Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds
Abstract
1. Introduction
2. Characterization and Types of Phenolic Compounds
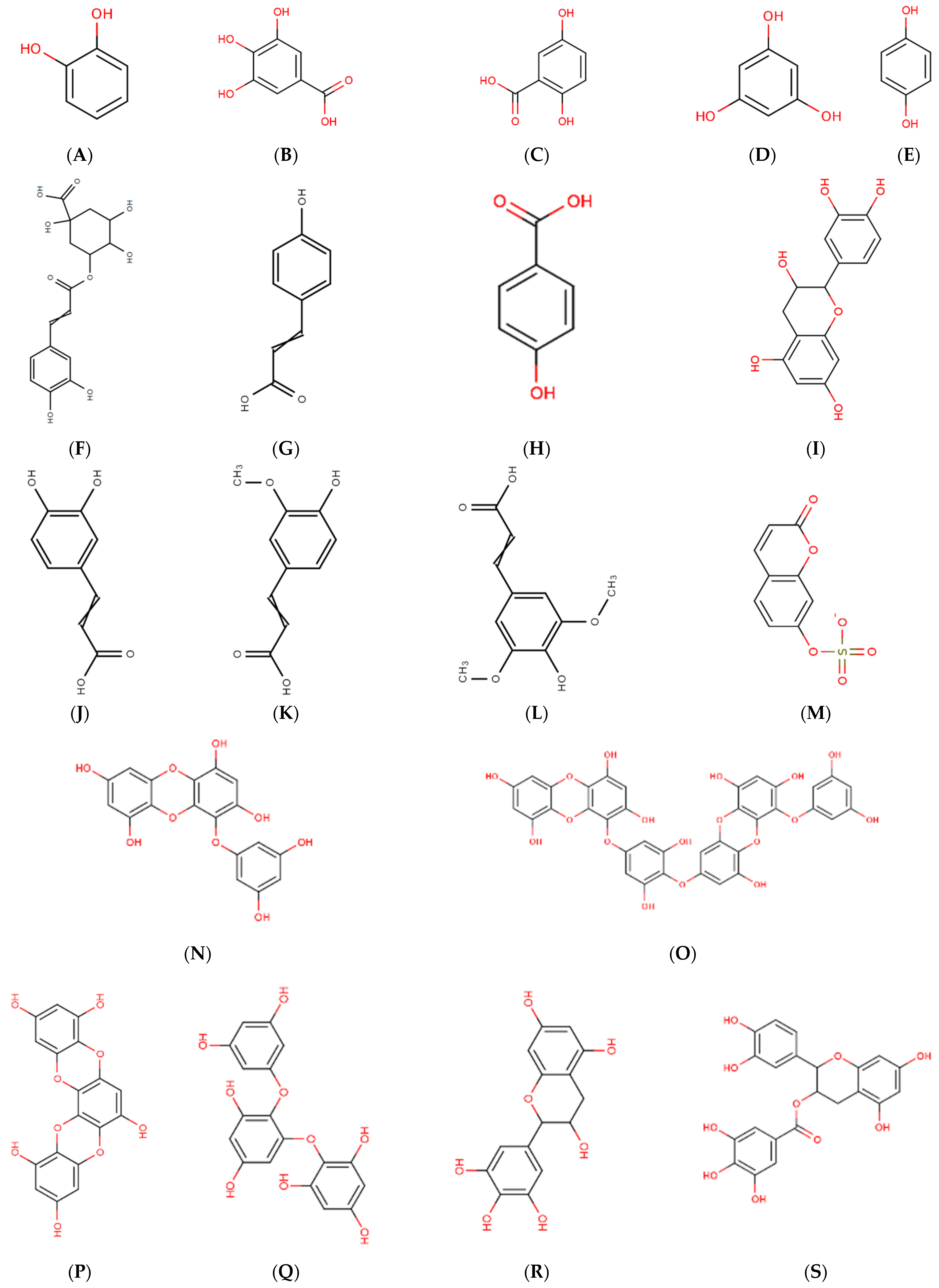
2.1. Polyphenolic Compounds
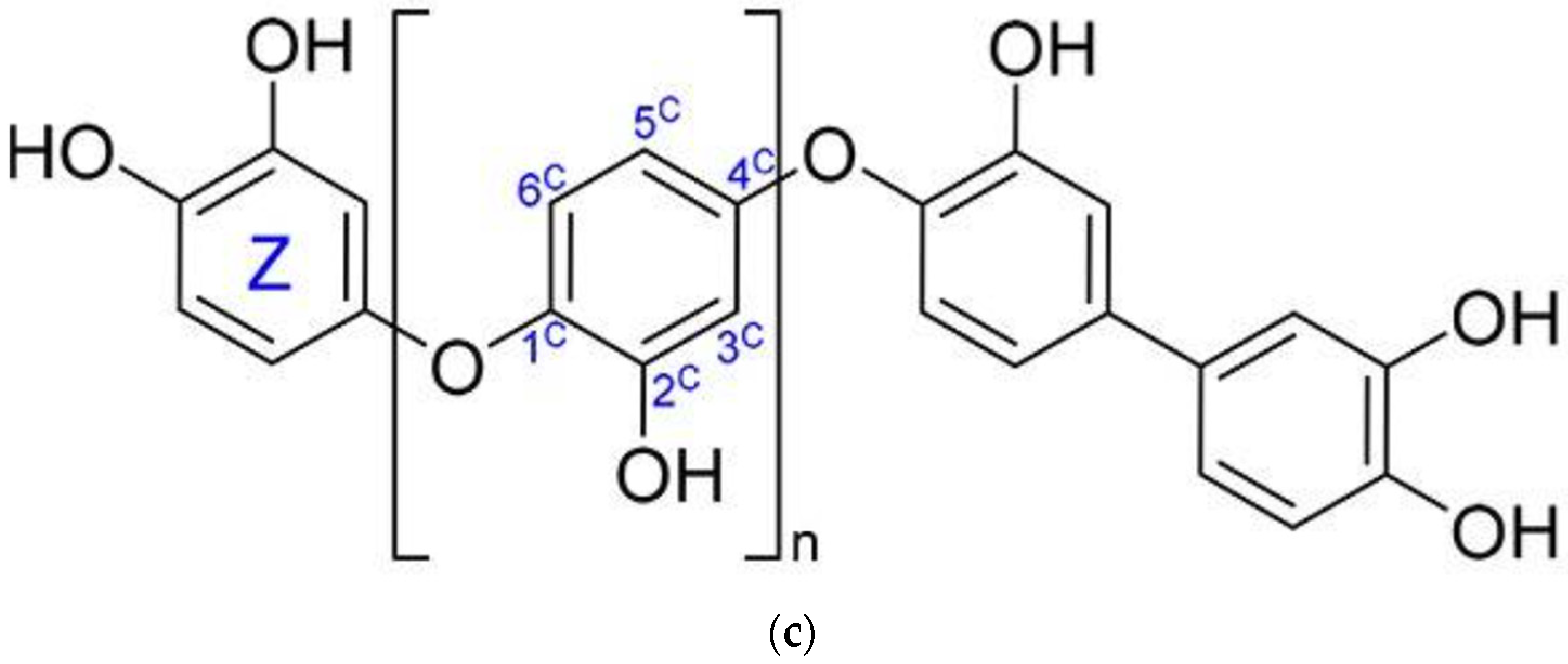
2.2. Lignans
2.3. Phlorotannins
2.4. Bromophenols
2.5. Flavonoids
2.6. Phenolic Terpenoids
2.7. Mycosporine-like Amino Acid
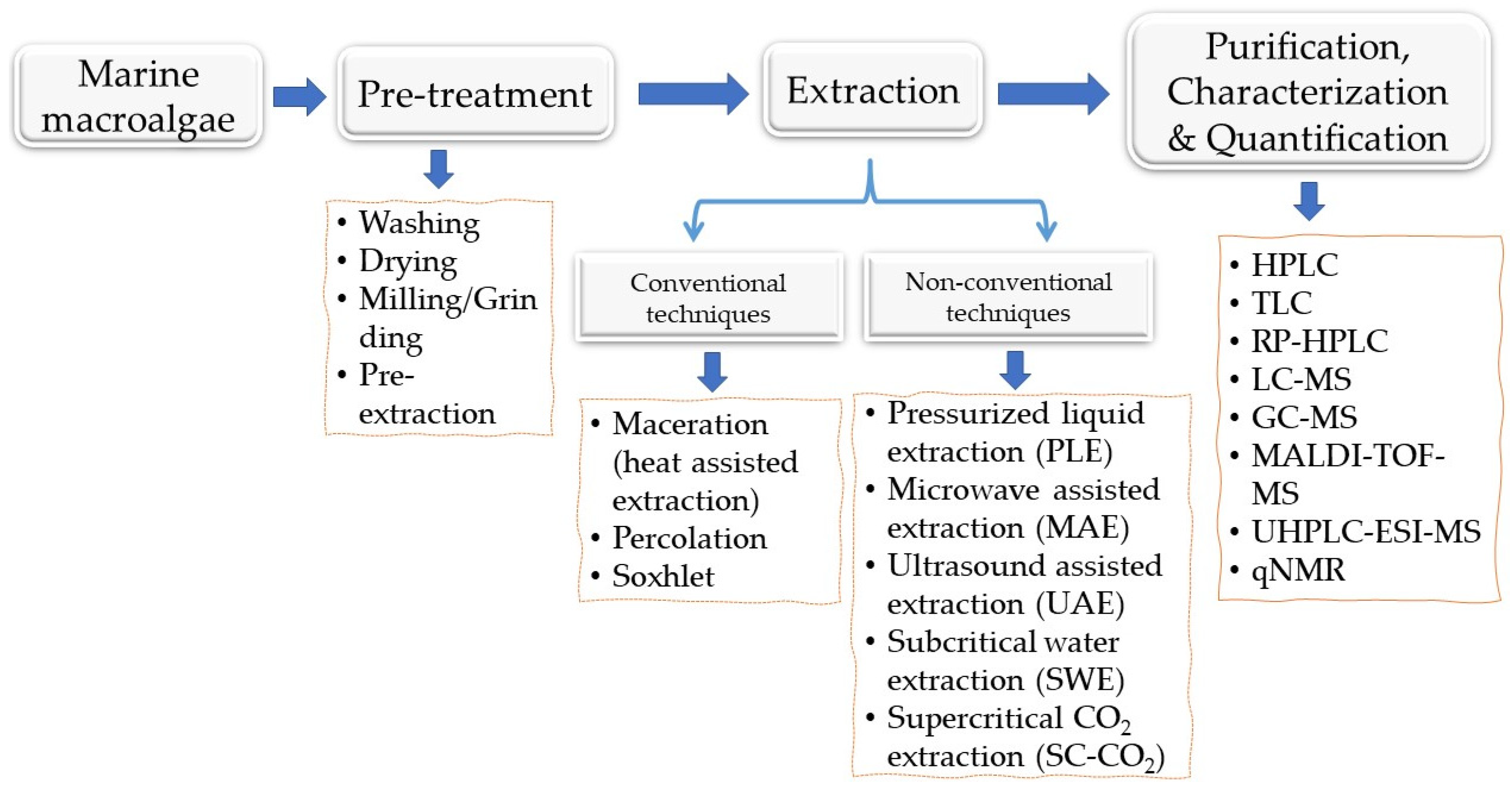
3. Extraction of Phenolic Compounds
4. Commercial Availability of Seaweed-Based Cosmetic Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. Mo. Med. 2011, 108, 60. [Google Scholar] [PubMed]
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharmacol. 2005, 37, 155. [Google Scholar] [CrossRef]
- Draelos, Z.D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.N.; Hayes, R.B.; Linet, M.S.; Li, G.L.; Dosemeci, M.; Travis, L.B.; Zhang, Z.N.; Li, D.G.; Chow, W.H.; Wacholder, S.; et al. An expanded cohort study of cancer among benzene-exposed workers in China. Benzene Study Group. Environ. Health Perspect. 1996, 104 (Suppl. S6), 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]
- Zhang, L.; Robertson, M.L.; Kolachana, P.; Davison, A.J.; Smith, M.T. Benzene metabolite, 1,2,4-benzenetriol, induces micronuclei and oxidative DNA damage in human lymphocytes and HL60 cells. Environ. Mol. Mutagen. 1993, 21, 339–348. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Llompart, M.; Sánchez-Prado, L.; García-Jares, C.; Lores, M. Photochemical behavior of UV filter combinations. In Cosmetics: Types, Allergies and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; p. 1. [Google Scholar]
- Knowland, J.; McKenzie, E.A.; McHugh, P.J.; Cridland, N.A. Sunlight-induced mutagenicity of a common sunscreen ingredient. FEBS Lett. 1993, 324, 309–313. [Google Scholar] [CrossRef]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. Comptes Rendus. Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- Mowad, C.M. Allergic contact dermatitis caused by parabens: 2 case reports and a review. Am. J. Contact Dermat. 2000, 11, 53–56. [Google Scholar] [CrossRef]
- Hafeez, F.; Maibach, H. An overview of parabens and allergic contact dermatitis. Skin Ther. Lett. 2013, 18, 5–7. [Google Scholar]
- Barrett, J. Chemical Exposures: The Ugly Side of Beauty Products. Environ. Health Perspect. 2005, 113, A24. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.D.; Alam, M.N. Cosmetics and their associated adverse effects: A review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Warbanski, M. The ugly side of the beauty industry. Herizons 2007, 21, 24–28. [Google Scholar]
- Kaličanin, B.; Velimirović, D. A study of the possible harmful effects of cosmetic beauty products on human health. Biol. Trace Elem. Res. 2016, 170, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Shin, K.H.; Kim, S.K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498755382. [Google Scholar]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M. Diverse applications of marine macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Algae. Litoral of Viana do Castelo; Câmara Municipal de Viana do Castelo: Viana do Castelo, Portugal, 2010; pp. 7–8. ISBN 978-972-588-217-7. [Google Scholar]
- Pereira, L. Guia Ilustrado das Macroalgas—Conhecer e Reconhecer Algumas Espécies da Flora Portuguesa; Universityde Coimbra Press: Coimbra, Portugal, 2009; p. 91. ISBN 978-989-26-0002-4. [Google Scholar]
- Pereira, L. Chapter 4—Cytological and cytochemical aspects in selected carrageenophytes (Gigartinales, Rhodophyta). In Advances in Algal Cell Biology; Heimann, K., Katsaros, C., Eds.; De Gruyter: Berlin, Germany, 2012; pp. 81–104. ISBN 978-3-11-022960-8. [Google Scholar]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Springer: Boston, MA, USA, 2012; pp. 55–98. [Google Scholar]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Osteoporosis treatment: Marine algal compounds. Adv. Food Nutr. Res. 2011, 64, 417–427. [Google Scholar]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Gam, D.H.; Park, J.H.; Hong, J.W.; Jeon, S.J.; Kim, J.H.; Kim, J.W. Effects of Sargassum thunbergii extract on skin whitening and anti-wrinkling through inhibition of TRP-1 and MMPs. Molecules 2021, 26, 7381. [Google Scholar] [CrossRef] [PubMed]
- Querellou, J.; Børresen, T.; Boyen, C.; Dobson, A.; Höfle, M.; Ianora, A.; Jaspars, M.; Kijjoa, A.; Olafsen, J.; Rigos, G. Marine biotechnology: Realising the full potential of Europe. VLIZ Spec. Publ. 2010, 47, 21. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Chisté, R.C.; Godoy, H.T.; Prado, M.A. The phenolic compounds and the antioxidant potential of infusion of herbs from the Brazilian Amazonian region. Food Res. Int. 2013, 53, 875–881. [Google Scholar]
- Nurilmala, M.; Hidayat, T.; Sudirdjo, F. Characteristics of seaweed as raw materials for cosmetics. Aquat. Procedia 2016, 7, 177–180. [Google Scholar]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the Essential Oil and Polar Extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K. Pharmacological properties of marine macroalgae-associated heterotrophic bacteria. Arch. Microbiol. 2019, 201, 505–518. [Google Scholar] [CrossRef]
- Holmquist, B.; Bunning, P.; Riordan, J.F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 1979, 95, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin-I converting enzyme) in-vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Nasab, S.B.; Homaei, A.; Pletschke, B.I.; Salinas-Salazar, C.; Castillo-Zacarias, C.; Parra-Saldívar, R. Marine resources effective in controlling and treating diabetes and its associated complications. Process Biochem. 2020, 92, 313–342. [Google Scholar] [CrossRef]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Hwang, H.J. Skin elasticity and sea polyphenols. Seanol Sci. Centre Rev. 2010, 1, 17. [Google Scholar]
- Malakar, B.; Mohanty, K. The budding potential of algae in cosmetics. In Algae; Springer: Singapore, 2021; pp. 181–199. [Google Scholar]
- Sakthivel, R.; Devi, K.P. Antioxidant, anti-inflammatory and anticancer potential of natural bioactive compounds from seaweeds. Stud. Nat. Prod. Chem. 2019, 63, 113–160. [Google Scholar]
- Panzella, L. Natural phenolic compounds for health, food and cosmetic applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef]
- Tang, H.; Inoue, M.; Uzawa, Y.; Kawamura, Y. Anti-tumorigenic components of a sea weed, Enteromorpha clathrata. BioFactors 2004, 22, 107–110. [Google Scholar] [CrossRef]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Ardekani, M.R.S.; Nabavi, S.M.B.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn. Mag. 2012, 8, 60. [Google Scholar] [PubMed]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of antioxidant and antimicrobial activity in relation to total phenolic content of green algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- Ko, S.C.; Ding, Y.; Kim, J.; Ye, B.R.; Kim, E.A.; Jung, W.K.; Heo, S.J.; Lee, S.H. Bromophenol (5-bromo-3, 4-dihydroxybenzaldehyde) isolated from red alga Polysiphonia morrowii inhibits adipogenesis by regulating expression of adipogenic transcription factors and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Phytother. Res. 2019, 33, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Yadav, S.; Mishra, A. Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol. Biol. Rep. 2020, 47, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.; Meyer, J.J.M. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-like amino acids for skin photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2019, 18, 35. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef]
- Shim, S.Y.; Choi, J.S.; Byun, D.S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorg. Med. Chem. 2009, 17, 4734–4739. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, E.H.; Park, K.; Kwon, J.Y.; Kim, P.H.; Jung, W.K.; Kim, Y.M. Eckol from Eisenia bicyclis inhibits inflammation through the Akt/NF-κB signaling in Propionibacterium acnes-induced human keratinocyte Hacat cells. J. Food Biochem. 2017, 41, e12312. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Park, D.C.; Ji, C.I.; Kim, S.H.; Jung, K.J.; Lee, T.G.; Kim, I.S.; Park, Y.H.; Kim, S.B. Characteristics of tyrosinase inhibitory extract from Ecklonia stolonifera. Fish. Aquat. Sci. 2000, 3, 195–199. [Google Scholar]
- Kilic, M.; Orhan, I.E.; Eren, G.; Okudan, E.S.; Estep, A.S.; Bencel, J.J.; Tabanca, N. Insecticidal activity of forty-seven marine algae species from the Mediterranean, Aegean, and Sea of Marmara in connection with their cholinesterase and tyrosinase inhibitory activity. S. Afr. J. Bot. 2021, 143, 435–442. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-binding inhibition of tyrosinase by Ecklonia cava phlorotannins. Mar. Drugs 2019, 17, 359. [Google Scholar] [CrossRef]
- Cha, S.H.; Ko, C.I.; Kim, D.; Jeon, Y.J. Protective effects of phlorotannins against ultraviolet B radiation in zebrafish (Danio rerio). Vet. Dermatol. 2012, 23, 51-e12. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Santos, S.; Félix, R.; Pais, A.; Rocha, S.M.; Silvestre, A. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Parys, S.; Rosenbaum, A.; Kehraus, S.; Reher, G.; Glombitza, K.W.; König, G.M. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J. Nat. Prod. 2007, 70, 1865–1870. [Google Scholar] [CrossRef]
- Ktari, L.; Mdallel, C.; Aoun, B.; Chebil Ajjabi, L.; Sadok, S. Fucoxanthin and Phenolic Contents of Six Dictyotales From the Tunisian Coasts With an Emphasis for a Green Extraction Using a Supercritical CO2 Method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive properties of marine phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran. J. Pharm. Res. IJPR 2014, 13, 163. [Google Scholar] [PubMed]
- Cho, M.; Kang, I.J.; Won, M.H.; Lee, H.S.; You, S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J. Med. Food 2010, 13, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Hong, J.; Mi, J.; Sun, B.; Zhang, J.; Li, C.; Yang, W. Polyphenols extracted from Enteromorpha clathrata alleviates inflammation in lipopolysaccharide-induced RAW 264.7 cells by inhibiting the MAPKs/NF-κB signaling pathways. J. Ethnopharmacol. 2022, 286, 114897. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Dale, B.; Gagaring, K.; McNamara, C.W.; Quave, C.L.; Soapi, K.; Kubanek, J. Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. J. Org. Chem. 2019, 84, 5035–5045. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2008; pp. 1–34. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in plants. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 263–284. [Google Scholar]
- Santoso, J.; Yoshie, Y.; Suzuki, T. Polyphenolic compounds from seaweeds: Distribution and their antioxidative effect. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2004; Volume 42, pp. 169–177. [Google Scholar]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Ishii, T.; Okino, T.; Suzuki, M.; Machiguchi, Y. Tichocarpols A and B, Two Novel Phenylpropanoids with Feeding-Deterrent Activity from the Red Alga Tichocarpus c rinitus. J. Nat. Prod. 2004, 67, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) krasser. Molecules 2018, 23, 2735. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Handique, J.G.; Baruah, J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Mouritsen, O.G. The science of seaweeds: Marine macroalgae benefit people culturally, industrially, nutritionally, and ecologically. Am. Sci. 2013, 101, 458–466. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 113–129. [Google Scholar]
- Kim, M.M.; Kim, S.K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Franco, C.; Su, P.; Zhang, W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J. Appl. Phycol. 2015, 27, 2049–2058. [Google Scholar] [CrossRef]
- Chang, M.Y.; Byon, S.H.; Shin, H.C.; Han, S.E.; Kim, J.Y.; Byun, J.Y.; Lee, J.D.; Park, M.K. Protective effects of the seaweed phlorotannin polyphenolic compound dieckol on gentamicin-induced damage in auditory hair cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 31–36. [Google Scholar] [CrossRef]
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective Effect of Diphlorethohydroxycarmalol against Ultraviolet B Radiation-Induced DNA Damage by Inducing the Nucleotide Excision Repair System in HaCaT Human Keratinocytes. Mar. Drugs 2015, 13, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Yang, H.M.; Kim, A.D.; Jeon, Y.J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Tang, Y.; Kim, Y.S.; Hwang, J.W.; Choi, E.J.; Lee, J.H.; Lee, S.H.; Jeon, Y.J.; Park, P.J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs 2015, 13, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, J.H.; Park, S.A.; Joo, N.R.; Lee, B.H.; Lee, K.B.; Oh, S.M. Dieckol or phlorofucofuroeckol extracted from Ecklonia cava suppresses lipopolysaccharide-mediated human breast cancer cell migration and invasion. J. Appl. Phycol. 2020, 32, 631–640. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 278–334. [Google Scholar]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2007; pp. 255–261. [Google Scholar]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Choi, J.W.; Utsuki, T.; Kim, J.I.; Jang, B.C.; Kim, H.R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines via inhibition of nuclear factor-κB, c-Jun NH2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef]
- Ryu, B.; Ahn, B.N.; Kang, K.H.; Kim, Y.S.; Li, Y.X.; Kong, C.S.; Kim, S.K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B Biol. 2015, 153, 352–357. [Google Scholar] [CrossRef]
- Kumar, L.R.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharmacal Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of Antioxidant, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase of The Extract and Fraction From Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188. [Google Scholar] [CrossRef]
- Bak, S.S.; Sung, Y.K.; Kim, S.K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 789–793. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; De Souza, M.D.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013; pp. 287–306. [Google Scholar]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono-and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef]
- Colon, M.; Guevara, P.; Gerwick, W.H.; Ballantine, D. 5’-Hydroxyisoavrainvilleol, a new diphenylmethane derivative from the tropical green alga Avrainvillea nigricans. J. Nat. Prod. 1987, 50, 368–374. [Google Scholar] [CrossRef]
- Carte, B.K.; Troupe, N.; Chan, J.A.; Westley, J.W.; Faulkner, D.J. Rawsonol, an inhibitor of HMG-CoA reductase from the tropical green alga Avrainvillea rawsoni. Phytochemistry 1989, 28, 2917–2919. [Google Scholar] [CrossRef]
- Estrada, D.M.; Martin, J.D.; Perez, C. A new brominated monoterpenoid quinol from Cymopolia barbata. J. Nat. Prod. 1987, 50, 735–737. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Jeyaprakash, R.R.K. HPLC Analysis of flavonoids in Acanthophora specifera (red seaweed) collected from Gulf of Mannar, Tamilnadu, India. Int. J. Sci. Res. 2017, 6, 69–72. [Google Scholar]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2 H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2018, 32, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Joe, C.; Robert, M.C. 1.16—Sesquiterpenes. In Comprehensive Natural Products II; Ben, H.-W., Mander, L.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 609–641. ISBN 9780080453828. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and Analysis of Mycosporine-Like Amino Acids in Marine Algae. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Suh, S.S.; Oh, S.K.; Lee, S.G.; Kim, I.C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264. [Google Scholar] [CrossRef]
- Pavia, H.; Brock, E. Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T. Variation in natural selection for growth and phlorotannins in the brown alga Fucus vesiculosus. J. Evol. Biol. 2004, 17, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Riani, M.K.L.; Anwar, E.; Nurhayati, T. Antioxidant and anti-collagenase activity of Sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacogn. J. 2018, 10, 932–936. [Google Scholar]
- Busetti, A.; Maggs, C.A.; Gilmore, B.F. Marine macroalgae and their associated microbiomes as a source of antimicrobial chemical diversity. Eur. J. Phycol. 2017, 52, 452–465. [Google Scholar] [CrossRef]
- Handelman, G.J. The evolving role of carotenoids in human biochemistry. Nutrition 2001, 17, 818–822. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H. Evaluation of antioxidant activity of marine algae-extracts from Korea. J. Aquat. Food Prod. Technol. 2015, 24, 227–240. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Hsieh, Y.P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J.-Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Cho, S.H.; Kang, S.E.; Cho, J.Y.; Kim, A.R.; Park, S.M.; Hong, Y.K.; Ahn, D.H. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J. Med. Food 2007, 10, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleraceae) following application of commercial seaweed extracts of the brown seaweed, (Ascophyllum nodosum). Agric. Food Sci. 2013, 22, 288–295. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Chakraborty, K.; Maneesh, A.; Makkar, F. Antioxidant activity of brown seaweeds. J. Aquat. Food Prod. Technol. 2017, 26, 406–419. [Google Scholar] [CrossRef]
- Vimaladevi, S.; Mahesh, A.; Dhayanithi, B.N.; Karthikeyan, N. Mosquito larvicidal efficacy of phenolic acids of seaweed Chaetomorpha antennina (Bory) Kuetz. against Aedes aegypti. Biologia 2012, 67, 212–216. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Micro-Macroalgae Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 9780128035818. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Lee, W.; Jeon, Y.J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; PP Oliveira, M.B. Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 345–378. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Green Confertii Extract-NS—The Garden of Naturalsolution—Datasheet. Available online: https://cosmetics.specialchem.com/product/i-natural-solution-green-confertii-extract-ns (accessed on 10 April 2020).
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Cha, S.H.; Kim, E.A.; Kim, K.N.; Heo, S.J.; Jun, H.S.; Jeon, Y.J. Prolonged exposure of marine algal phlorotannins with whitening effect did not cause inflammatory hyperpigmentation in zebrafish larva. Gen 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, H.S.; Lee, W.; Han, E.J.; Kim, S.Y.; Fernando, I.S.; Ahn, G.; Kim, K.N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Shibata, T.; Yamaguchi, K.; Nagayama, K.; Kawaguchi, S.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against glycosidases from the viscera of the turban shell Turbo cornutus. Eur. J. Phycol. 2002, 37, 493–500. [Google Scholar] [CrossRef]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.; Ahn, B.N.; Kim, S.K. Potential effect of phloroglucinol derivatives from Ecklonia cava on matrix metalloproteinase expression and the inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages. Fish. Sci. 2011, 77, 867–873. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialization. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Ryu, B.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Exhibitory effects of compounds from brown alga Ecklonia cava on the human osteoblasts. J. Biotech. 2008, 136, 577–588. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Im, S.T.; Myung, S.; Kim, H.S.; Lee, S.H. Diphlorethohydroxycarmalol inhibits melanogenesis via protein kinase A/cAMP response element-binding protein and extracellular signal-regulated kinase-mediated microphthalmia-associated transcription factor downregulation in α-melanocyte stimulating hormone-stimulated B16F10 melanoma cells and zebrafish. Cell Biochem. Funct. 2021, 39, 546–554. [Google Scholar] [PubMed]
- Song, T.Y.; Chen, C.H.; Yang, N.C.; Fu, C.S. The correlation of in vitro mushroom tyrosinase activity with cellular tyrosinase activity and melanin formation in melanoma cells A2058. J. Food Drug Anal. 2009, 17, 4. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Cha, S.H.; Lee, S.H.; Kang, D.H.; Jung, W.K.; Affan, A.; Oh, C.; Jeon, Y.J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Soleimani, S.; Babaei Mahani Nezhad, S.; Yousefzadi, M. Role of Phlorotannins Derived from Brown Alga Padina sp. as a Protective Agent against Ultraviolet Radiation and Oxidative Stress. J. Mar. Med. 2021, 3, 97–106. [Google Scholar]
- Ko, S.C.; Cha, S.H.; Heo, S.J.; Lee, S.H.; Kang, S.M.; Jeon, Y.J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.S.R.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I. UV sensitivity of vegetative and reproductive tissues of two Antarctic Brown Algae is related to differential allocation of phenolic substances. Photochem. Photobiol. 2015, 91, 1382–1388. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Islam, M.N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Ahn, G.; Roh, S.W.; Lee, W.; Kim, D.; Jeon, Y.J. Whitening effect of octaphlorethol A isolated from Ishige foliacea in an in vivo zebrafish model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, A.; Picon, A.; Nuñez, M. High pressure processing for the extension of Laminaria ochroleuca (kombu) shelf-life: A comparative study with seaweed salting and freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 420–428. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Bacchetta, C.; Rossi, A.; Cazenave, J.; Drago, S.R. Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J. Appl. Phycol. 2019, 31, 1455–1465. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285. [Google Scholar] [CrossRef]
- Li, Y.; Xia, C.; Yao, G.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Protective effects of liquiritin on UVB-induced skin damage in SD rats. Int. Immunopharmacol. 2021, 97, 107614. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, J.M.; Shin, D.B.; Lee, N.H. The antioxidant activity and tyrosinase inhibitory activity of phloro-tannins in Ecklonia cava. Food Sci. Biotechnol. 2004, 13, 476–480. [Google Scholar]
- Okeke, E.S.; Nweze, E.J.; Chibuogwu, C.C.; Anaduaka, E.G.; Chukwudozie, K.I.; Ezeorba, T.P.C. Aquatic Phlorotannins and Human Health: Bioavailability, Toxicity, and Future Prospects. Nat. Prod. Commun. 2021, 16, 1934578X211056144. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K.; Ryu, B.; Ngo, D.H.; Yoon, N.Y.; Bach, L.G.; Hang, N.T.N.; Ngo, D.N. The suppressive activity of fucofuroeckol-A derived from brown algal Ecklonia stolonifera Okamura on UVB-induced mast cell degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Jang, J.; Ye, B.R.; Heo, S.J.; Oh, C.; Kang, D.H.; Kim, J.H.; Affan, A.; Yoon, K.T.; Choi, Y.U.; Park, S.C.; et al. Photo-oxidative stress by ultraviolet-B radiation and antioxidative defense of eckstolonol in human keratinocytes. Environ. Toxicol. Pharmacol. 2012, 34, 926–934. [Google Scholar] [CrossRef]
- Lee, J.H.; Eom, S.H.; Lee, E.H.; Jung, Y.J.; Kim, H.J.; Jo, M.R.; Son, K.T.; Lee, H.J.; Kim, J.H.; Lee, M.S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, K.; Jeon, J.S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Lee, M.S.; Yoon, H.D.; Kim, J.I.; Choi, J.S.; Byun, D.S.; Kim, H.R. Dioxinodehydroeckol inhibits melanin synthesis through PI3K/Akt signalling pathway in α-melanocyte-stimulating hormone-treated B16F10 cells. Exp. Dermatol. 2012, 21, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Jacobo-Velázquez, D.A. Classification of phenolic compounds. In Phenolic Compounds in Food; CRC Press: Boca Raton, FL, USA, 2018; pp. 3–20. [Google Scholar]
- Padilla, M.; Palma, M.; Barroso, C.G. Determination of phenolics in cosmetic creams and similar emulsions. J. Chromatogr. A 2005, 1091, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S. Natural products from marine algae: Methods and protocols. Nat. Prod. Mar. Algae Methods Protoc. 2015, 1308, 1–439. [Google Scholar]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. In Natural Products Isolation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 427–464. [Google Scholar]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.; Ferreira, I.C.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crops Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kalil, S.J.; Moraes, C.C.; Sala, L.; Burkert, C.A. Bioproduct extraction from microbial cells by conventional and nonconventional techniques. In Food Bioconversion; Academic Press: Cambridge, MA, USA, 2017; pp. 179–206. [Google Scholar]
- Cikoš, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; SimalGandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Dos Santos, D.Y. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]




| No. | Name of Marine Algae | Seaweed-Based Bioactive Compounds | Cosmetic Properties/Benefits | References |
|---|---|---|---|---|
| 1 | Macroalgal species | Catechins, Flavanols, Flavanol glycosides, Gallic acid, Epicatechin, Phloroglucinol, Pyro catechol, Gallate, Flavonoids, Anthocyanins, Stilbenes, Lignans | Matrix Metalloproteinase (MMP) inhibitors, Reduce collagen degradation | [145,146,147] |
| 2 | Corallina pilulifera (R) | - | Inhibit the expression of MMP2 and MMP-9 | [148] |
| 3 | Sargassum horneri (B) | Sargachromanol E | Antiaging | [149] |
| 4 | Phycocalidia vietnamensis (R) | Mycosporine-like amino acids (MAAs) | UV absorber | [150] |
| 5 | Ecklonia cava (B) | Phlorotannins | Skin whitener, Tyrosinase inhibition | [151] |
| 6 | Macroalgal species | - | Antioxidant activity | [152,153] |
| 7 | Macroalgal species | Phlorotannins | Anti-wrinkle, Antiaging | [154,155] |
| 8 | Sargassum fusiforme (as Hizikia fusiformis) (B) | Phlorotannins | Tyrosinase inhibition, Skin whitener | [156] |
| 9 | Corallina pilulifera (R) | Phlorotannins, Eckol, Fucols, Fucophorethols, Fuhalols, Phlorethols | Antiaging, Antiphotoaging, Antioxidant, Tyrosinase inhibition | [157,158,159,160] |
| 10 | Macroalgal species | Phlorotannins | Inhibit melanin synthesis, Anti UVB photodamage | [161] |
| 11 | Ecklonia cava (B) | Phlorotannins | Melanin synthesis, UV protector | [162,163] |
| 12 | Ecklonia cava (B) | Phlorotannins such as eckstolonol, dieckol | Antioxidant, photoprotective, UV protector | [164] |
| 13 | Brown algae species | Phlorotannins such as Phloroeckol, Tetrameric phloroglucinol | Anti-skin aging, Antioxidant | [165] |
| 14 | Corallina pilulifera (R) | Phlorotannins | Metalloproteinase inhibitors, and UV protectors, Prevent collagen degradation, Wrinkle formation | [166] |
| 15 | Ulva clathrata, Ulva compressa (as Enteromorpha compressa), Ulva intestinalis, Ulva linza, Ulva flexuosa, Ulva australis, Capsosiphon fulvescens, Chaetomorpha moniligera (G) | Bromophenols, Flavonoids | Highly radical scavenger | [167,168] |
| 16 | E. cava (B) | Phlorotannins | UVB protector | [169] |
| 17 | Saccharina japonica (as Laminaria japonica), Ecklonia cava, (B) | Phlorotannins | Utilized in facial masks, UV protectors, Anti-acne | [170,171,172] |
| 18 | Ulva compressa (as Enteromorpha compressa) (G) | Flavonoids, Tannins, Phlorotannins | Antioxidant effect, Anti-aging | [173] |
| 19 | Fucus vesiculosus (B) | Flavonoids, Phenols, HQ, Saponin | Tyrosinase inhibitor, Melanin Inhibition | [174] |
| 20 | Ecklonia cava (B) | Phlorotannins; Eckol, Dieckol, Dioxinodehydroeckol, 7- phloroeckol, Phloroglucinol | Tyrosinase inhibition (Skin whitener) | [175,176,177] |
| 21 | Ericaria selaginoides (as Cystoseira tamariscifolia), Gongolaria usneoides (as Cystoseira usneoides), Fucus spiralis, Gongolaria nodicaulis (as Cystoseira nodicaulis (B) | Phlorotannins, Fucophloroethol, Bieckol, Phlorofucofuroeckol, 7-phloroeckol | Antioxidant, Anti-aging, anti-wrinkling, Hyaluronidase inhibition, Lipid peroxidase inhibition | [178] |
| 22 | Ecklonia bicyclis (as Eisenia bicyclis) (B) | Phlorotannins (Phlorofucofuroeckol- A, Dieckol, Eckol, Phloroglucinol, 8,8′ bieckol | Hyaluronidase inhibitor, Anti-wrinkle | [179] |
| 23 | Ecklonia kurome (B) | Phlorofucofuroeckol A, 8-8 bieckol, Dieckol, Eckol, Phloroglucinol | Hyaluronidase inhibition, Anti-wrinkle | [180] |
| 24 | Ecklonia stolonifera (B) | Phlorotannins: Eckol, Phlorofucofuroeckol A, Dieckol, Eckstolonol | Tyrosinase inhibitor, Skin whitener Metalloproteinase inhibitors, Anti-wrinkle | [181] |
| 25 | Ecklonia stolonifera (B) | Phlorotannins: phlorofucofuroeckol A | Anti-inflammatory | [182] |
| 26 | Ecklonia cava (B) | Phlorotannins | UVB protector | [183] |
| 27 | Ecklonia cava (B) | Phlorotannins, 6,6′-Bieckol, dioxinodehydroeckol | Metalloproteinase inhibitors, Anti-wrinkle | [183] |
| 28 | Fucus vesiculosus, Ecklonia cava, Corallina pilulifera (R) | Eckols, Fucols, Fuhalols, Phlorethols, Fucolphlorethols | Antiphotoaging, Antiaging, Antioxidants, UV protector, Tyrosinase inhibition, Hyaluronidase inhibition | [184,185,186,187] |
| 29 | Ishige foliacea (B) | Octaphlorethol A | Tyrosinase inhibitor (whitening effect) | [188] |
| 30 | Ishige okamurae (B) | Diphlorethohydroxycarmalol | Antioxidant, UV protector | [189] |
| 31 | Sargassum horneri (B) | Sargachromanol E | Antiaging, Metalloproteinase inhibitors | [189] |
| 32 | Gracilaria gracilis (R) | Phenol | Antioxidant, ROS scavenger | [190] |
| 33 | Sargassum polycystum (B) | Flavonoids, Tannins, Terpenoids, Phenols, Saponins | Anti-melanogenesis (skin whitener) | [190,191] |
| 34 | Laurencia sp. ® | Bromophenols | Antioxidant | [192] |
| 35 | Halidrys siliquosa, Ecklonia cava, Ascoseira mirabilis, Cystosphaera jacquinotii, Ishige okamurae, (B) | Phlorotannins: diphlorethol, triphloroethol, trifuhalol and tetrafuhalol, phloroglucinol, eckol, eckstolonol | Antioxidant, UV protector | [193,194,195,196,197,198] |
| 36 | Fucus vesiculosus (B) | high polyphenol content | Increased brightness and skin age spot reduction, UV protector, and soothing benefit | [198] |
| 37 | Sargassum polycystum, Ecklonia cava subsp. stolonifera (as Ecklonia stolonifera), Ecklonia cava, Sargassum siliquastrum (B) | Unspecified flavonoids, Tannins, Phlorotannins | Tyrosinase inhibition, Anti melanogenesis | [199,200,201] |
| 38 | Eisenia bicyclis, Ecklonia cava subsp. kurome (as Ecklonia kurome), Ecklonia cava (B) | Phlorofucofuroeckol-A, Phlorotannins | Hyaluronidase inhibition Anti-inflammatory Inhibit melanin synthesis, Antioxidant | [202] |
| 39 | Ecklonia stolonifera (B) | Phlorofucofuroeckol A and B | Anti-inflammation, Antiaging (Metalloproteinase inhibitors) | [203] |
| 40 | Sargassum fusiforme (as Hizikia fusiformis) (B) | Fucosterol | Antiaging, Metalloproteinase inhibitors | [204] |
| 41 | Ecklonia cava (B) | Eckol, dieckol | Skin whitener | [204] |
| 42 | Ishige foliacea (B) | Phlorotannin | Downregulation of tyrosinase and melanin synthesis | [205,206] |
| 43 | Laminaria ochroleuca (B) | Polyphenol | Antioxidant | [207] |
| 44 | Macrocystis pyrifera (B) | Phlorotannin | Antioxidant, ROS scavenger | [208] |
| 45 | Saccharina latissima (B) | Phenol | Antioxidant | [209] |
| 46 | Sargassum serratifolium (B) | Sargachromenol | Anti-melanogenic | [210] |
| 47 | Schizymenia dubyi (R) | Phenol | Anti-melanogenic, tyrosinase inhibition | [210] |
| 48 | Sargassum thunbergia (B) | Thunbergol | Antioxidant | [211] |
| 49 | Pyropia columbina (R) | Phenol | Antioxidant | [212] |
| 50 | Rhodomela confervoides (R) | Bromophenol | Antioxidant | [213] |
| 51 | Ulva prolifera (G) | Phenol, flavonoid | Antioxidant | [214] |
| 52 | Ulva rigida (G) | Phenol | Antioxidant | [215] |
| 53 | Ecklonia cava (B) | Dioxinodehydroeckol | UVB protector | [216] |
| 54 | Eisenia bicyclis, Ecklonia cava subsp. stolonifera (as E. stolonifera) (B) | Ecokol | Anti-inflammatory, Tyrosinase inhibition | [217,218,219] |
| 55 | Ecklonia cava subsp. stolonifera (as E. stolonifera) (B) | Fucofuroeckol-A | UVB protector | [220] |
| 56 | Cystoseira compressa (B) | Fuhalol | Antioxidant | [221] |
| 57 | Fucus vesiculosus (B) | Fucophloroethol | Antioxidant | [222] |
| 58 | Ecklonia cava (B) | Eckstolonol | Antioxidant | [223] |
| 59 | Ishige foliacea (B) | Octaphlorethol-A | Antioxidant effects | [224] |
| 60 | Eisenia bicyclis, Ecklonia cava subsp. stolonifera (as E. stolonifera) (B) | Phlorofucofuroeckol-A | Hepatoprotective against oxidative stress, Tyrosinase inhibition | [225,226] |
| 61 | Ecklonia cava (B) | 2-phloroeckol, 2-O-(2,4,6-Trihydroxyphenyl)-6,6′-bieckol | Tyrosinase inhibition | [227] |
| 62 | Ascophyllum nodosum, Fucus serratus, Himanthalia elongata, Halidrys siliquosa, (B) | Phlorotannins | Antioxidant, Photoprotective | [228,229,230] |
| 63 | Ecklonia cava subsp. stolonifera (as E. stolonifera) (B) | Dioxinodehydroeckol | Downregulation of melanogenic enzymes that are namely TYR, TRP1, and TRP2 | [231] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalasariya, H.S.; Pereira, L. Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds. Appl. Sci. 2022, 12, 11954. https://doi.org/10.3390/app122311954
Kalasariya HS, Pereira L. Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds. Applied Sciences. 2022; 12(23):11954. https://doi.org/10.3390/app122311954
Chicago/Turabian StyleKalasariya, Haresh S., and Leonel Pereira. 2022. "Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds" Applied Sciences 12, no. 23: 11954. https://doi.org/10.3390/app122311954
APA StyleKalasariya, H. S., & Pereira, L. (2022). Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds. Applied Sciences, 12(23), 11954. https://doi.org/10.3390/app122311954







