Dietary Supplemented Anthocyanin Reduced Serum Amyloid Beta Oligomers and Improved Cognitive Dysfunction Scores in Elderly Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Signalment
2.2. Information on Cognitive Dysfunction Preventive Pet Food and Animal Maintenance
2.3. General Blood Test
2.4. CDS
2.5. Serum Anti-Inflammatory and Antioxidative Indexes
2.6. Serum AβO
2.7. Statistical Analysis
3. Results
3.1. Physical Examination
3.2. Complete Blood Count (CBC), Chemistry, and Electrolyte
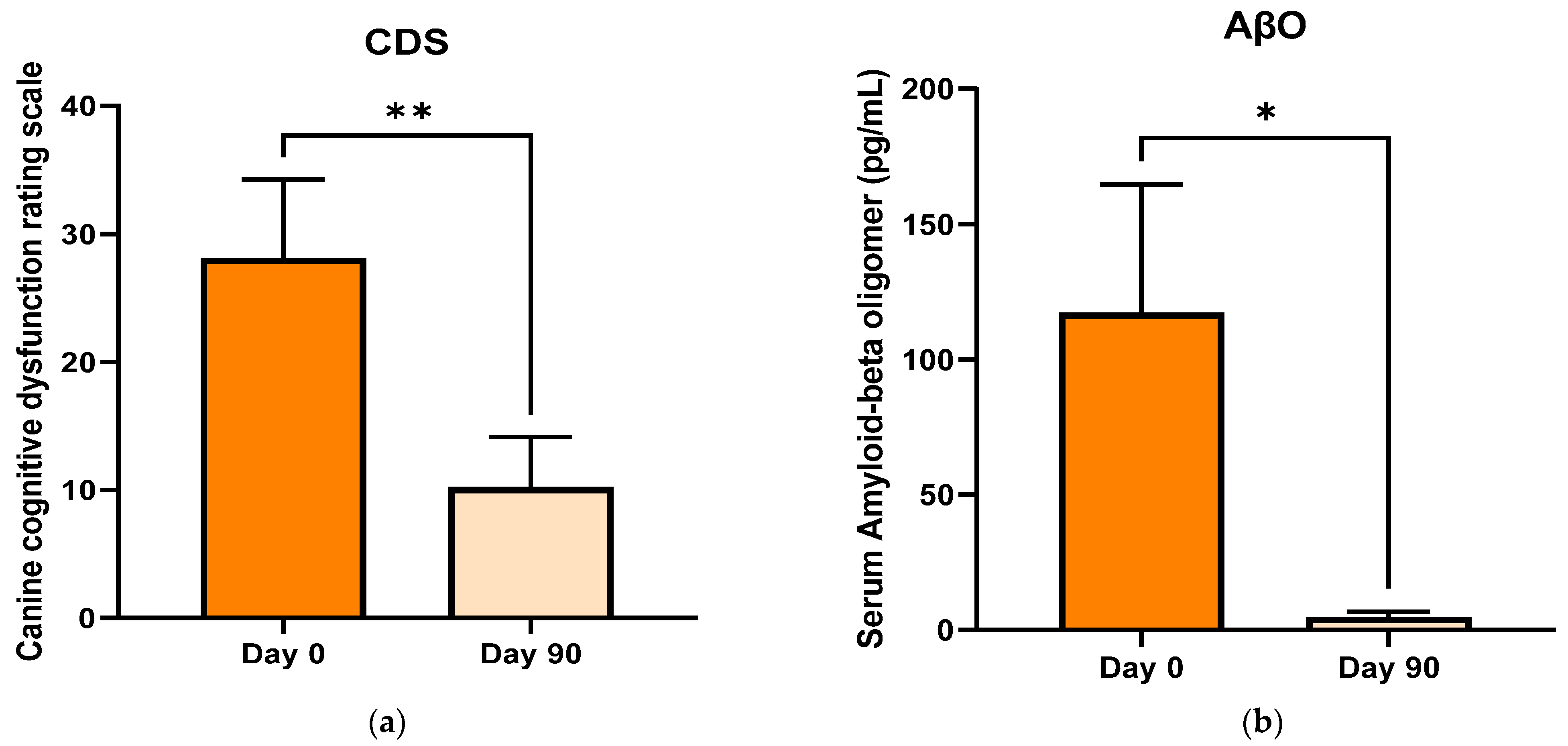
3.3. Cognitive Dysfunction Score (CDS)
3.4. Serum Amyloid-Beta Oligomers (AβO)
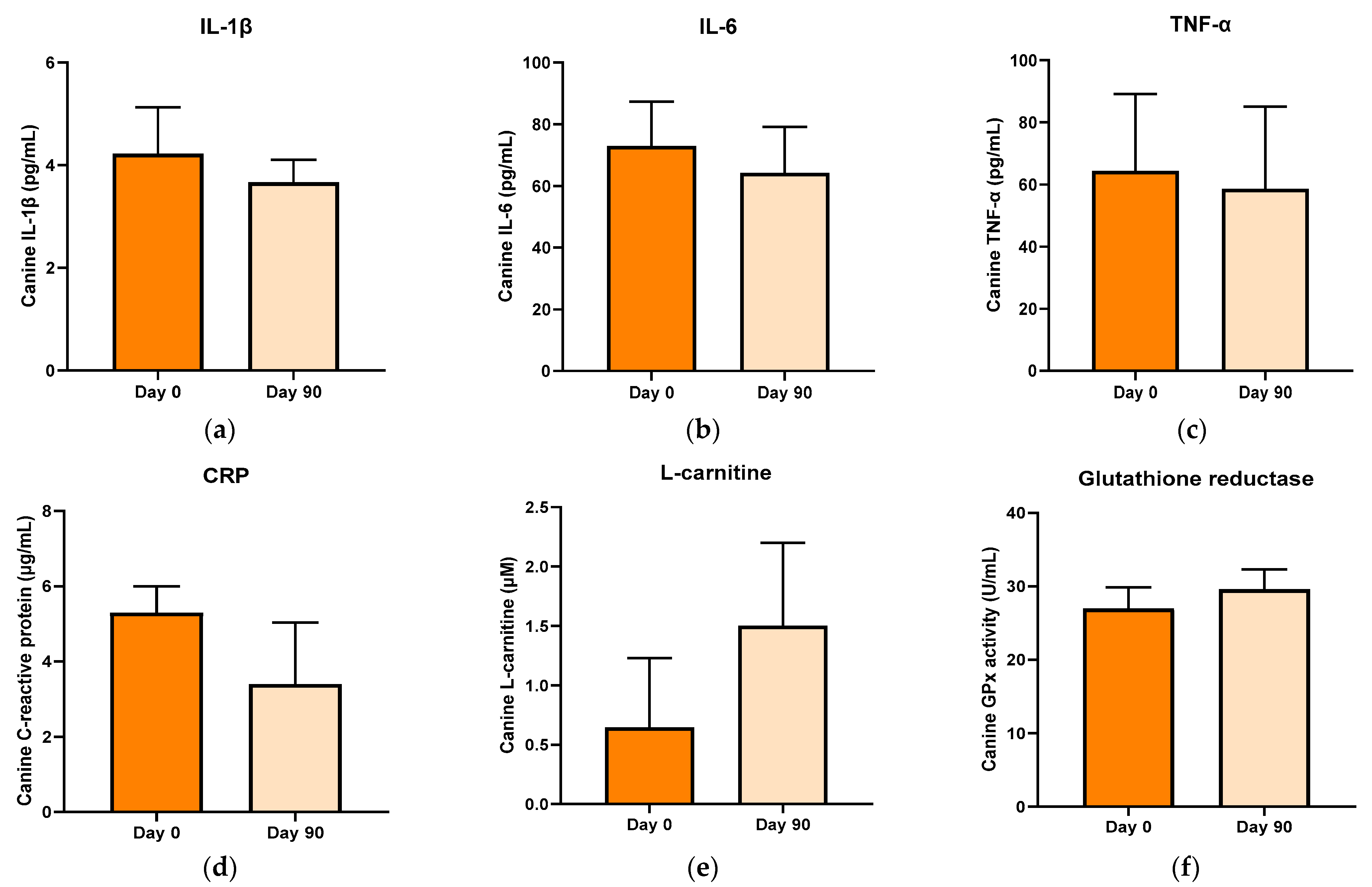
3.5. Anti-Inflammatory and Antioxidative Indices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? eLife 2021, 10, e62852. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.E.; Warr, P. Age and working memory: The role of perceptual speed, the central executive, and the phonological loop. Psychol. Aging 1996, 11, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.O.; Cooper, S.A.; Salvador-Carulla, L. Intelligence and specific cognitive functions in intellectual disability: Implications for assessment and classification. Curr. Opin. Psychiatry 2018, 31, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.; Singh-Manoux, A.; Batty, G.D.; Ebmeier, K.P.; Jokela, M.; Harmer, C.J.; Kivimäki, M. The level of cognitive function and recognition of emotions in older adults. PLoS ONE 2017, 12, e0185513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Fast, R.; Schütt, T.; Toft, N.; Møller, A.; Berendt, M. An observational study with long-term follow-up of canine cognitive dysfunction: Clinical characteristics, survival, and risk factors. J. Vet. Intern. Med. 2013, 27, 822–829. [Google Scholar] [CrossRef]
- Cummings, S.R.; Black, D.M.; Nevitt, M.C.; Browner, W.; Cauley, J.; Ensrud, K.; Genant, H.K.; Palermo, L.; Scott, J.; Vogt, T.M. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993, 341, 72–75. [Google Scholar] [CrossRef]
- Cummings, B.J.; Cotman, C.W. Image analysis of beta-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet 1995, 346, 1524–1528. [Google Scholar] [CrossRef]
- Dodart, J.C.; Bales, K.R.; Gannon, K.S.; Greene, S.J.; DeMattos, R.B.; Mathis, C.; DeLong, C.A.; Wu, S.; Wu, X.; Holtzman, D.M.; et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat. Neurosci. 2002, 5, 452–457. [Google Scholar] [CrossRef]
- Ozawa, M.; Chambers, J.K.; Uchida, K.; Nakayama, H. The Relation between canine cognitive dysfunction and age-related brain lesions. J. Vet. Med. Sci. 2016, 78, 997–1006. [Google Scholar] [CrossRef]
- Schütt, T.; Toft, N.; Berendt, M. Cognitive Function, Progression of Age-related Behavioral Changes, Biomarkers, and Survival in Dogs More Than 8 Years Old. J. Vet. Intern. Med. 2015, 29, 1569–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.G.; Lee, H.; Lee, S.B. Mechanisms of protein toxicity in neurodegenerative diseases. Cell. Mol. Life Sci. 2018, 75, 3159–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef] [PubMed]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Bonyadi, N.; Dolatkhah, N.; Salekzamani, Y.; Hashemian, M. Effect of berry-based supplements and foods on cognitive function: A systematic review. Sci. Rep. 2022, 12, 3239. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Men, J.; Fu, H.; Xu, T. The preventive efficacy of vitamin B supplements on the cognitive decline of elderly adults: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 367. [Google Scholar] [CrossRef]
- Farina, N.; Llewellyn, D.; Isaac, M.G.; Tabet, N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 1, CD002854. [Google Scholar] [CrossRef]
- Malouf, M.; Grimley, E.J.; Areosa, S.A. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst. Rev. 2003, CD004514. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef]
- Meng, Q.; Yin, H.; Wang, S.; Shang, B.; Meng, X.; Yan, M.; Li, G.; Chu, J.; Chen, L. The effect of combined cognitive intervention and physical exercise on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 261–276. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-O-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a Therapeutic Diet on Dogs With Signs of Cognitive Dysfunction Syndrome (CDS): A Prospective Double Blinded Placebo Controlled Clinical Study. Front. Nutr. 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. Jama 2015, 313, 1924–1938. [Google Scholar] [CrossRef] [Green Version]
- Rijal Upadhaya, A.; Kosterin, I.; Kumar, S.; von Arnim, C.A.; Yamaguchi, H.; Fändrich, M.; Walter, J.; Thal, D.R. Biochemical stages of amyloid-β peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain A J. Neurol. 2014, 137, 887–903. [Google Scholar] [CrossRef]
- Viola, K.L.; Bicca, M.A.; Bebenek, A.M.; Kranz, D.L.; Nandwana, V.; Waters, E.A.; Haney, C.R.; Lee, M.; Gupta, A.; Brahmbhatt, Z.; et al. The Therapeutic and Diagnostic Potential of Amyloid β Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front. Neurosci. 2022, 15, 1798. [Google Scholar] [CrossRef]
- McGeer, P.L.; Guo, J.P.; Lee, M.; Kennedy, K.; McGeer, E.G. Alzheimer’s Disease Can Be Spared by Nonsteroidal Anti-Inflammatory Drugs. J. Alzheimer’s Dis. JAD 2018, 62, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical antioxidants modulate mammalian cellular epigenome: Implications in health and disease. Antioxid. Redox Signal. 2012, 17, 327–339. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
| Nutrition | Per Pet Food 100 g |
|---|---|
| Protein | 24.43 g |
| Fat | 13.53 g |
| Carbohydrate (NFE) | 46.81 g |
| Calcium | 1.20 g |
| Phosphorus | 0.83 g |
| Sodium | 0.40 g |
| Magnesium | 0.13 g |
| Omega 6 | 2.61 g |
| Omega 3 | 0.95 g |
| L-carnitine | 30.00 mg |
| Vitamin E | 75.00 mg |
| Taurine | 0.30 g |
| Cyanidin-3-glucoside (from honeyberry) | 10.50 mg |
| Metabolizable energy | 364.30 kcal |
| Items (Unit) | Day 0 | Day 90 |
|---|---|---|
| Body weight (Kg) | 9.89 ± 9.49 | 9.85 ± 9.38 |
| Body temperature (°C) | 38.80 ± 0.35 | 38.59 ± 0.31 |
| Heart rate (per minute) | 124.44 ± 7.06 | 127.11 ± 5.93 |
| Respiration rate (per minute) | 31.11 ± 8.01 | 28.44 ± 5.64 |
| Category | Items (Unit) | Day 0 | Day 90 | Reference |
|---|---|---|---|---|
| CBC | WBC (109/L) | 9.01 ± 3.54 | 7.92 ± 2.95 | 5.05–16.76 |
| RBC (1012/L) | 6.53 ± 0.74 | 6.52 ± 0.97 | 5.65–8.87 | |
| HGB (g/dL) | 14.36 ± 1.74 | 14.66 ± 2.47 | 13.1–20.5 | |
| PCV (%) | 44.70 ± 5.35 | 43.41 ± 6.82 | 37.1–65.0 | |
| MCV (fL) | 68.30 ± 2.74 | 66.74 ± 2.88 | 61.6–73.5 | |
| MCH (pg) | 21.93 ± 1.40 | 20.70 ± 5.26 | 21.2–25.9 | |
| MCHC (g/dL) | 32.08 ± 1.67 | 33.90 ± 3.26 | 32.0–37.9 | |
| PLT (103/μL) | 365.33 ± 124.18 | 388.38 ± 141.18 | 148–484 | |
| Chemistry | AST (U/dL) | 24.71 ± 7.76 | 25.86 ± 8.93 | 15–43 |
| ALT (U/dL) | 63.56 ± 34.96 | 84.50 ± 50.68 | 19–70 | |
| ALP (U/dL) | 205.11 ± 301.53 | 208.88 ± 396.89 | 15.0–127.0 | |
| BUN (mg/dL) | 18.68 ± 4.05 | 19.44 ± 9.24 | 8–26 | |
| CRE (mg/dL) | 0.87 ± 0.23 | 0.84 ± 0.27 | 0.5–1.3 | |
| Electrolytes | Na (mmol/L) | 148.44 ± 6.21 | 142.88 ± 11.37 | 144–154 |
| K (mmol/L) | 4.73 ± 0.81 | 4.24 ± 0.58 | 4.1–5.3 | |
| Cl (mmol/L) | 95.99 ± 42.57 | 113.50 ± 3.02 | 105.0–116.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Park, M.; Lee, H.-J. Dietary Supplemented Anthocyanin Reduced Serum Amyloid Beta Oligomers and Improved Cognitive Dysfunction Scores in Elderly Dogs. Appl. Sci. 2022, 12, 12130. https://doi.org/10.3390/app122312130
Lee M-J, Park M, Lee H-J. Dietary Supplemented Anthocyanin Reduced Serum Amyloid Beta Oligomers and Improved Cognitive Dysfunction Scores in Elderly Dogs. Applied Sciences. 2022; 12(23):12130. https://doi.org/10.3390/app122312130
Chicago/Turabian StyleLee, Mi-Jin, Miey Park, and Hae-Jeung Lee. 2022. "Dietary Supplemented Anthocyanin Reduced Serum Amyloid Beta Oligomers and Improved Cognitive Dysfunction Scores in Elderly Dogs" Applied Sciences 12, no. 23: 12130. https://doi.org/10.3390/app122312130





