, J.-L. Functionalized Mn(II)-MOF based on host-guest interaction for selective and rapid capture of Congo red from water. J. Solid State Chem. 2019, 270, 697–704. [Google Scholar] [CrossRef]




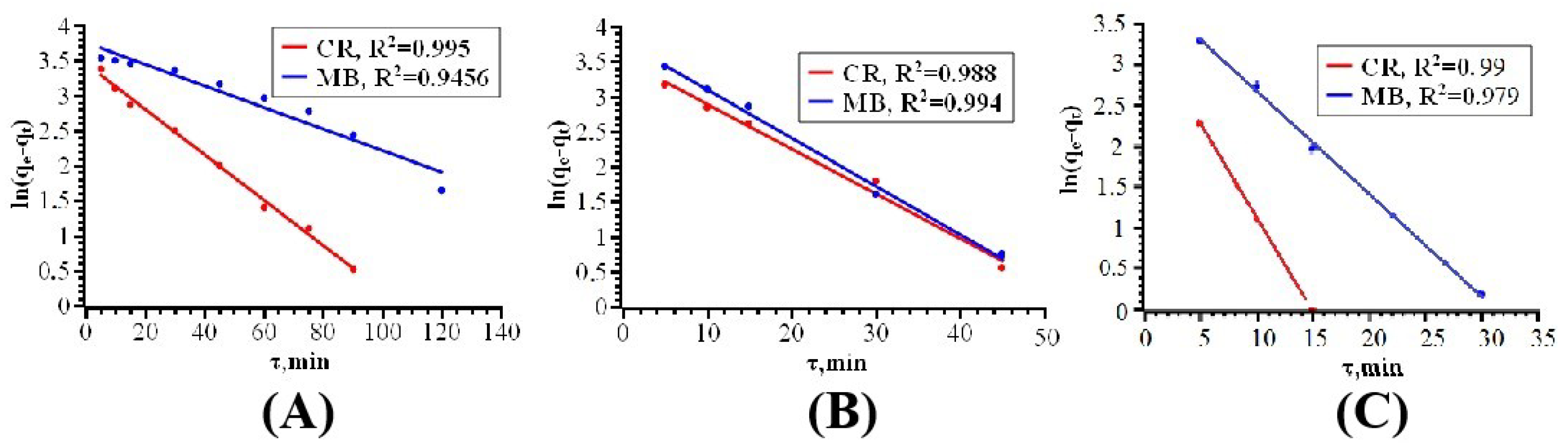
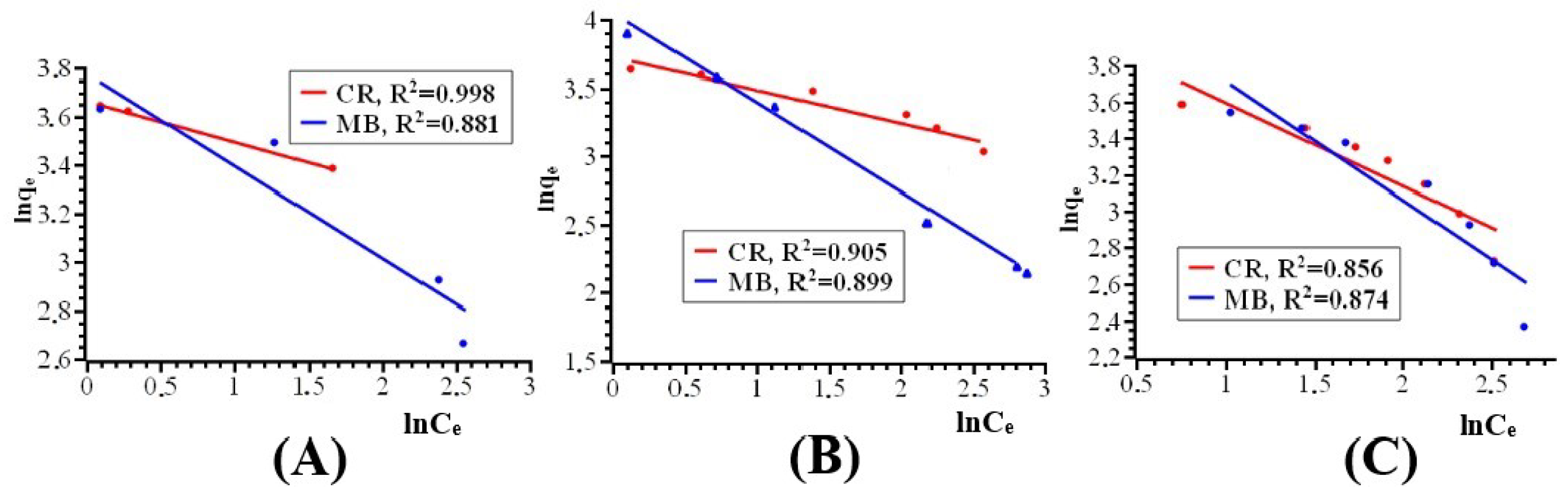




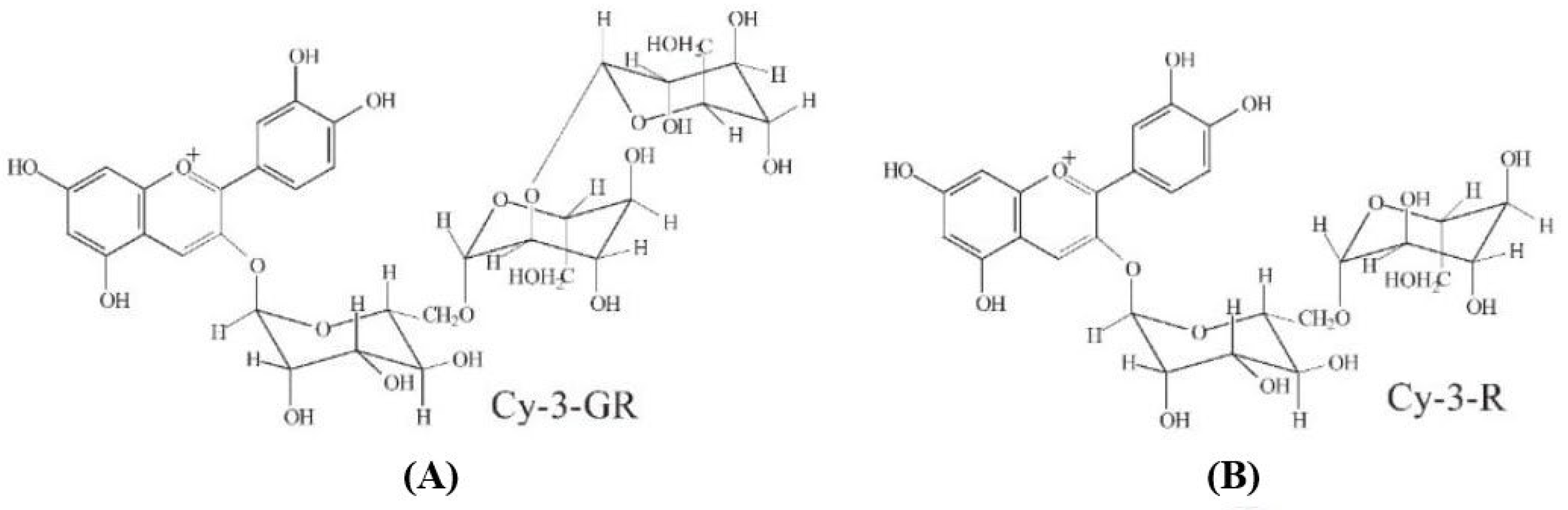
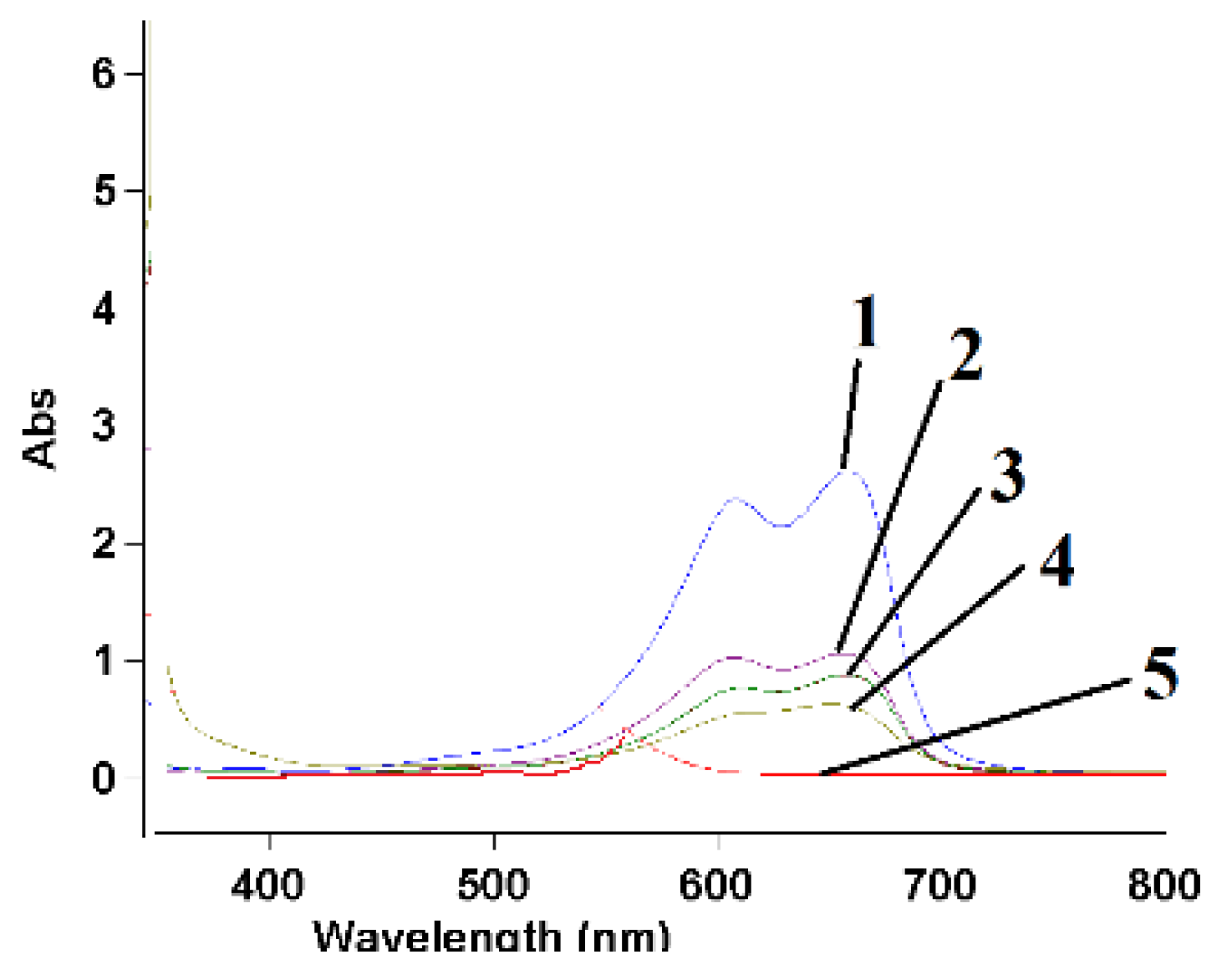


| Dyes | T [K] | Pseudo First-Order Kinetics Constant k1 [min−1] | Langmuir Model | Freundlich Model | ||
|---|---|---|---|---|---|---|
| KL [L/mg] | R2 | KF [mg−1/n L1/n g−1] | R2 | |||
| CR | 283 | 0.0173 | 0.93 | 0.996 | 3.17 | 0.912 |
| 291 | 0.0555 | 2.16 | 0.991 | 4.12 | 0.956 | |
| 308 | 0.317 | 3.12 | 0.998 | 5.4 | 0.974 | |
| MB | 283 | 0.078 | 0.85 | 0.895 | 2.76 | 0.889 |
| 291 | 0.08 | 1.96 | 0.981 | 3.94 | 0.954 | |
| 308 | 0.082 | 2.74 | 0.915 | 4.22 | 0.963 | |
| Dyes | T [°C] | KD |
|---|---|---|
| CR | 283 | 2.4·104 |
| MB | 283 | 3.5·103 |
| CR | 291 | 3.2·104 |
| MB | 291 | 1.5·104 |
| CR | 308 | 4.9·104 |
| MB | 308 | 1.9·104 |
| Dyes | ∆G0 [kJ/mol] | ∆H0283 [kJ/mol] | ∆S0283 [J/mol K] | ||
|---|---|---|---|---|---|
| 283 K | 291 K | 308 K | |||
| MB | −7.9 | −8.8 | −11 | −57 | 17.3 |
| CR | −5.9 | −8.8 | −11.16 | −65 | 20.8 |
| Salt | Content [g/kg] |
|---|---|
| NaCl | 23.476 |
| KCl | 0.664 |
| CaCl2 | 1.102 |
| MgCl2 | 4.981 |
| Na2SO4 | 3.917 |
| NaHCO3 | 0.192 |
| KBr | 0.096 |
| SrCl2 | 0.025 |
| NaF | 0.003 |
| H3BO3 | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharisov, B.; Kharissova, O.; Zhinzhilo, V.; Bryantseva, J.; Uflyand, I. Solid-Phase Extraction of Organic Dyes on Mixed-Ligand Zr(IV) Metal–Organic Framework. Appl. Sci. 2022, 12, 12219. https://doi.org/10.3390/app122312219
Kharisov B, Kharissova O, Zhinzhilo V, Bryantseva J, Uflyand I. Solid-Phase Extraction of Organic Dyes on Mixed-Ligand Zr(IV) Metal–Organic Framework. Applied Sciences. 2022; 12(23):12219. https://doi.org/10.3390/app122312219
Chicago/Turabian StyleKharisov, Boris, Oxana Kharissova, Vladimir Zhinzhilo, Julia Bryantseva, and Igor Uflyand. 2022. "Solid-Phase Extraction of Organic Dyes on Mixed-Ligand Zr(IV) Metal–Organic Framework" Applied Sciences 12, no. 23: 12219. https://doi.org/10.3390/app122312219
APA StyleKharisov, B., Kharissova, O., Zhinzhilo, V., Bryantseva, J., & Uflyand, I. (2022). Solid-Phase Extraction of Organic Dyes on Mixed-Ligand Zr(IV) Metal–Organic Framework. Applied Sciences, 12(23), 12219. https://doi.org/10.3390/app122312219







