Abstract
Electrochemical synthesis of metal-organic frameworks (MOFs) has proven to possess many environmental advantages over traditional synthesis methods such as reduced energy use and shorter reaction times. However, the use of toxic, fossil fuel derived solvents such as N,N-dimethylformamide (DMF) presents a challenge to the environmental credentials of this method that has yet to be dealt with. Here, we investigate bioderived solvents, CyreneTM and γ-valerolactone (GVL), as an alternative for the synthesis of a range of MOFs via the anodic deposition method. The obtained MOF materials are characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM) to confirm their identities and morphologies and for comparison with MOFs synthesized using the traditional DMF-based solvent systems. When using CyreneTM and GVL solvents, crystalline MOF materials were obtained of comparable quality to those afforded using DMF. However, in several cases, using CyreneTM or GVL led to the formation of less stable, higher porosity MOF structures than those obtained using DMF, indicating that the larger bio solvent molecules may also play a templating role during the synthesis. This study successfully demonstrates the first-time electrochemical synthesis of MOFs has been performed using bio solvents and has highlighted that the use of bio solvents can provide a route to obtaining lower density, higher porosity MOF phases than those obtained using traditional solvents.
1. Introduction
Over the years, numerous environmental issues have had a detrimental impact to humanity on a global scale. This includes ozone layer depletion, global warming and the exhaustion of natural resources, amongst others. Climate emergencies are now being declared in many countries [1] which illustrates the cruciality of change that needs to happen to tackle this crisis.
The chemical industry in particular plays a significant role in humanities’ contribution to climate change. The sector is responsible for 15% and 9% of total primary oil consumption and gas consumption, respectively [2], making it the largest industrial consumer of these fossil fuels. As a result, carbon dioxide emissions from the chemical industry continue to rise [2]; chemical companies need to address these consumption issues and governmental action is required. One strategy chemical companies could better adopt are the principles of green chemistry, which aim to create products and processes which do not harm the environment and human wellbeing [3]. Particularly key and relevant principles include less precarious chemical syntheses (Principle Three), safer solvents and auxiliaries (e.g., separating agents, Principle Five) and use of renewable feedstocks (Principle Seven) [4]. Solvents are ubiquitous in chemical processes and their use is often one of the major stumbling blocks to implementing green chemistry principles which motivates research into safer, cleaner and greener solvent alternatives such as bioderived solvents.
One type of material which requires the use of unsustainable solvents as part of its synthesis are metal-organic frameworks (MOFs) and particularly a subset known as zeolitic-imidazolate frameworks (ZIFs) [5]. MOFs are a pioneering type of highly ordered crystalline and porous material that have attracted much attention within scientific research in recent years. They consist of metal ions, or metal ion clusters, and heteroatom-containing organic molecules linked together via multiple coordination bonds to form complex architectures [6]. The discovery of their immensely practical properties such as a large distinct surface area [7], variable morphologies [8] and tunable structures [7] has opened an abundance of potential applications, many of which are perhaps yet to be discovered. Current functions of MOFs that are also under study include natural gas storage [9], drug delivery [10] and chromatography [11].
The traditional method of MOF production is solvothermal synthesis [12]. Solvothermal synthesis of MOFs utilizes solvents like N,N-dimethylformamide (DMF), extremely high temperatures and high pressures [13]; all extremely unattractive from a green chemistry perspective. New methods for MOF synthesis that address some of these problems have been developed such as microwave-assisted synthesis [14] and mechanochemical synthesis [15], but problems remain with regard to the low yields that such methods achieve [15]. Electrochemical MOF synthesis is another novel synthetic route for MOFs that does not suffer from the same issues around low yield, which can proceed via either a cathodic or anodic mechanism [16,17]. The anodic method is particularly interesting from a green chemistry perspective as only the organic linker reactant is dissolved into the solvent, compared to a more complex cocktail of chemicals required for the cathodic method [17].
Electrochemical MOF synthesis offers significant green chemistry related advantages compared to alternative synthesis routes. The process is continuous and requires shorter reaction times lower temperatures and reduced pressures, meaning less energy input is needed. However, toxic [18,19] and fossil fuel derived [20] solvents such as DMF are still currently required. Replacing these with more benign, bio-derived solvents would therefore be a significant step in improving the green credentials of this synthesis method.
Very little research has been conducted to combine electrochemical synthesis and bio-derived solvents, and none focusing on the electrochemical synthesis of MOFs. Despite this, some promising early work describes the use of such solvents in traditional solvothermal MOF synthesis. Dihydrolevoglucosenone (commercially known as CyreneTM) is a bio-derived solvent made from waste cellulose [21,22] which possesses similar properties to that of DMF and has previously proven effective as a solvent for the solvothermal synthesis of a variety of MOFs [23,24]. γ-Valerolactone (GVL) has also shown similar promise, particularly for the synthesis of ZIFs [25,26].
In this work we demonstrate the feasibility of replacing the traditional, toxic, fossil fuel derived solvents in electrochemical MOF synthesis with safer, greener and cleaner alternatives. Specifically, we utilize GVL and CyreneTM to electrochemically synthesize a wide range of MOFs with either Cu, Zn or Co as the metal component and either 1,3,5-benzenetricarboxylic acid (BTC), imidazole (IM), benzimidazole (bIM), 2-methylimidazole (mIM) or 2-ethylimidazole (eIM) as the organic linker component. The MOFs formed are characterized using X-ray diffraction (XRD) to confirm their identity and scanning electron microscopy (SEM) to compare their morphology and crystal quality. Excitingly, we also demonstrate that less stable, higher porosity MOF structures are obtained when utilizing these novel, bio-derived solvents instead of DMF, indicating that the larger bio solvent molecules may also play a templating role during the synthesis.
2. Materials and Methods
2.1. Materials
All materials were used as received for the following experiments. Cu sheet (99.98+%) was obtained from Advent Research Materials. Co foil (99.9%) and CyreneTM were obtained from Sigma Aldrich. Zn foil (99.9%), BTC (98%), IM (99%), bIM (98%), mIM (99%, eIM (99%), Ethanol (HPLC grade), DMF (HPLC grade), Water (HPLC grade) and GVL (98%) were obtained from Fisher Scientific. Tributylmethylammonium methyl sulphate (TBMAMS) (>95%) was obtained from Santa Cruz Biotechnology.
2.2. MOF Coating Synthesis
MOF coatings were synthesized electrochemically via anodic deposition. Two metal electrodes (each with a geometric area of ~4 cm2) were held around 1 cm apart and partially immersed into a heated, nitrogen de-aerated 0.064 mol dm−3 TBMAMS solution prepared in a solvent mixture, which acts as the electrolyte. A Metrohm Autolab PGSTAT204 potentiostat supplied a constant potential difference of 2.5 V between the metal anode and the cathode for a fixed amount of time, thus generating a MOF coating around the anode. Table A1 summarizes the range of conditions used to form the MOFs using traditional solvents (ethanol or DMF) [6], Table 1 and Table 2 show the range of conditions used to form the coatings utilizing the novel bio-derived solvents CyreneTM and GVL, respectively. These conditions were selected in each case after a rigorous optimization process assessing linker concentration, ratio of organic solvent to water, reaction temperature and reaction duration to optimize the quality of the MOF obtained. The coatings were rinsed with ethanol to remove any remaining unreacted linker and electrolyte solution before analysis.

Table 1.
Summary of the conditions used to produce a range of MOF coatings using CyreneTM.

Table 2.
Summary of the conditions used to produce a range of MOF coatings using GVL.
2.3. Characterisation of MOF Coatings
The identities and crystallographic information of the MOF coatings were obtained via X-Ray Diffraction (XRD) using a Rigaku Miniflex X-ray diffractometer. The XRD patterns were acquired from the coatings formed on top of the anode surface using Cu radiation at 40 kV and 15 mA in the range 3–35 2θ° (with a step size of 0.0025 2θ° and scan step time of 2.0 s) whilst spinning at 10 revolutions per minute. Data were backgrounded using the inbuilt background removal tool within the SmartLab Studio software to remove the influence of the underlying metal electrode on the diffraction patterns. Predicted peak positions for different phases were obtained based on literature crystallographic information files using SmartLab Studio software. The morphology of the MOF coatings were characterized on the anode surface using a Thermo Scientific Phenom Pro Desktop Scanning Electron Microscope (SEM). All images were obtained at 10 kV under a reduced vacuum using the charge reduction sample holder. The back-scattered electron detector was utilized.
3. Results
3.1. Synthesis with Traditional Solvents
Using traditional solvents, the XRD patterns (Figure A1) show that the MOF coatings obtained were HKUST-1 for Cu and BTC, ZIF-8 for Zn and mIM, ZIF-7 for Zn and bIM in DMF, ZIF-67 for Co and mIM, ZIF-14 for Zn and eIM and ZIF-zni for Zn and IM. These are the expected structures based on prior literature and are confirmed by comparison of the patterns to the predicted peak positions generated from the crystallographic information files [27,28]. It is worth noting that some peaks in the pattern for the MOF obtained from Zn and eIM (Figure A1E) do not correspond to ZIF-14 and instead correspond to an alternative, higher porosity phase with a rhombohedral RHO structure [29].
The SEM images (Figure A2) support the identification of the MOFs with the coatings observed to either be composed of crystals of the expected morphology (e.g., cubic octahedral in the case of HKUST-1) or of crystals too small for the shape to be accurately determined. It is worth noting that in the case of ZIF-14, crystals with two distinct morphologies (icositetrahedral on the one hand and plate like on the other) can be observed (Figure A2E). This corroborates the observations from the XRD where peaks corresponding to two different crystal phases can be observed.
3.2. Synthesis with CyreneTM
Using the synthesis conditions described in the previous literature for use with traditional solvents as a starting point [6], the quality of the crystalline MOF coatings obtained when using CyreneTM as the solvent were optimized by varying linker concentration, solvent ratios, reaction temperature and reaction duration. The conditions detailed in Table 1 are the result of this optimization process.
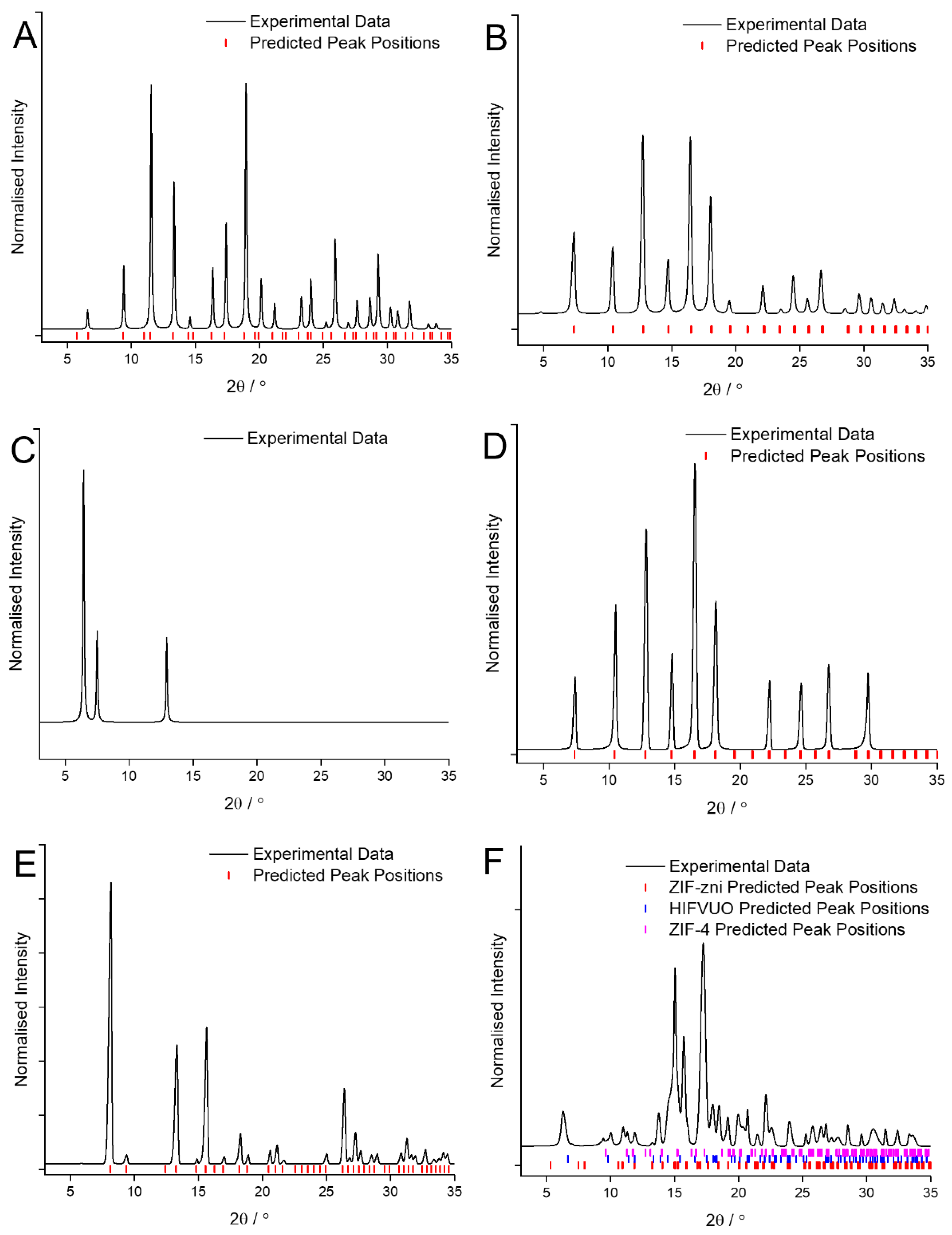
The XRD patterns of the MOF coatings obtained when using CyreneTM for Cu and BTC, Zn and mIM, Zn and bIM, Co and mIM, Zn and eIM and Zn and IM are shown in Figure 1. In three cases, Cu and BTC, Zn and mIM and Co and mIM, the crystalline MOF coatings were the same phase as obtained using traditional solvents as confirmed by comparison with the predicted peak positions [27,28]. It is worth noting that the pattern obtained, of HKUST-1, ZIF-8 and ZIF-67, respectively, displayed comparatively low peak intensities at low angle (Figure 1A,B,D) in comparison to that obtained with the traditional solvents (Figure A1A,B,D). This is attributed to difficulties encountered with background correcting the data caused by the greater influence of the underlying metal electrode on some of the samples.
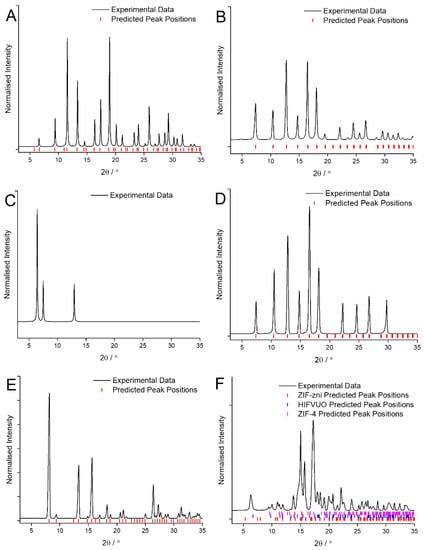
Figure 1.
XRD patterns of the MOF coatings obtained using CyreneTM as the solvent for: (A) Cu and BTC; (B) Zn and mIM; (C) Zn and bIM; (D) Co and mIM; (E) Zn and eIM; (F) Zn and IM compared against predicted peak position values [27,28,30,31].
In the other three cases the use of CyreneTM as the solvent has led to significant changes in the phase of MOF obtained as the coating. In the case of Zn and eIM (Figure 1E) the presence of the bio-solvent has led to the formation of a purer coating composed entirely of ZIF-14 with no trace of the RHO phase previously observed detected [27,28]. Conversely with Zn and IM (Figure 1F) changing the solvent has led to the formation of a coating that appears to be composed of a mixture of three different phases [27,28,30]. This ability to apparently select which phase is formed simply as a function of the solvent used is of interest as much research has been devoted the use of additives for achieving this [31]. Zn and bIM also form a completely different phase than expected in the presence of CyreneTM (Figure 1C) but unfortunately due in part to the small amount of material obtained identification of this material has not yet been possible.
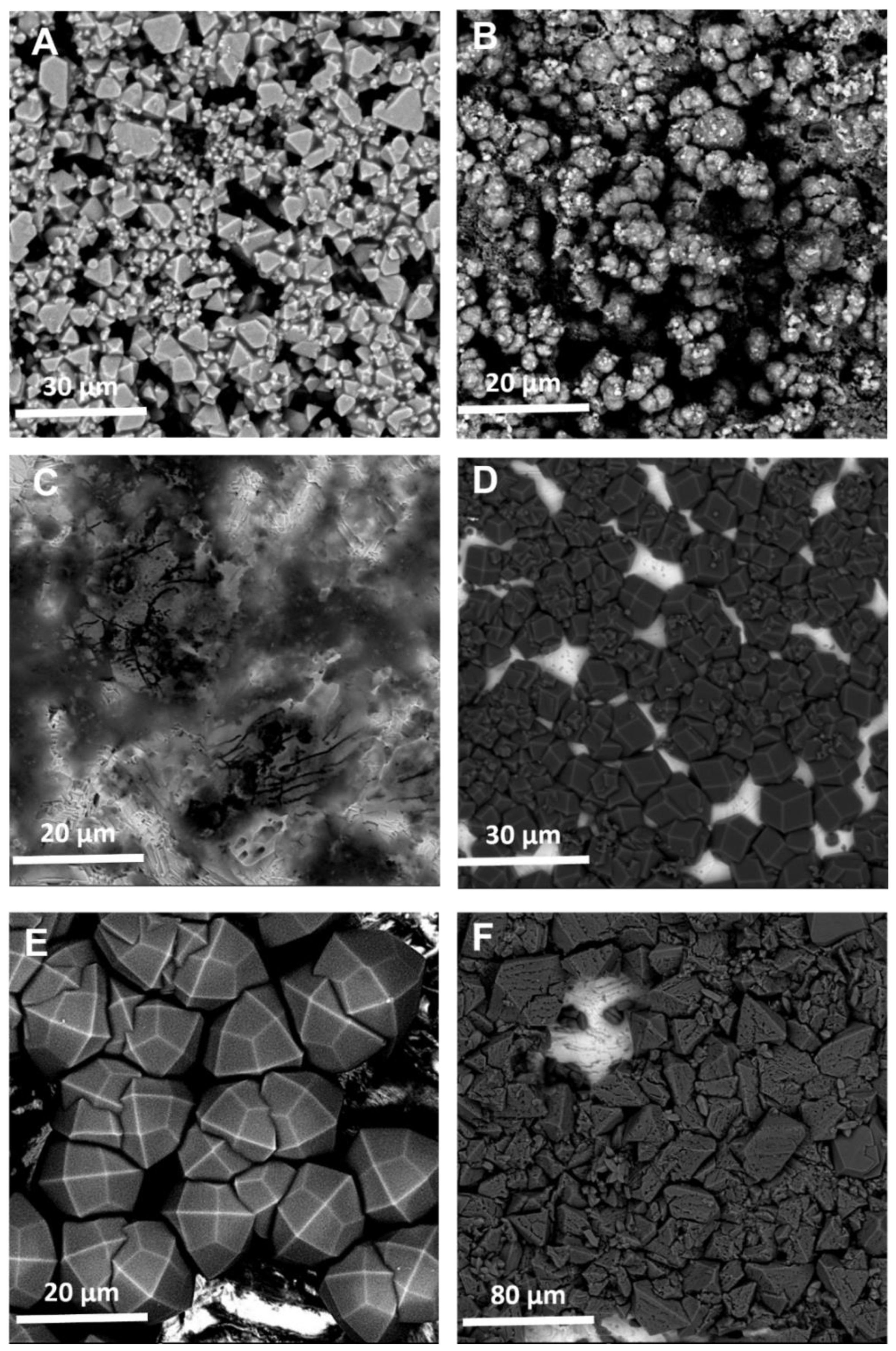
The SEM images of these coatings (Figure 2) corroborate much of what was observed in the XRD data. The images generally show sparser coatings, with more bare metal electrode visible, which explains the issue with backgrounding and Figure 2C clearly shows little material was synthesized in the case of Zn and bIM. It is also clear that the crystalline quality of the coating is significantly improved when using CyreneTM, with this particularly apparent when comparing Figure 2D–F to Figure A2D–F.
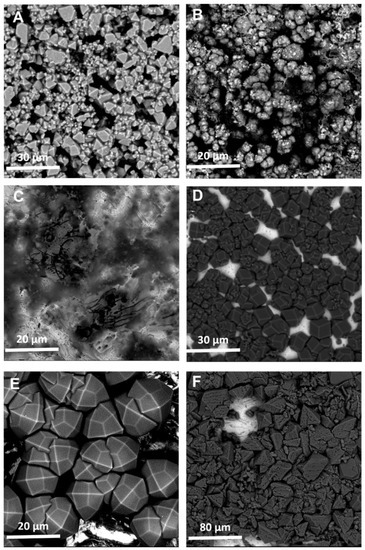
Figure 2.
SEM images of the MOF coatings using CyreneTM as the solvent for: (A) Cu and BTC; (B) Zn and mIM; (C) Zn and bIM; (D) Co and mIM; (E) Zn and eIM; (F) Zn and IM.
3.3. Synthesis with GVL
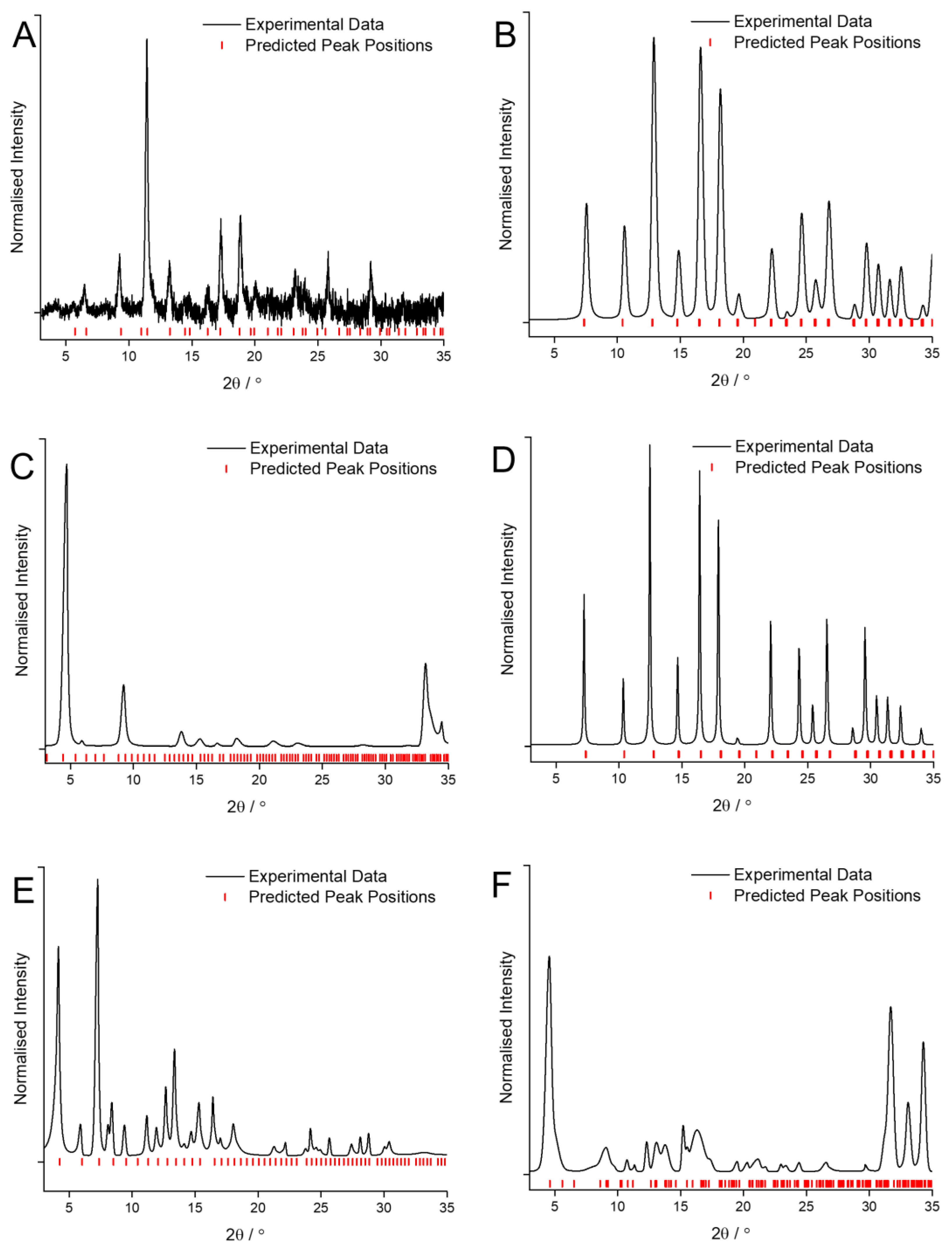
Using the synthesis conditions described in the previous literature for use with traditional solvents as a starting point [6], the quality of the crystalline MOF coatings obtained when using GVL as the solvent were optimized by varying linker concentration, solvent ratios, reaction temperature and reaction duration. The conditions detailed in Table 2 are the result of this optimization process. XRD patterns of the MOF coatings obtained when using GVL for Cu and BTC, Zn and mIM, Zn and bIM, Co and mIM, Zn and eIM and Zn and IM are shown in Figure 3.
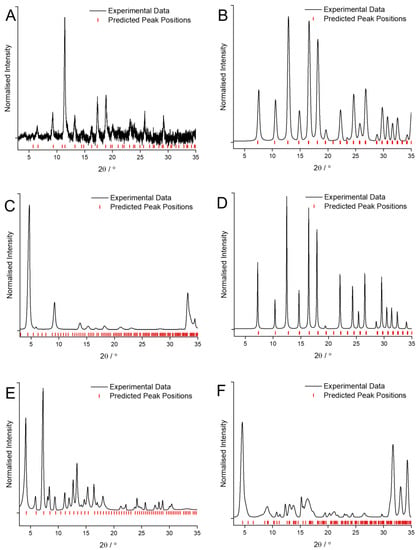
Figure 3.
XRD patterns of the MOF coatings obtained using GVL as the solvent for: (A) Cu and BTC; (B) Zn and mIM; (C) Zn and bIM; (D) Co and mIM; (E) Zn and eIM; (F) Zn and IM compared against predicted peak position values [27,28,31,32,33].
Similarly to the case when CyreneTM was used, replacing the traditional solvents with GVL results in no change to the phase being formed when reacting Cu with BTC (Figure 3A), Zn with mIM (Figure 3B) and Co with mIM (Figure 3D). The patterns are clearly of HKUST-1, ZIF-8 and ZIF-67, respectively [27,28] however it is noticeable that the signal to noise ratio for HKUST-1 (Figure 3A) is much lower than previously and that the peaks for ZIF-67 (Figure 3D) are much narrower than those for ZIF-8 (Figure 3B).
More interestingly, again just like when utilising CyreneTM, the use of GVL as a solvent has led to the formation of three entirely different MOF phases when reacting Zn with bIM (Figure 3C), Zn with eIM (Figure 3E) and Zn with IM (Figure 3F). In the case of Zn and bIM rather than forming the SOD phase ZIF-7, the RHO phase ZIF-11 has been obtained with high purity [31]. Reacting Zn and eIM in GVL has led to the formation of the RHO phase, previously observed as minority product when using traditional solvents, almost exclusively [32]. Finally, the use of GVL as a solvent has caused Zn and IM to form high purity ZIF-10, as opposed to any of the other phases observed when using either the traditional solvents or CyreneTM [33]. In each of these three cases using GVL as a solvent has led to the formation of a higher porosity, lower stability MOF phase than would otherwise have been obtained.
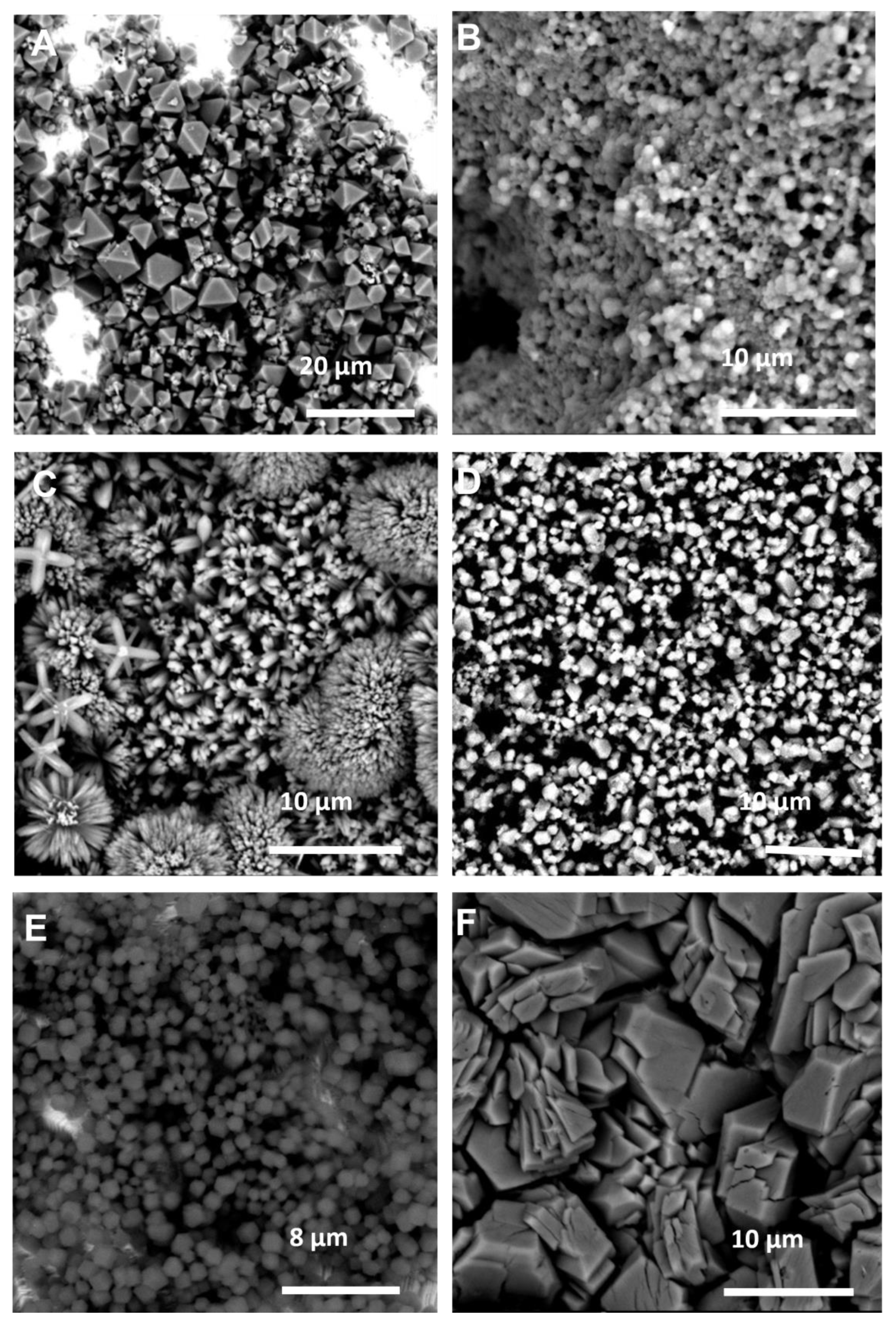
The SEM images of these coatings (Figure 4) corroborate much of what was observed in the XRD data. The HKUST-1 coating (Figure 4A) is very thin, with areas of bare Cu metal clearly observable, which explains the low signal to noise ratio observed in the XRD (Figure 3A), and the observed crystal morphologies in Figure 4C, E and F are completely different to those observed when using the same reactants with CyreneTM as the solvent (Figure 2C,E,F). The use of GVL has also seemingly led to an improvement in the overall crystal quality, indeed all six products in this case exhibit clearly defined crystals with discernable morphologies.
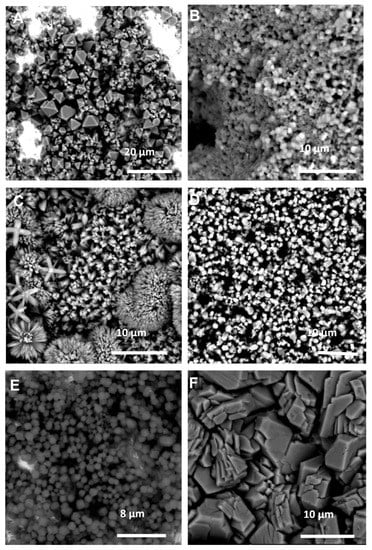
Figure 4.
SEM images of the MOF coatings using GVL as the solvent for: (A) Cu and BTC; (B) Zn and mIM; (C) Zn and bIM; (D) Co and mIM; (E) Zn and eIM; (F) Zn and IM.
4. Discussion
Overall, it is clear from these results that the replacement of traditional solvents with bio-derived solvents such as CyreneTM and GVL for the electrochemical synthesis of MOFs is absolutely viable. Across a range of metals and with a variety of linkers, crystalline MOF materials are obtained when using these solvents that are broadly of a higher crystallinity, as verified by the larger crystal sizes and more clearly discernible morphologies observed in the SEM analysis. The MOF phases obtained by using these new solvents are either the same as those obtained using traditional methods or, more excitingly, are actually higher porosity phases suggesting that the bio-derived solvent molecules are playing a templating role during the MOF synthesis. This opens the possibility of simply using the choice of bio-derived solvent for a reaction as way of obtained the desired MOF phase, with a good example being the reaction of Zn and eIM. Whilst using traditional solvents results in an impure mixture of two phases, using a bioderived solvent allows either phase to be selected for and formed with high purity. Furthermore, during optimization of the synthetic conditions for the Co and mIM reaction in both CyreneTM and GVL it was noted that, whilst ZIF-67 was produced across the electrode surface during the reaction, the greatest quantity and quality of material was formed towards the top of the electrode at the solid/liquid/gas interface as opposed to at the purely solid/liquid interface. To the authors knowledge, this has not been observed previously in electrochemical synthesis and investigations are underway both into the mechanism of this modified version of the anodic dissolution synthesis and its potential.
5. Conclusions
The viability of replacing toxic, fossil fuel derived solvents such as DMF with bioderived solvents such as CyreneTM and GVL for the electrochemical synthesis of MOFs has been clearly demonstrated, with the MOF materials obtained generally of a higher crystallinity as demonstrated by increasing particle sizes and clearly discernable morphologies. The use of bioderived solvents also provides a facile method for MOF phase selection during synthesis, with both more desirable higher porosity phases and importantly control over which phase is formed readily achieved. Evidence of an improved methodology for obtaining Co MOF coatings has also been obtained which, if realized, could lead to improved electrodes for MOF based supercapacitor devices.
Author Contributions
Conceptualization, S.D.W.; methodology, S.D.W.; formal analysis, M.B., L.M.; investigation, M.B., L.M.; data curation, M.B., L.M.; writing—original draft preparation, M.B.; writing—review and editing, M.B., L.M., J.H., M.J.D., S.D.W.; supervision, M.J.D., S.D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw X-ray diffraction and scanning electron microscopy data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
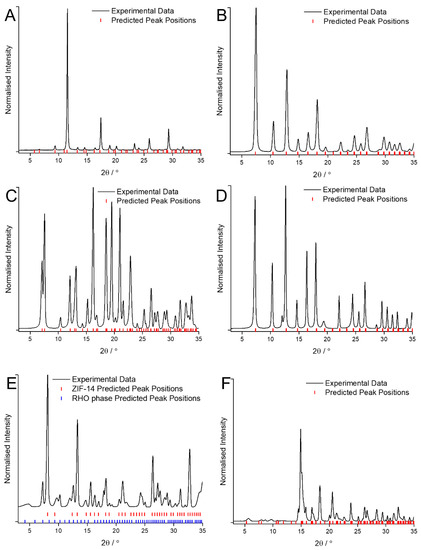
Figure A1.
XRD patterns of the MOF coatings obtained using traditional solvents confirming their identities as: (A) HKUST-1; (B) ZIF-8; (C) ZIF-7; (D) ZIF-67; (E) ZIF-14; (F) ZIF-zni; compared against predicted peak position values [27,28].
Figure A1.
XRD patterns of the MOF coatings obtained using traditional solvents confirming their identities as: (A) HKUST-1; (B) ZIF-8; (C) ZIF-7; (D) ZIF-67; (E) ZIF-14; (F) ZIF-zni; compared against predicted peak position values [27,28].
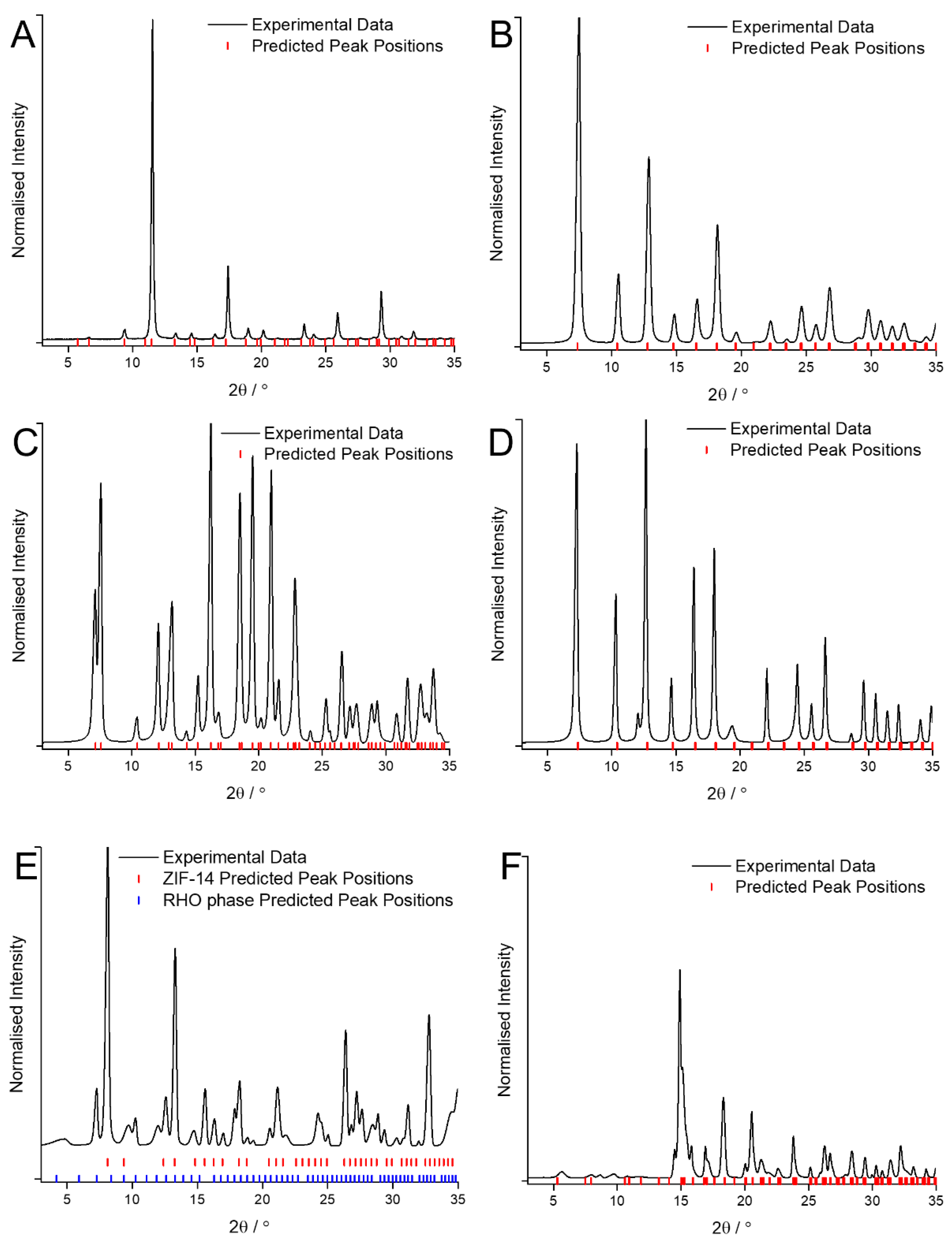
Figure A2.
SEM images of the MOF coatings obtained using traditional solvents demonstrating the expected morphologies for: (A) HKUST-1; (B) ZIF-8; (C) ZIF-7; (D) ZIF-67; (E) ZIF-14; (F) ZIF-zni.
Figure A2.
SEM images of the MOF coatings obtained using traditional solvents demonstrating the expected morphologies for: (A) HKUST-1; (B) ZIF-8; (C) ZIF-7; (D) ZIF-67; (E) ZIF-14; (F) ZIF-zni.

Table A1.
Summary of the conditions used to produce a range of MOF coatings using traditional solvents [6].
Table A1.
Summary of the conditions used to produce a range of MOF coatings using traditional solvents [6].
| Metal Electrode | Linker (mol dm−3) | Solvent | Vol % Organic Solvent/ H2O | Temperature (°C) | Time (min) |
|---|---|---|---|---|---|
| Cu | BTC 0.048 | Ethanol | 50/50 | 55 | 60 |
| Zn | IM 0.30 | DMF | 25/75 | 85 | 60 |
| Zn | bIM 0.52 | DMF | 100/0 | 55 | 120 |
| Zn | mIM 3.00 | N/A | 0/100 | 55 | 60 |
| Zn | eIM 0.20 | DMF | 28/72 | 85 | 60 |
| Co | mIM 0.24 | DMF | 75/25 | 100 | 210 |
References
- Manfredi, C.; Nail, S. Climate and environmental emergency: A case for a humanities approach. E-Rea 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Levi, P.; Vass, T.; Mandová, H.; Gouy, A. Chemicals: Tracking Report. IEA. 2020. Available online: https://www.iea.org/reports/chemicals (accessed on 4 August 2022).
- Lewandowski, T.A. Green Chemistry. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 798–799. [Google Scholar]
- El-Din, H.; Saleh, H.; Koller, M. Introductory Chapter. In Principles of Green Chemistry, 1st ed.; Saleh, H., Ed.; IntechOpen Limited: London, UK, 2018; pp. 1–26. [Google Scholar]
- Biswal, B.; Panda, T.; Banerjee, R. Solution mediated phase transformation (RHO to SOD) in porous imidazolate based zeolitic frameworks with high water stability. Chem. Commun. 2012, 48, 11868–11870. [Google Scholar] [CrossRef] [PubMed]
- Worrall, S.; Mann, H.; Rogers, A.; Bissett, M.; Attfield, M.; Dryfe, R. Electrochemical deposition of zeolitic imidazolate framework electrode coatings for supercapacitor electrodes. Electrochim. Acta 2016, 197, 228–240. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.; Skinner, W.; Wang, Z.; Jiang, H. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Mason, J.; Veenstra, M.; Long, J. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, G.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Han, S.B.; Wei, Y.H.; Valenta, C.; Lagzi, I.; Gassensmith, J.J.; Coskun, A.; Stoddart, J.F.; Grzybowski, B.A. Chromatography in a Single Metal-Organic Framework (MOF) Crystal. J. Am. Chem. Soc. 2010, 132, 16358–16361. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.; Imaz, I.; Maspoch, D.; Hill, M. New production routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Butova, V.V.; Soltadov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 287. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.C. Recent progress in the synthesis of metal-organic frameworks. Sci. Tech. Adv. Mater. 2015, 16, 4–8. [Google Scholar] [CrossRef]
- Preicel, P.; Lopez-Sanchez, J. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorisation of Biobased Chemicals. ACS Sus. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Campagnol, N.; van Assche, T.; Li, M.; Stappers, L.; Dinca, M.; Denayer, J.; Vos, D.; Fransaer, J. On the electrochemical deposition of metal-organic frameworks. J. Mater. Chem. A 2016, 10, 3914. [Google Scholar] [CrossRef]
- Xie, S.; Monnens, W.; Wan, K.; Zhang, W.; Guo, W.; Xu, M.; Vankelecom, I.; Zhang, X.; Fransaer, J. Cathodic Electrodeposition of MOF Films Using Hydrogen Peroxide. Angew. Chem. Int. Ed. 2021, 60, 24950–24957. [Google Scholar] [CrossRef]
- Jyothi, K.; Kalyani, D.; Nachiappan, V. Effect of acute exposure of N,N-dimethylformamide, an industrial solvent on lipid peroxidation and antioxidants in liver and kidney of rats. Ind. J. Biochem. Biphys. 2012, 49, 279–284. [Google Scholar]
- Luo, J.C.; Cheng, T.J.; Kuo, H.W. Abnormal liver function associated with occupational exposure to dimethylformamide and glutathione S-transferase polymorphisms. Biomarkers 2005, 10, 464–474. [Google Scholar] [CrossRef]
- Weissermel, K.; Arpe, H.J. Industrial Organic Chemistry: Important Raw Materials and Intermediates, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 45–46. [Google Scholar]
- Sherwood, J.; de Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.; Farmer, T.; Duncan, T.; Raverty, W.; Hunt, A.; Clark, J. Diydrolevoglucosenone (CyreneTM) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Oklu, N.; Matsinha, L.; Makhubela, B. Biosolvents: Synthesis, Industrial Production and Applications. In Solvents, Ionic Liquids and Solvent Effects, 1st ed.; Glossman-Mitnik, D., Maciejewska, M., Eds.; IntechOpen Limited: London, UK, 2019; p. 7. [Google Scholar]
- Zhang, J.; White, G.; Ryan, M.; Hunt, A.; Katz, M. Dihydrolevoglucosenone (CyreneTM) As a Green Alternative to N’N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sust. Chem. Eng. 2016, 4, 7186–7192. [Google Scholar] [CrossRef]
- Skrjanc, A.; Byrne, C.; Logar, N. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules 2021, 26, 1573. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Strappaveccia, G.; Luciani, L.; Bartollini, E.; Marrocchi, A.; Pizzo, F.; Vaccaro, L. γ-Valerolactone as an alternative biomass-derived medium for the Sonogashira reaction. Green Chem. 2015, 17, 1071–1076. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.; Uribe-Romo, F.; Knobler, C.; O’Keefe, M.; Yaghi, O. Synthesis, Structure and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Ahmed, A.; Hodgson, N.; Barrow, M.; Clowes, R.; Robertson, C.; Steiner, A.; McKeown, P.; Bradshaw, D.; Myers, P.; Zhang, H. Macroporous metal-organic framework microparticles with improved liquid phase separation. J. Mater. Chem. A 2014, 2, 9085–9090. [Google Scholar] [CrossRef]
- Huang, X.; Lin, Y.; Zhang, J.; Chen, X. Ligand-Directed Strategy for Zeolite-Type Metal–Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Chen, Z.; Zhang, G.; Weng, L.; Zhao, D. Design and Generation of Extended Zeolitic Metal–Organic Frameworks (ZMOFs): Synthesis and Crystal Structures of Zinc(II) Imidazolate Polymers with Zeolitic Topologies. Chem. Eur. J. 2007, 13, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yao, J. Toluene-assisted synthesis of RHO-type zeolitic imidazolate frameworks: Synthesis and formation mechanism of ZIF-11 and ZIF-12. Dalton Trans. 2013, 42, 16608–16613. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Diaz, A.; Villarroel-Rocha, J.; Ting, V.; Bimbo, N. Flexible ZIFs- probing guest-induced flexibility with CO2, N2 and Ar adsorption. J. Chem. Toxicol. Biotech. 2019, 94, 3787–3792. [Google Scholar] [CrossRef]
- Park, K.; Ni, Z.; Côté, A.; Choi, J.; Huang, R.; Uribe-Romo, F.; Chae, H.; O’Keefe, M.; Yaghi, O. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).