In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
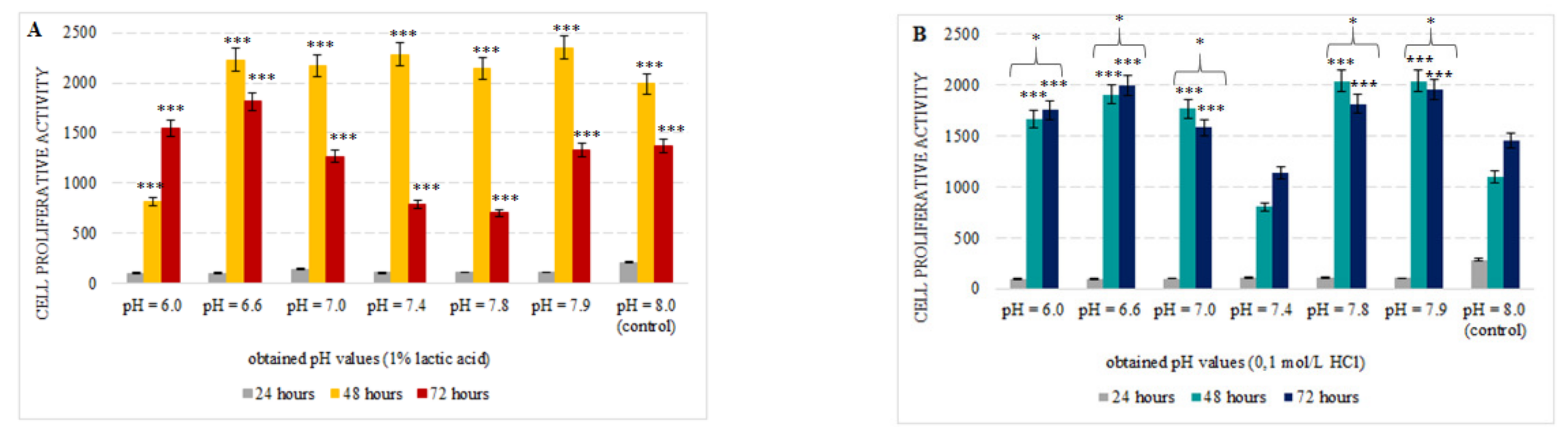
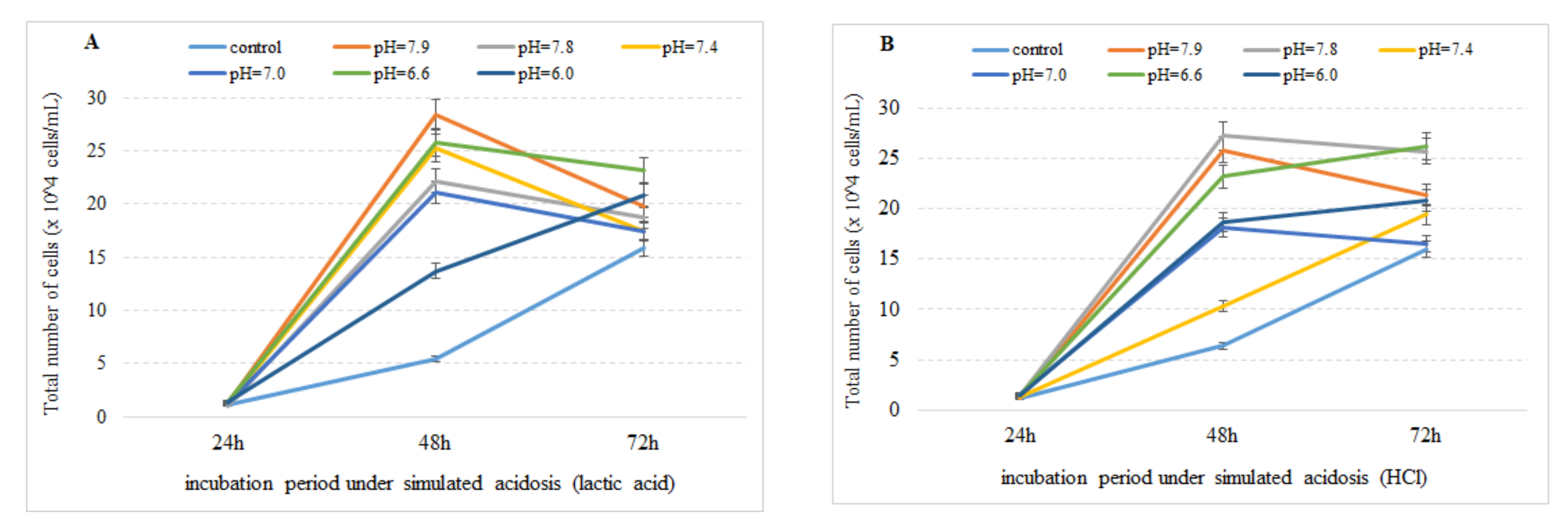
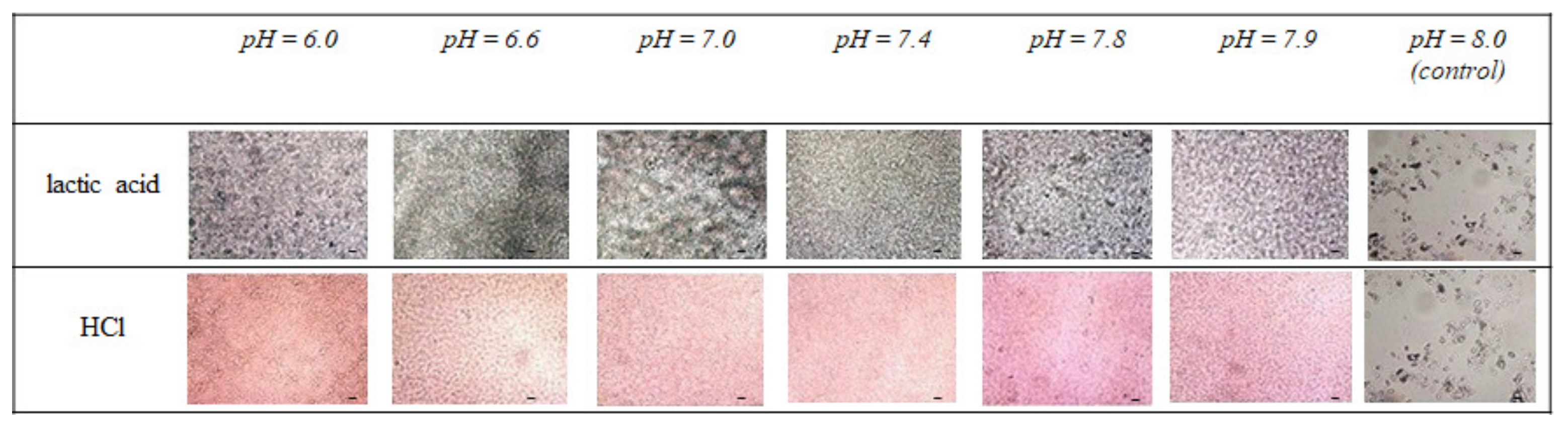
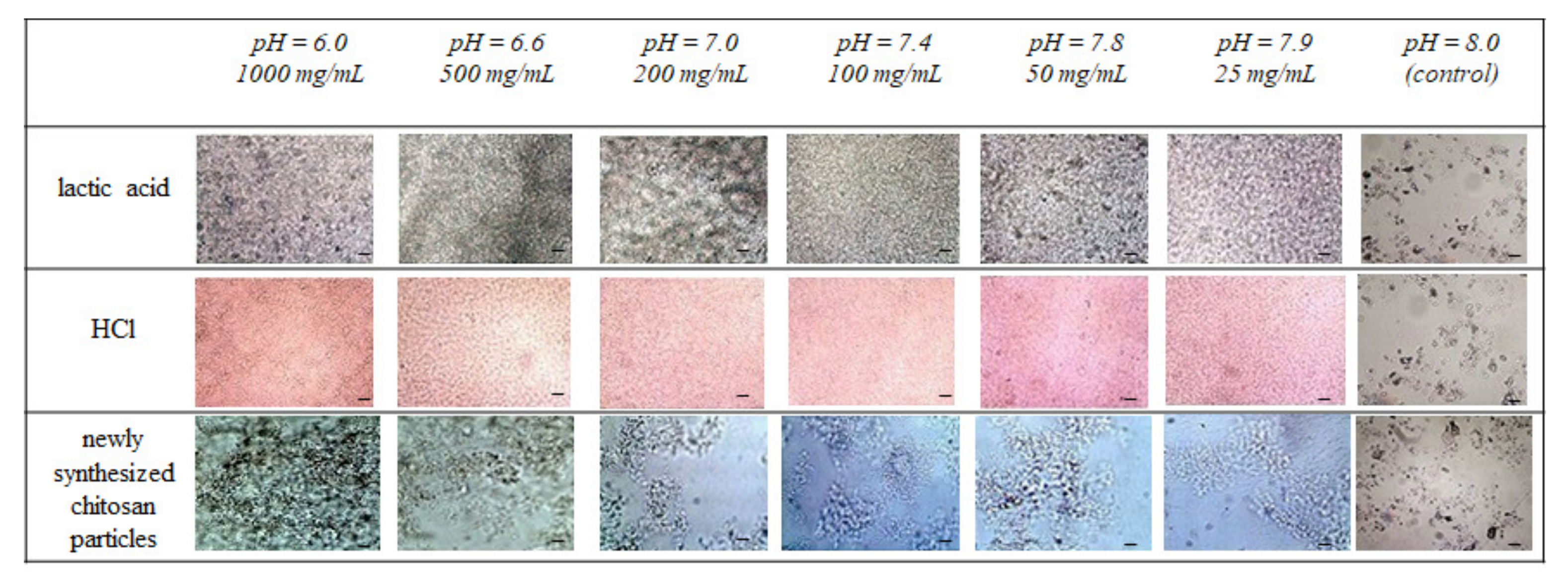
3.1. Effect of Simulated Acidosis on Cell Proliferation Activity
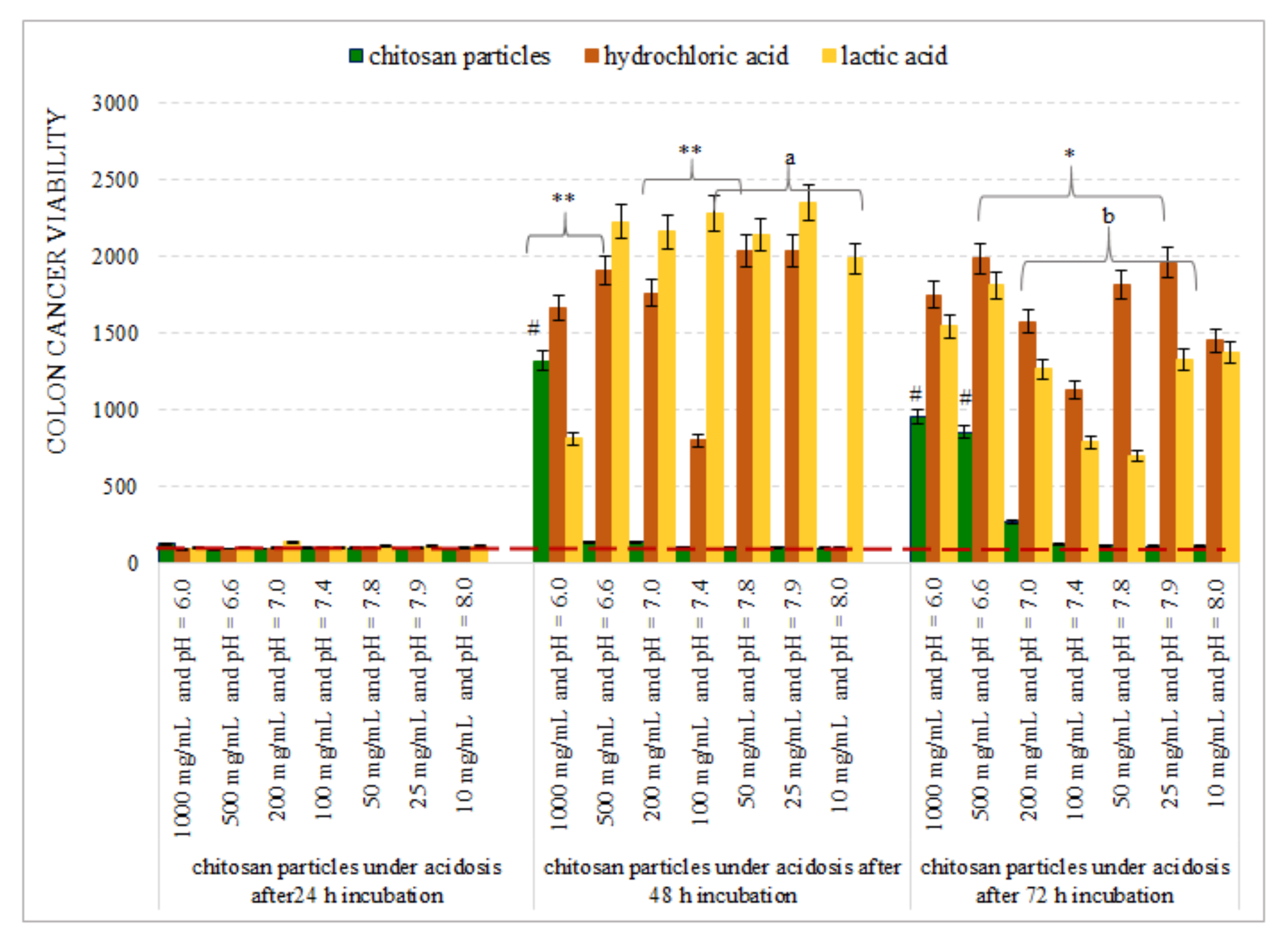
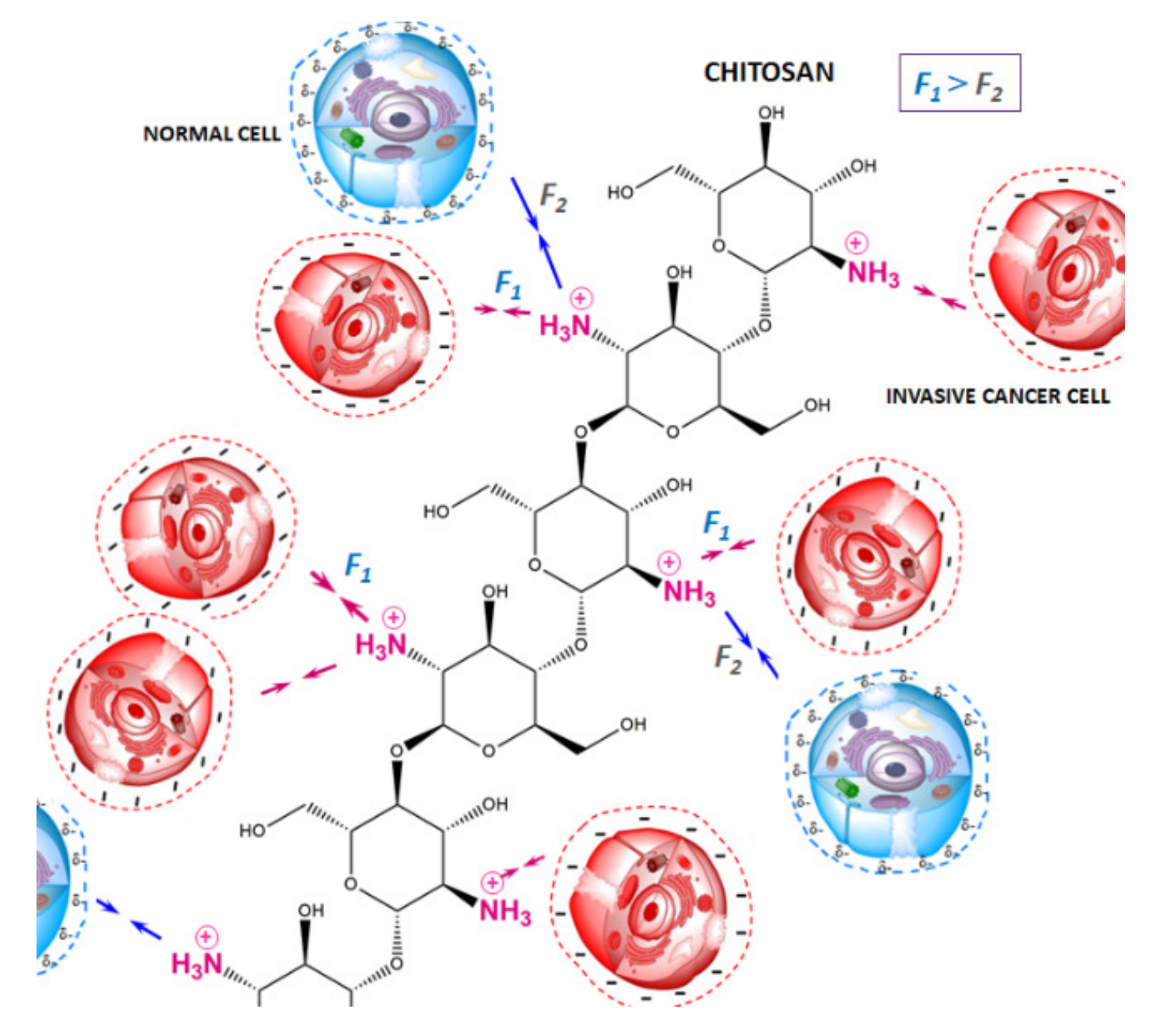
3.2. Chitosan Microparticles as a Potential Model for the Examination of Cancer Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, J.; Zeng, D.; Sun, H.; Rong, X.; Shi, M.; Bin, J.; Liao, Y.; Liao, W. Immune cell infiltration as a biomarker for the diagnosis and prognosis of stage I–III colon cancer. Cancer Immunol. Immunother. 2018, 68, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, J.; Zhang, J.; Yang, Z.; Jin, W.; Li, Y.; Jin, L.; Yin, L.; Liu, H.; Wang, Z. Integrated bioinformatics analysis of key genes involved in progress of colon cancer. Mol. Genet. Genom. Med. 2019, 7, e00588. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Nascimento, C.; Ferreira, F. Tumor microenvironment of human breast cancer, and feline mammary carcinoma as a potential study model. Biochem. Biophys. Acta Rev. Cancer 2021, 1876, 188587. [Google Scholar] [CrossRef]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial–mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- Barathova, M.; Takacova, M.; Holotnakova, T.; Gibadulinova, A.; Ohradanova, A.; Zatovicova, M.; Hulikova, A.; Kopacek, J.; Parkkila, S.; Supuran, C.T.; et al. Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumour phenotype. Br. J. Cancer 2007, 98, 129–136. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The Role of Carbonic Anhydrase 9 in Regulating Extracellular and Intracellular pH in Three-dimensional Tumor Cell Growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, H.; Ross, B.D. Intra- and extracellular pH in solid tumors. In Antiangiogenic Agents in Cancer Therapy; Humana: Totowa, NJ, USA, 1999; pp. 51–64. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Publishing: New York, NY, USA, 2002. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Juneau, P.L.; Masserant, S. Influence of tumor physiochemical conditions on interleukin-2-stimulated lymphocyte proliferation. Br. J. Cancer 1992, 66, 619–622. [Google Scholar] [CrossRef]
- Carswell, K.S.; Papoutsakis, E.T. Extracellular pH Affects the proliferation of cultured human T cells and their expression of the Interleukin-2 receptor. J. Immunother. 2000, 23, 669–674. [Google Scholar] [CrossRef]
- McAdams, T.A.; Miller, W.M.; Papoutsakis, E.T. PH is a potent modulator of erythroid differentiation. Br. J. Haematol. 1998, 103, 317–325. [Google Scholar] [CrossRef]
- Kim, N.; Minami, N.; Yamada, M.; Imai, H. Immobilized pH in culture reveals an optimal condition for somatic cell reprogramming and differentiation of pluripotent stem cells. Reprod. Med. Biol. 2017, 16, 58–66. [Google Scholar] [CrossRef]
- Teo, A.; Mantalaris, A.; Lim, M. Influence of culture pH on proliferation and cardiac differentiation of murine embryonic stem cells. Biochem. Eng. J. 2014, 90, 8–15. [Google Scholar] [CrossRef]
- Miller, W.M.; Blanch, H.W.; Wilke, C.R. A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture. Biotechnol. Bioeng. 1988, 32, 965–977. [Google Scholar] [CrossRef]
- McQueen, A.; Bailey, J.E. Effect of ammonium ion and extracellular pH on hybridoma cell metabolism and antibody production. Biotechnol. Bioeng. 1990, 35, 1067–1077. [Google Scholar] [CrossRef]
- Gaitanaki, C.J.; Sugden, P.H.; Fuller, S.J. Stimulation of protein synthesis by raised extracellular pH in cardiac myocytes and perfused hearts. FEBS Lett. 1990, 260, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Nordberg, J.; Skowronski, E.; Babior, B.M. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA 1996, 93, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: Origins and roles. Cell Death Differ. 2004, 11, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.-E.; Ardenkjær-Larsen, J.H.; Zandt, R.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Wike-Hooley, J.L.; Berg, A.P.V.D.; van der Zee, J.; Reinhold, H.S. Human tumour pH and its variation. Eur. J. Cancer Clin. Oncol. 1985, 21, 785–791. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Q.; Cao, J.; Wang, Y.; Yang, X.; Xu, Q.; Liang, Q.; Sun, Y. Recent advances in microfluidic-aided chitosan-based multifunctional materials for biomedical applications. Int. J. Pharm. 2021, 600, 120465. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan Films and Scaffolds for Regenerative Medicine Applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef] [PubMed]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2020, 21, 101318. [Google Scholar] [CrossRef]
- Yaroslavov, A.; Efimova, A.; Krasnikov, E.; Trosheva, K.; Popov, A.; Melik-Nubarov, N.; Krivtsov, G. Chitosan-based multi-liposomal complexes: Synthesis, biodegradability and cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455–462. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Chitosan promotes ROS-mediated apoptosis and S phase cell cycle arrest in triple-negative breast cancer cells: Evidence for intercalative interaction with genomic DNA. RSC Adv. 2017, 7, 43141–43150. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. BioResearch Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef]
- Chang, P.-H.; Sekine, K.; Chao, H.-M.; Hsu, S.-H.; Chern, E. Chitosan promotes cancer progression and stem cell properties in association with Wnt signaling in colon and hepatocellular carcinoma cells. Sci. Rep. 2017, 7, 45751. [Google Scholar] [CrossRef]
- Solanki, N.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Hansbro, N.G.; Tambuwala, M.M.; Aljabali, A.A.; Paudel, K.R.; Liu, G.; Satija, S.; et al. Antiproliferative effects of boswellic acid-loaded chitosan nanoparticles on human lung cancer cell line A549. Futur. Med. Chem. 2020, 12, 2019–2034. [Google Scholar] [CrossRef]
- Llobet, L.; Montoya, J.; López-Gallardo, E.; Ruiz-Pesini, E. Side Effects of Culture Media Antibiotics on Cell Differentiation. Tissue Eng. Part C Methods 2015, 21, 1143–1147. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Chien, S.; Lv, Y. Suspension state promotes metastasis of breast cancer cells by up-regulating cyclooxygenase-2. Theranostics 2018, 8, 3722–3736. [Google Scholar] [CrossRef]
- Lyons, S.M.; Alizadeh, E.; Mannheimer, J.; Schuamberg, K.; Castle, J.; Schroder, B.; Turk, P.; Thamm, D.; Prasad, A. Changes in cell shape are correlated with metastatic potential in murine and human osteosarcomas. Biol. Open 2016, 5, 289–299. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y. Suspension state increases reattachment of breast cancer cells by up-regulating lamin A/C. Biochem. Biophys. Acta Mol. Cell Res. 2017, 1864, 2227–2282. [Google Scholar] [CrossRef]
- Whipple, R.A.; Cheung, A.M.; Martin, S.S. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp. Cell Res. 2007, 313, 1326–1336. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. Acid-Mediated Tumor Invasion: A Multidisciplinary Study. Cancer Res. 2006, 66, 5216–5223. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.O.; Li, Z.; Shim, H.-E.; Cho, I.-S.; Nurunnabi; Park, H.; Lee, K.Y.; Moon, S.-H.; Kim, K.-S.; Kang, S.-W.; et al. Bioinspired tuning of glycol chitosan for 3D cell culture. NPG Asia Mater. 2016, 8, e309. [Google Scholar] [CrossRef]
- Florczyk, S.; Liu, G.; Kievit, F.; Lewis, A.M.; Wu, J.D.; Zhang, M. 3D Porous Chitosan-Alginate Scaffolds: A New Matrix for Studying Prostate Cancer Cell-Lymphocyte Interactions In Vitro. Adv. Heal. Mater. 2012, 1, 590–599. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.; Zhang, M. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Dadou, S.M.; El-Barghouthi, M.I.; Alabdallah, S.K.; Badwan, A.A.; Antonijevic, M.D.; Chowdhry, B.Z. Effect of Protonation State and N-Acetylation of Chitosan on Its Interaction with Xanthan Gum: A Molecular Dynamics Simulation Study. Mar. Drugs 2017, 15, 298. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X.; Han, Y.; Wei, Y.; Guo, Q.; Yang, S.; Zhang, Y.; Liao, W.; Gao, Y. Effect of chitosan molecular weight on zein-chitosan nanocomplexes: Formation, characterization, and the delivery of quercetagetin. Int. J. Biol. Macromol. 2020, 164, 2215–2223. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Rodríguez-Núñez, J.R. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.; Tacheva, T.; Semkova, S.; Panovska, R.; Yaneva, Z. In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles. Appl. Sci. 2022, 12, 12029. https://doi.org/10.3390/app122312029
Ivanova D, Tacheva T, Semkova S, Panovska R, Yaneva Z. In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles. Applied Sciences. 2022; 12(23):12029. https://doi.org/10.3390/app122312029
Chicago/Turabian StyleIvanova, Donika, Tanya Tacheva, Severina Semkova, Radmila Panovska, and Zvezdelina Yaneva. 2022. "In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles" Applied Sciences 12, no. 23: 12029. https://doi.org/10.3390/app122312029
APA StyleIvanova, D., Tacheva, T., Semkova, S., Panovska, R., & Yaneva, Z. (2022). In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles. Applied Sciences, 12(23), 12029. https://doi.org/10.3390/app122312029





