MRI Assessment of the Bi-Leaflet Mechanical Heart Valve: Investigating the EOA Using the Acoustic Source Term Method
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
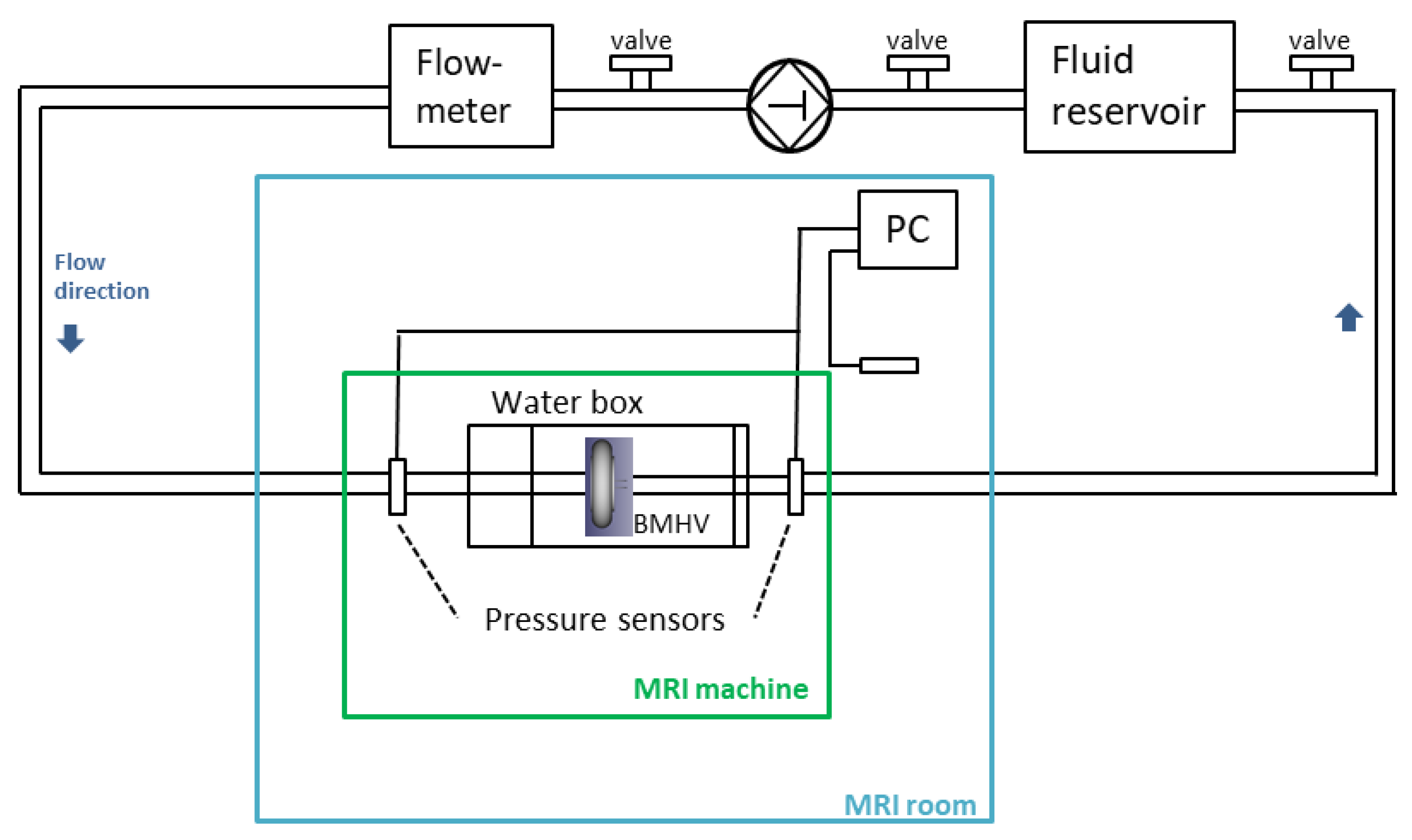
2.1. Experimental Setup
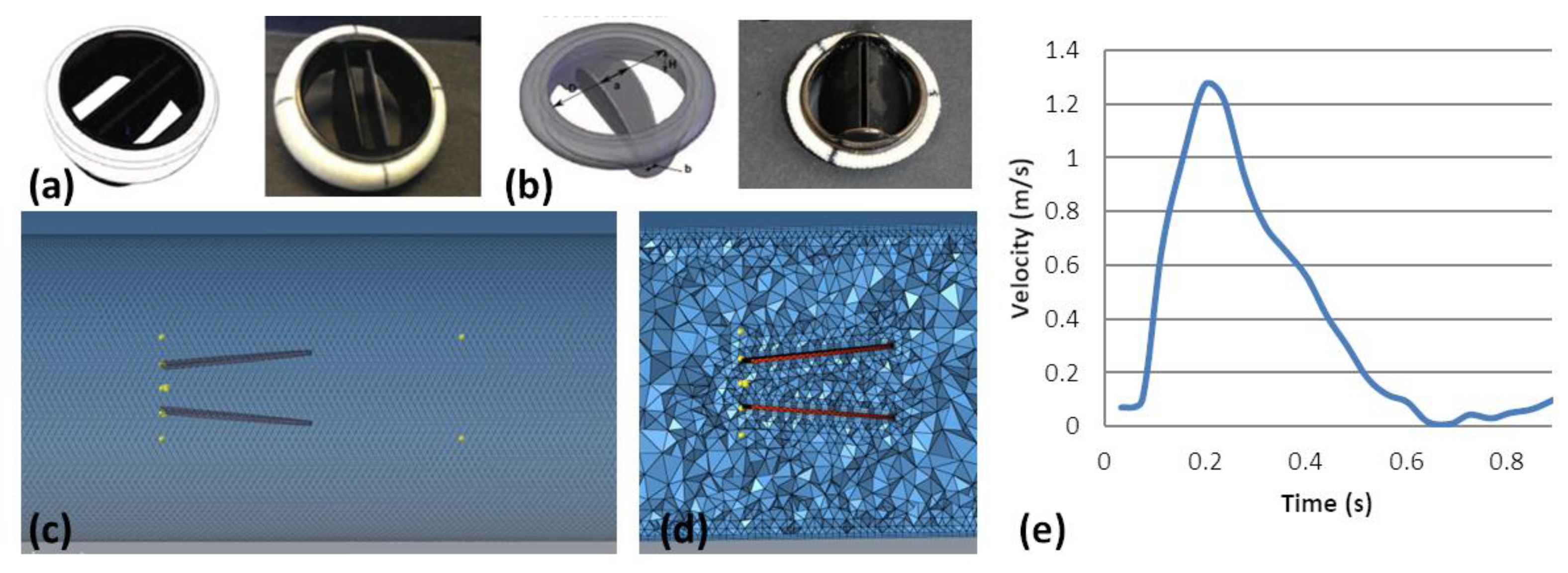
2.2. Numerical Simulation
2.3. MRI Acquisition and Post-Processing
2.4. EOA Computation
2.4.1. Continuity EOA Computation
2.4.2. AST Computation and EOAAST Measurement
2.5. Statistical Analysis
3. Results
3.1. Numerical Simulations
3.1.1. Prosthesis Design and Patient Haemodynamic Parameters
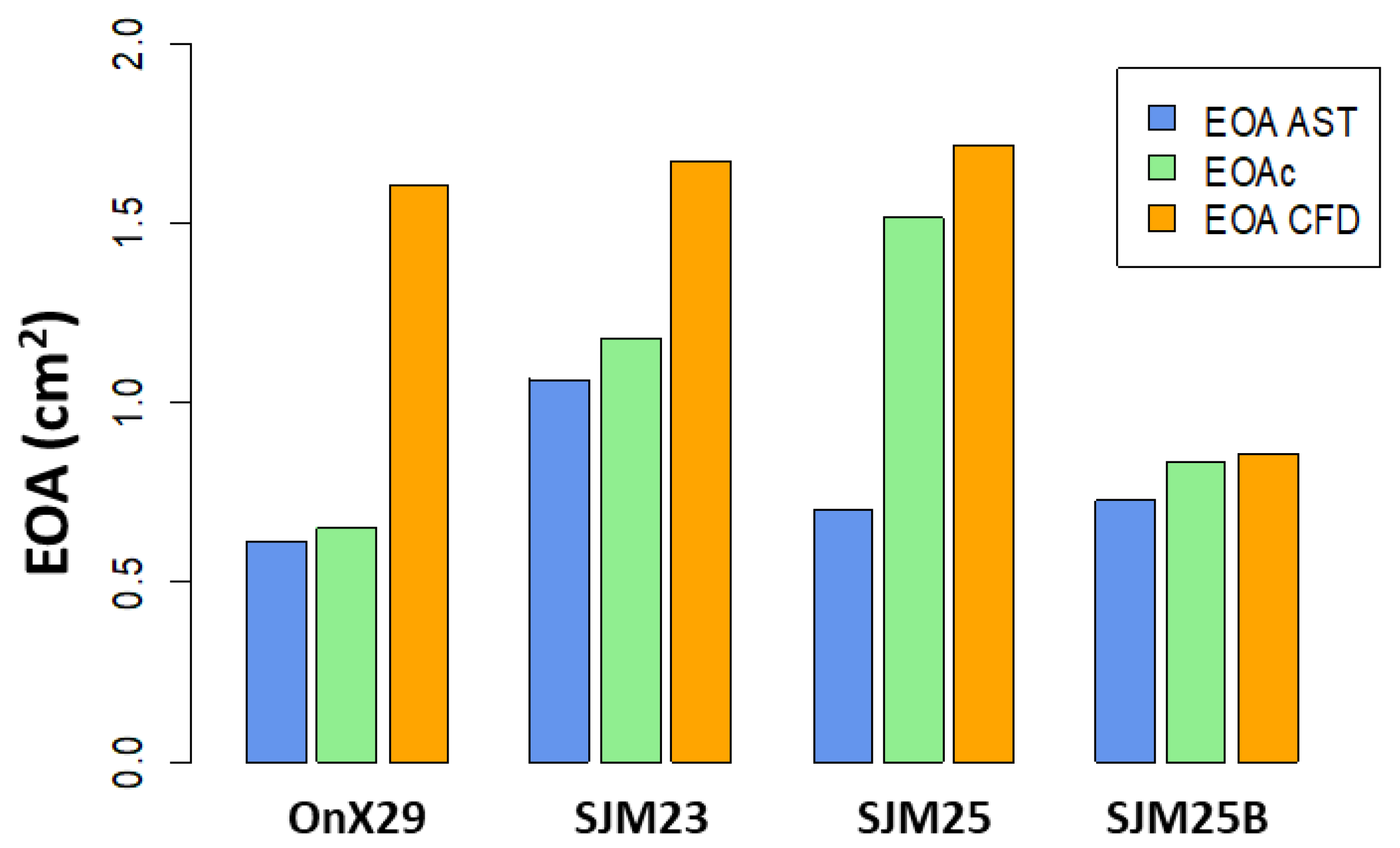
3.1.2. EOA Computation—Distance to Center
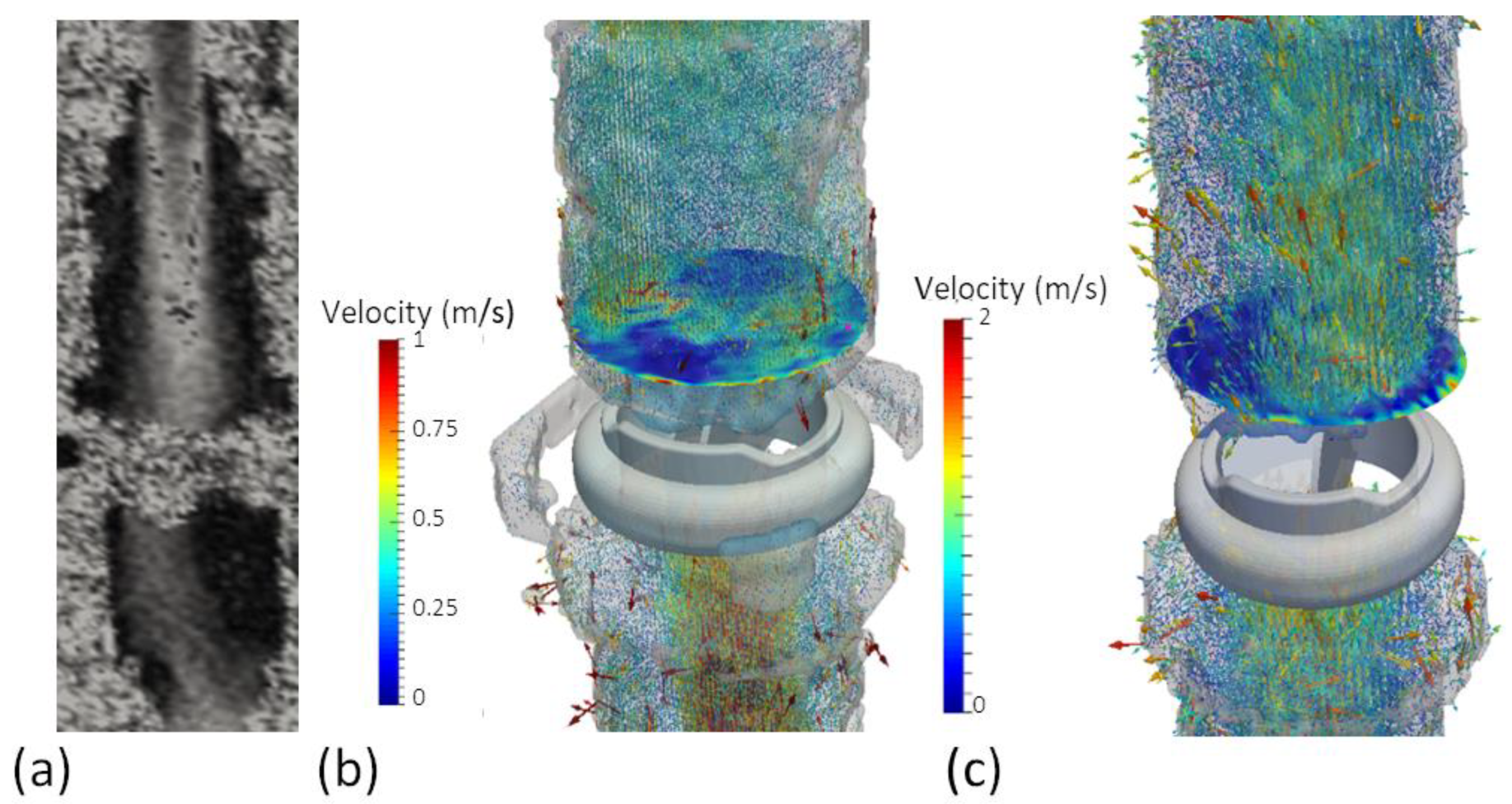
3.2. 4D Phase-Contrast Flow measurements
4. Discussion
4.1. EOA Computation with MRI
4.2. Toward Full 4D Flow Assessment by MRI
4.3. Clinical Perspectives
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.H.; Griffith, B.P.; Gammie, J.S. Isolated Aortic Valve Replacement in North America Comprising 108,687 Patients in 10 Years: Changes in Risks, Valve Types, and Outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D.; DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-Term Safety and Effectiveness of Mechanical versus Biologic Aortic Valve Prostheses in Older Patients: Results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation 2013, 127, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Suchá, D.; Chamuleau, S.A.J.; Symersky, P.; Meijs, M.F.L.; van den Brink, R.B.A.; de Mol, B.A.J.M.; Mali, W.P.T.M.; Habets, J.; van Herwerden, L.A.; Budde, R.P.J. Baseline MDCT Findings after Prosthetic Heart Valve Implantation Provide Important Complementary Information to Echocardiography for Follow-up Purposes. Eur. Radiol. 2016, 26, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Seward, J.B.; Tajik, A.J. The Echo Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 978-0-7817-4853-7. [Google Scholar]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D Flow Cardiovascular Magnetic Resonance Consensus Statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Habets, J.; Budde, R.P.; Symersky, P.; van den Brink, R.B.; de Mol, B.A.; Mali, W.P.; van Herwerden, L.A.; Chamuleau, S.A. Diagnostic Evaluation of Left-Sided Prosthetic Heart Valve Dysfunction. Nat. Rev. Cardiol. 2011, 8, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Adegbite, O.; Kadem, L.; Newling, B. Purely Phase-Encoded MRI of Turbulent Flow through a Dysfunctional Bileaflet Mechanical Heart Valve. Magn. Reson. Mater. Phys. Biol. Med. 2014, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Von Knobelsdorff-Brenkenhoff, F.; Karunaharamoorthy, A.; Trauzeddel, R.F.; Barker, A.J.; Blaszczyk, E.; Markl, M.; Schulz-Menger, J. Evaluation of Aortic Blood Flow and Wall Shear Stress in Aortic Stenosis and Its Association with Left Ventricular Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004038. [Google Scholar] [CrossRef] [PubMed]
- Von Knobelsdorff-Brenkenhoff, F.; Dieringer, M.A.; Greiser, A.; Schulz-Menger, J. In Vitro Assessment of Heart Valve Bioprostheses by Cardiovascular Magnetic Resonance: Four-Dimensional Mapping of Flow Patterns and Orifice Area Planimetry. Eur. J. Cardiothorac. Surg. 2011, 40, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Tanis, W.; Budde, R.P.J.; van der Bilt, I.A.C.; Delemarre, B.; Hoohenkerk, G.; van Rooden, J.-K.; Scholtens, A.M.; Habets, J.; Chamuleau, S. Novel Imaging Strategies for the Detection of Prosthetic Heart Valve Obstruction and Endocarditis. Neth. Heart J. 2016, 24, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Joannic, D.; Delassus, P.; Lalande, A.; Juillion, P.; Fontaine, J.-F. Comparison of the Strain Field of Abdominal Aortic Aneurysm Measured by Magnetic Resonance Imaging and Stereovision: A Feasibility Study for Prediction of the Risk of Rupture of Aortic Abdominal Aneurysm. J. Biomech. 2015, 48, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-S. Formula for the Viscosity of a Glycerol−Water Mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Garcia, D.; Kadem, L. What Do You Mean by Aortic Valve Area: Geometric Orifice Area, Effective Orifice Area, or Gorlin Area? J. Heart Valve Dis. 2006, 15, 601–608. [Google Scholar] [PubMed]
- Kadem, L.; Knapp, Y.; Pibarot, P.; Bertrand, E.; Garcia, D.; Durand, L.-G.; Rieu, R. A New Experimental Method for the Determination of the Effective Orifice Area Based on the Acoustical Source Term. Exp. Fluids 2005, 39, 1051–1060. [Google Scholar] [CrossRef]
- Gorlin, R.; Gorlin, S.G. Hydraulic Formula for Calculation of the Area of the Stenotic Mitral Valve, Other Cardiac Valves, and Central Circulatory Shunts. I. Am. Heart J. 1951, 41, 1–29. [Google Scholar] [CrossRef]
- Garcia, J.; Marrufo, O.R.; Rodriguez, A.O.; Larose, E.; Pibarot, P.; Kadem, L. Cardiovascular Magnetic Resonance Evaluation of Aortic Stenosis Severity Using Single Plane Measurement of Effective Orifice Area. J. Cardiovasc. Magn. Reson. 2012, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Powell, A. Theory of Vortex Sound. J. Acoust. Soc. Am. 1964, 36, 177–195. [Google Scholar] [CrossRef]
- Howe, M.S. Theory of Vortex Sound; Cambridge University Press: Cambridge, UK, 2003; ISBN 978-0-521-01223-2. [Google Scholar]
- Ha, H.; Lantz, J.; Ziegler, M.; Casas, B.; Karlsson, M.; Dyverfeldt, P.; Ebbers, T. Estimating the Irreversible Pressure Drop across a Stenosis by Quantifying Turbulence Production Using 4D Flow MRI. Sci. Rep. 2017, 7, 46618. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Kvitting, J.-P.; Dyverfeldt, P.; Ebbers, T. Validation of Pressure Drop Assessment Using 4D Flow MRI-Based Turbulence Production in Various Shapes of Aortic Stenoses. Magn. Reson. Med. 2019, 81, 893–906. [Google Scholar] [CrossRef] [PubMed]


| BMHV Model (Condition) | Pixel Spacing (mm) | Pix. Thickness (mm) | Label | Inner Diam. (mm) | Height (mm) | Op. Angle (°) | VENC (cm/s) | Blurring Distance (mm) | Blurring Distance Distal (mm) | Ratio Blurring Distal/Centre | EOAc (cm2) | Recovery Plane (nb(Dist. mm)) | EOAAST Mean (cm2) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONX29 | OnX (normal) | 0.781 | 2.5 | 29 | 23.4 | 14.2 | 90 | 300 | 20.2 | 12.1 | 0.60 | 1.74 | 5 (12.5) | 0.61 |
| SJM23 | St Jude Medical (normal) | 0.781 | 2.5 | 23 | 21.4 | 12.5 | 85 | 280 | 18.9 | 16.9 | 0.89 | 1.4 | 3 (7.5) | 0.70 |
| SJM25 | St Jude Medical (normal) | 0.781 | 2.5 | 25 | 23 | 13.9 | 85 | 260 | 23.4 | 32.7 | 1.40 | 1.55 | 2 (5) | 1.06 |
| SJM25B | St Jude Medical (blocked) | 0.938 | 3 | 25 | 23 | 13.9 | 85 | 310 | 21.4 | 25.4 | 1.19 | 1.01 | 4 (12) | 0.73 |
| Design of Experiment Parameters | Range | Increments | No. of Simulations |
|---|---|---|---|
| BMHV internal diameter (mm) | 20–24 | 1 | 5 |
| Distance between leaflet (mm) | 3–4 | 0.5 | 3 |
| Leaflet opening angles (°) | 80–90 | 5 | 3 |
| BMHV Dysfunction (opening angle of one leaflet °) | 0–15 | 15 | 2 × 2 |
| Stroke volume (mL) | 70–120 | 70; 90; 120 | 3 |
| Heart Rate (bpm) | 60–120 | 60; 70; 120 | 3 |
| Mesh size | 3 |
| Name | Opening Angle (°) | Diameter (mm) | Leaflet Space | HR (bpm) | SV (mL) | Mesh Size (mm) | EOA AST Mean (cm2) | EOA AST SD (cm2) | EOA AST min (cm2) | EOA AST Max (cm2) | Rec. Plane No. (Dist. mm) | EOAAST (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMHV_Leaflet85-21-30 | 85 | 21 | 3.0 | 70 | 90 | 0.5 | 1.62 | 0.20 | 0.99 | 1.75 | 8 (16) | 1.66 |
| BMHV_Leaflet85-22-35 | 85 | 22 | 3.5 | 70 | 90 | 0.5 | 1.65 | 0.21 | 0.99 | 1.77 | 3 (6) | 1.69 |
| BMHV_Leaflets85-23-35 | 85 | 23 | 3.5 | 70 | 90 | 0.5 | 1.34 | 0.38 | 0.76 | 1.74 | 3 (6) | 1.72 |
| BMHV_Leaflet85-24-40 | 85 | 24 | 4.0 | 70 | 90 | 0.5 | 1.76 | 0.06 | 1.58 | 1.83 | 2 (4) | 1.77 |
| BMHV_Leaflet90-20-30 | 90 | 20 | 4.0 | 70 | 90 | 0.5 | 1.49 | 0.23 | 0.91 | 1.64 | 4 (8) | 1.60 |
| BMHV_Leaflet90-21-30 | 90 | 21 | 4.0 | 70 | 90 | 0.5 | 1.62 | 0.17 | 1.07 | 1.73 | 3 (6) | 1.68 |
| BMHV_leaflet90-22-35 | 90 | 22 | 3.5 | 70 | 90 | 0.5 | 1.47 | 0.24 | 0.83 | 1.66 | 3 (6) | 1.66 |
| BMHV_Leaflet90-23-35 | 90 | 23 | 3.5 | 70 | 90 | 0.5 | 1.60 | 0.18 | 1.01 | 1.71 | 4 (8) | 1.61 |
| BMHV_leaflet90-24-40 | 90 | 24 | 4.0 | 70 | 90 | 0.5 | 1.54 | 0.20 | 0.89 | 1.67 | 5 (10) | 1.59 |
| BMHV_Leaflet90-22-35_HR60 | 90 | 22 | 3.5 | 60 | 90 | 0.5 | 1.45 | 0.23 | 0.83 | 1.69 | 5 (10) | 1.57 |
| BMHV_Leaflet90-22-35_HR120 | 90 | 22 | 3.5 | 120 | 90 | 0.5 | 1.45 | 0.25 | 0.84 | 1.70 | 5 (10) | 1.58 |
| BMHV_leaflet90-22-35_M03 | 90 | 22 | 3.5 | 70 | 90 | 0.3 | 1.69 | 0.21 | 0.99 | 1.80 | 2 (4) | 1.74 |
| BMHV_leaflet90-22-35_M07 | 90 | 22 | 3.5 | 70 | 90 | 0.7 | 1.49 | 0.19 | 0.87 | 1.67 | 3 (6) | 1.56 |
| BMHV_leaflet90-22-35_M09 | 90 | 22 | 3.5 | 70 | 90 | 0.9 | 0.89 | 0.11 | 0.75 | 1.08 | 5 (10) | 0.94 |
| BMHV_Leaflet90-22-35_SV70 | 90 | 22 | 3.5 | 70 | 70 | 0.5 | 1.44 | 0.23 | 0.83 | 1.61 | 6 (12) | 1.57 |
| BMHV_Leaflet90-22-35_SV120 | 90 | 22 | 3.5 | 70 | 120 | 0.5 | 1.60 | 0.18 | 1.01 | 1.71 | 3 (6) | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evin, M.; Joannic, D.; Monnet, A.; Fletcher, D.F.; Grieve, S.M.; Fontaine, J.-F.; Lalande, A. MRI Assessment of the Bi-Leaflet Mechanical Heart Valve: Investigating the EOA Using the Acoustic Source Term Method. Appl. Sci. 2022, 12, 11771. https://doi.org/10.3390/app122211771
Evin M, Joannic D, Monnet A, Fletcher DF, Grieve SM, Fontaine J-F, Lalande A. MRI Assessment of the Bi-Leaflet Mechanical Heart Valve: Investigating the EOA Using the Acoustic Source Term Method. Applied Sciences. 2022; 12(22):11771. https://doi.org/10.3390/app122211771
Chicago/Turabian StyleEvin, Morgane, David Joannic, Aurélien Monnet, David F. Fletcher, Stuart M. Grieve, Jean-François Fontaine, and Alain Lalande. 2022. "MRI Assessment of the Bi-Leaflet Mechanical Heart Valve: Investigating the EOA Using the Acoustic Source Term Method" Applied Sciences 12, no. 22: 11771. https://doi.org/10.3390/app122211771
APA StyleEvin, M., Joannic, D., Monnet, A., Fletcher, D. F., Grieve, S. M., Fontaine, J.-F., & Lalande, A. (2022). MRI Assessment of the Bi-Leaflet Mechanical Heart Valve: Investigating the EOA Using the Acoustic Source Term Method. Applied Sciences, 12(22), 11771. https://doi.org/10.3390/app122211771






