In Silico Evaluation of a Physiological Controller for a Rotary Blood Pump Based on a Sensorless Estimator
Abstract
1. Introduction
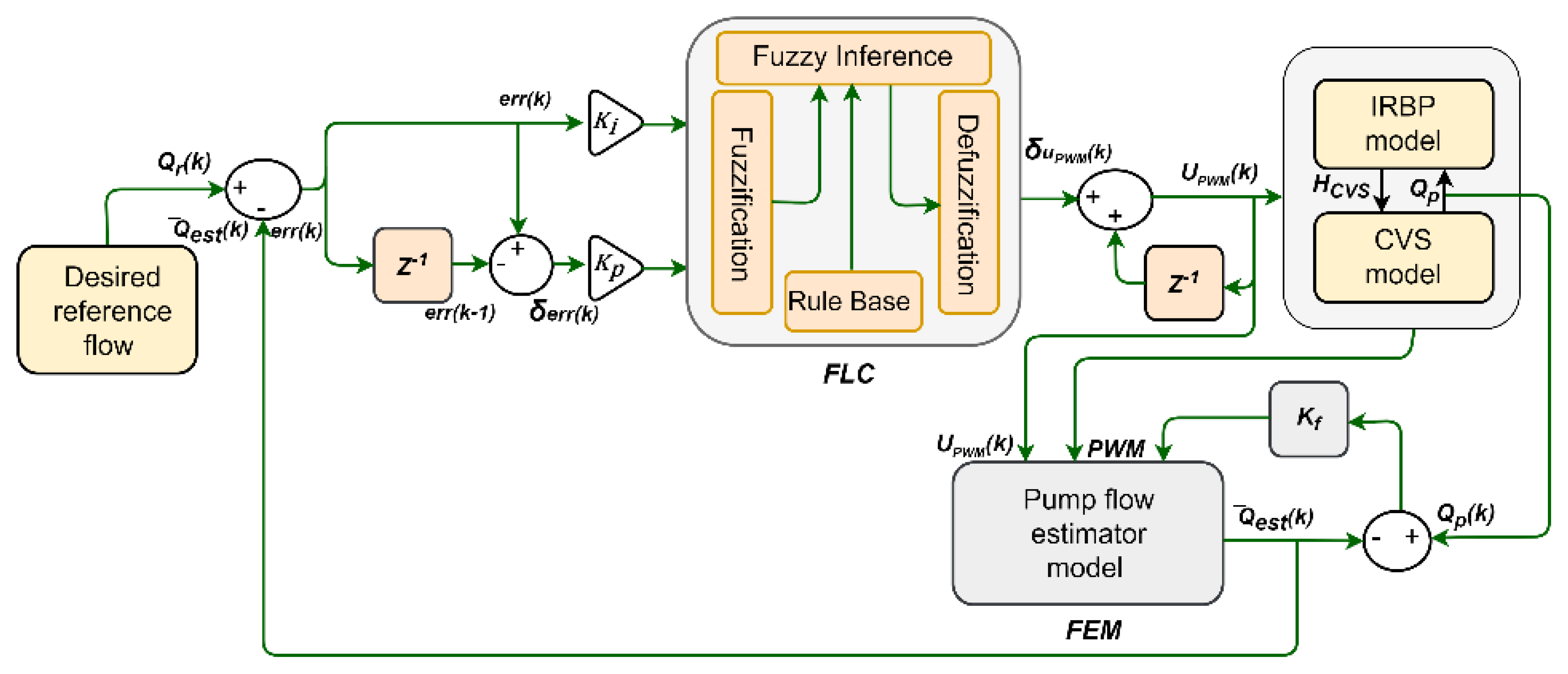
2. Physiological Controller Development
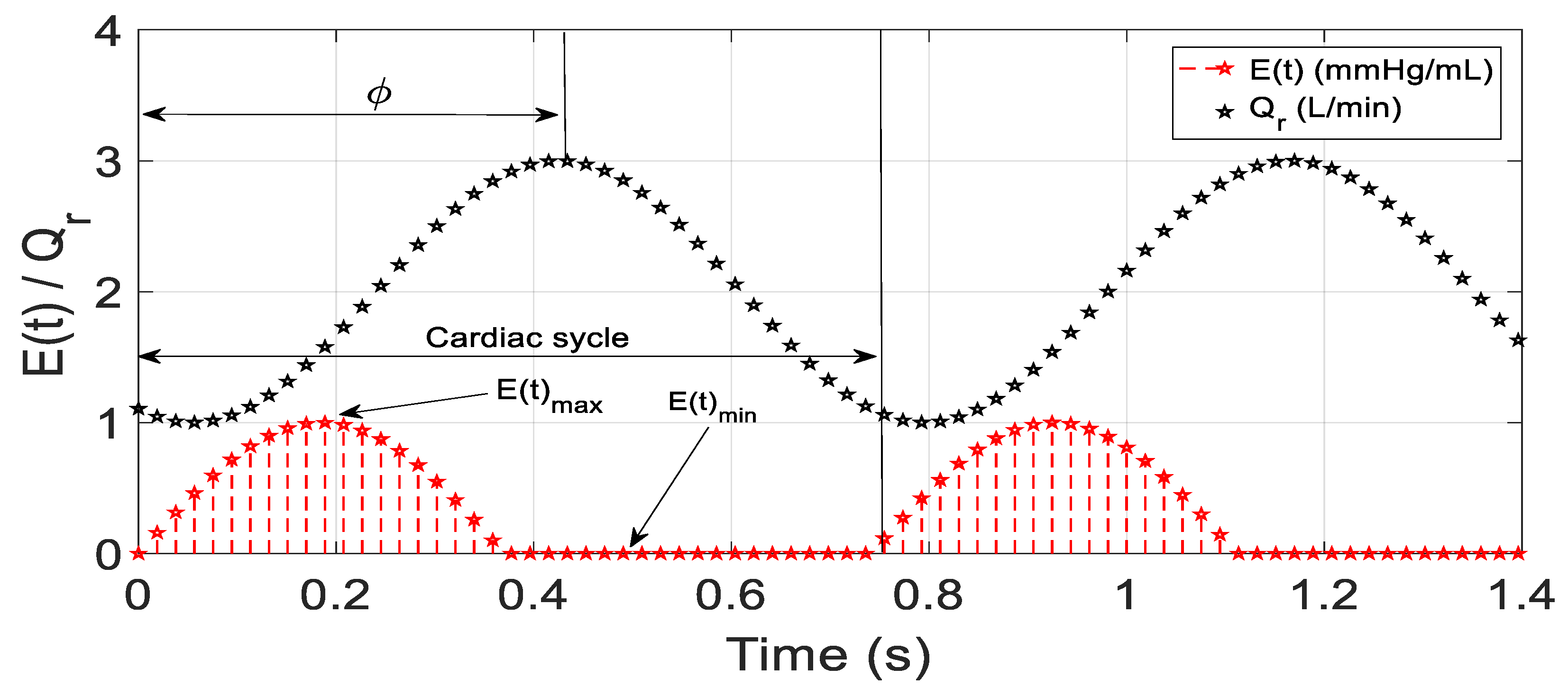
2.1. Software Model of CVS
- -
- The electrical motor winding:
- -
- The motor electromagnetic torque:
- -
- The hydraulic pump:
2.2. Flow Estimator Model
2.3. Controller Design
2.4. Desired Reference Flow
2.5. In Silico Protocols
3. Simulation Results
3.1. Constant Speed Controller
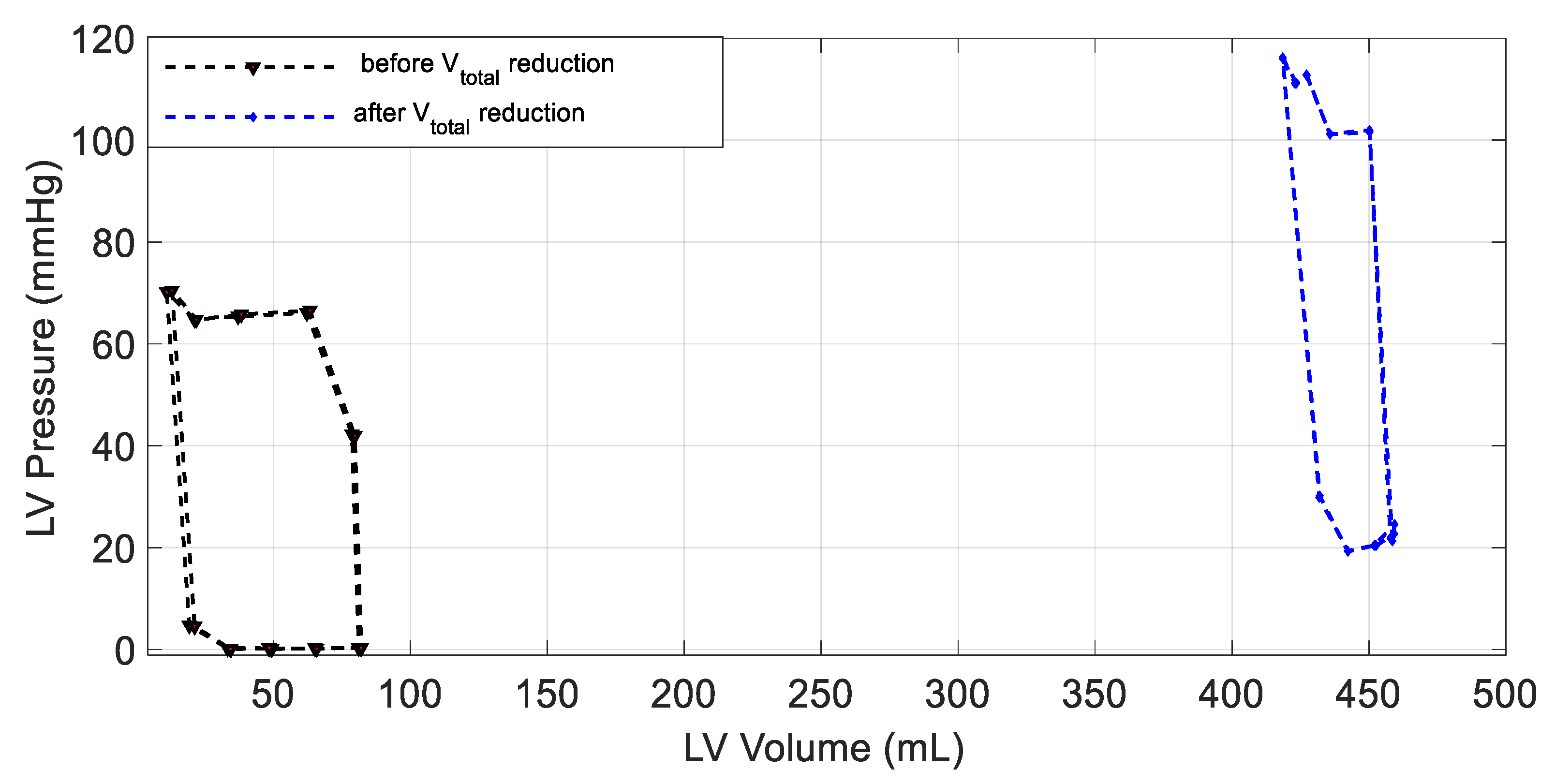
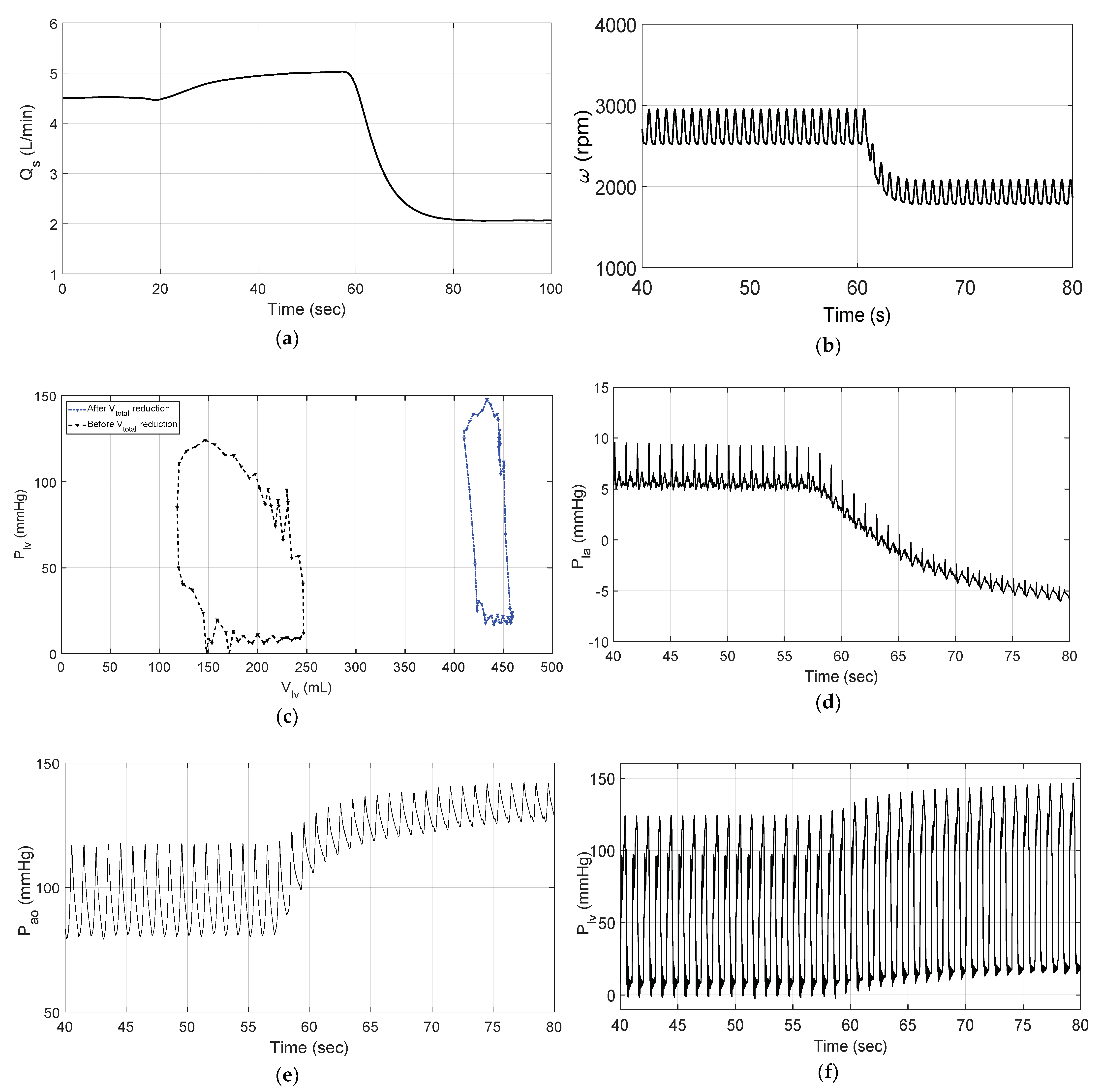
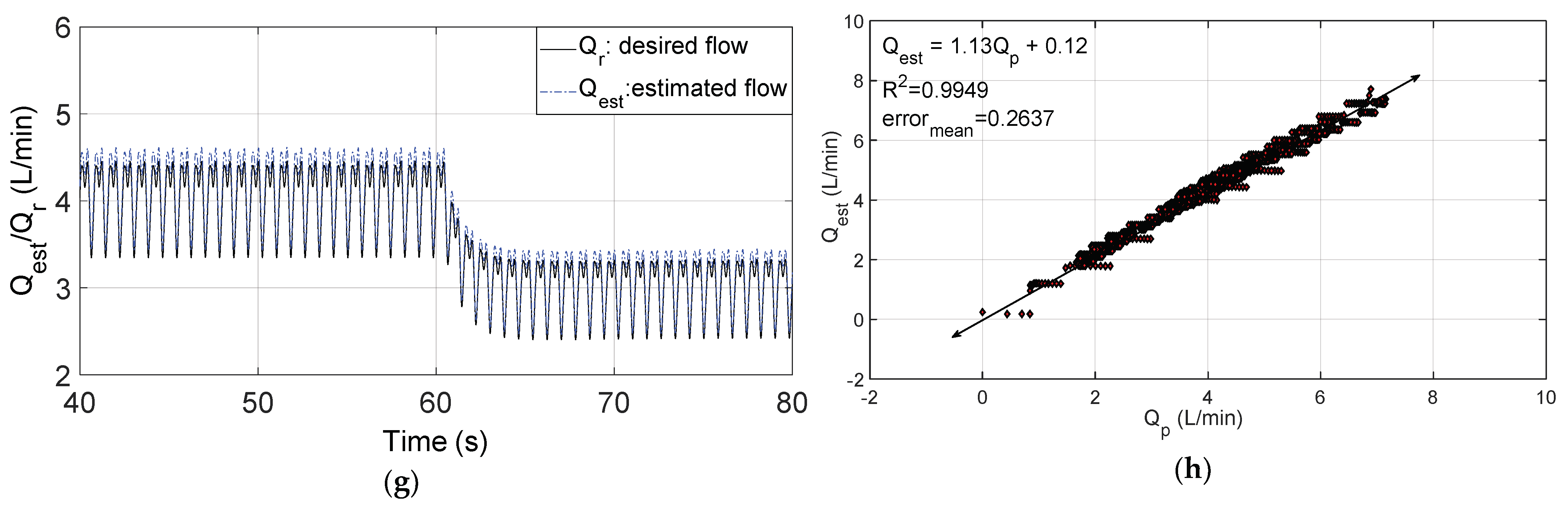
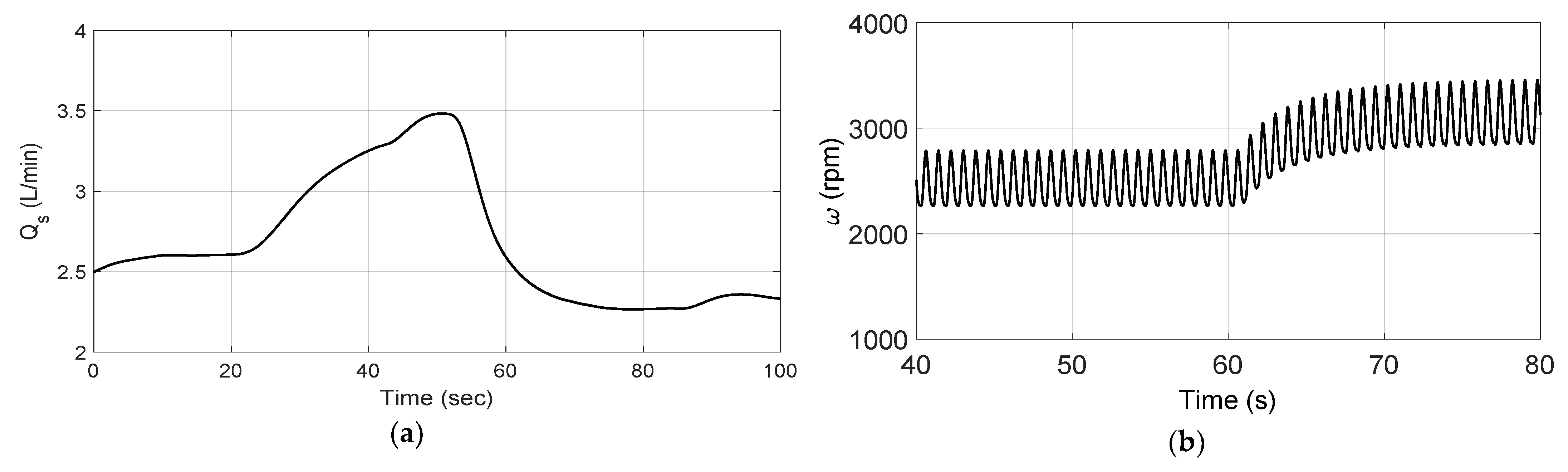
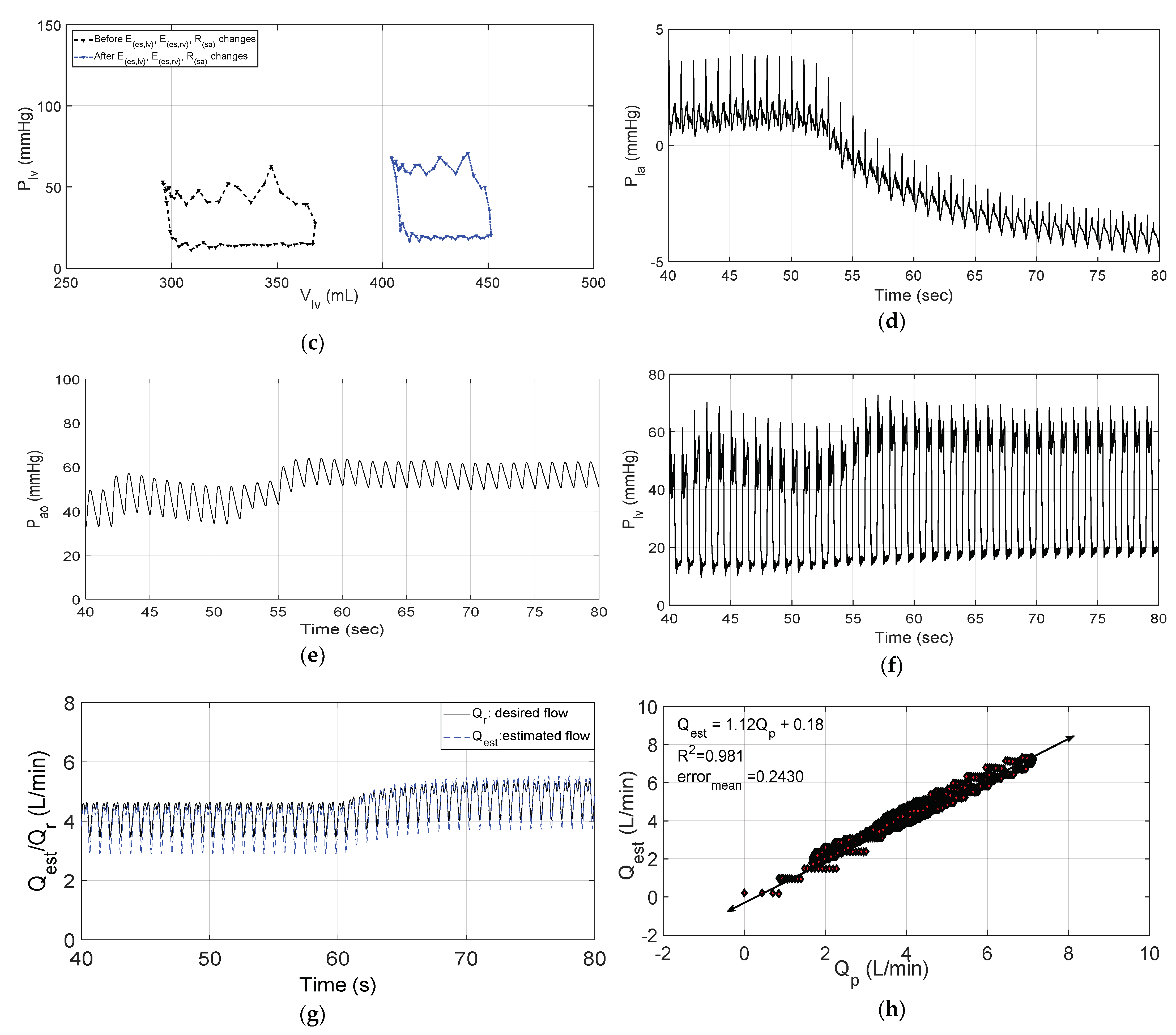
3.2. Changes in Patient Parameters to Demonstrate a Relaxation Test
3.3. Changes in Patient Parameters to Demonstrate an Exercise Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVS | Cardiovascular system |
| HF | Heart failure |
| ARX | Auto-regressive |
| IRBP | Implantable rotary blood pump |
| FLC | Fuzzy logic control |
| PI | Proportional-integral |
| FEM | Flow estimator model |
| PWM | Pulse-width modulation |
| Desired reference flow | |
| Estimated average pump flow | |
| Left atrium pressure | |
| Aortic pressure | |
| Left ventricle pressure | |
| ) | Elastance function |
| Total blood volume | |
| Left ventricle contractility | |
| Right ventricle contractility | |
| Systemic vascular resistance | |
| Systematic flow | |
| Actual flow | |
| Left ventricle volume | |
| Left ventricle pressure | |
| SV | Stroke volume |
References
- Wang, Y.; Shen, P.; Zheng, M.; Fu, P.; Liu, L.; Wang, J.; Yuan, L. Influence of Impeller Speed Patterns on Hemodynamic Characteristics and Hemolysis of the Blood Pump. Appl. Sci. 2019, 9, 4689. [Google Scholar] [CrossRef]
- Wu, P. Recent advances in the application of computational fluid dynamics in the development of rotary blood pumps. Med. Nov. Technol. Devices 2022, 28, 100177. [Google Scholar] [CrossRef]
- Bakouri, M. Physiological Control Law for Rotary Blood Pumps with Full-State Feedback Method. Appl. Sci. 2019, 9, 4593. [Google Scholar] [CrossRef]
- Jing, T.; Xin, T.; Wang, F.; Zhang, Z.; Zhou, L. Control Strategy Design of a Microblood Pump Based on Heart-Rate Feedback. Micromachines 2022, 13, 358. [Google Scholar] [CrossRef]
- Elenkov, M.; Lukitsch, B.; Ecker, P.; Janeczek, C.; Harasek, M.; Gföhler, M. Non-parametric dynamical estimation of blood flow rate, pressure difference and viscosity for a miniaturized blood pump. Int. J. Artif. Organs 2022, 45, 207–215. [Google Scholar] [CrossRef]
- Cysyk, J.; Jhun, C.S.; Newswanger, R.; Pae, W.; Izer, J.; Flory, H.; Reibson, J. Weiss, W.; Rosenberg, G. Vivo Evaluation of a Physiologic Control System for Rotary Blood Pumps Based on the Left Ventricular Pressure-Volume Loop. ASAIO J. 2022, 5, 791–799. [Google Scholar] [CrossRef]
- Ogawa, D.; Kobayashi, S.; Yamazaki, K.; Motomura, T.; Nishimura, T.; Shimamura, J.; Tsukiya, T.; Mizuno, T.; Takewa, Y.; Tatsumi, E.; et al. Evaluation of cardiac beat synchronization control for a rotary blood pump on valvular regurgitation with a mathematical model. Artif. Organs 2020, 45, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Sadatieh, S.; Dehghani, M.; Mohammadi, M.; Boostani, R. Extremum-seeking control of left ventricular assist device to maximize the cardiac output and prevent suction. Chaos Solitons Fractals 2021, 148, 111013. [Google Scholar] [CrossRef]
- Liang, L.; Qin, K.; El-Baz, A.S.; Roussel, T.J.; Sethu, P.; Giridharan, G.A.; Wang, Y. A Flow Sensor-Based Suction-Index Control Strategy for Rotary Left Ventricular Assist Devices. Sensors 2021, 21, 6890. [Google Scholar] [CrossRef]
- Yun, Z.; Li, K.; Jiang, H.; Tang, X. A Composite Flexible Sensor for Direct Ventricular Assist Device. Sensors 2022, 22, 2607. [Google Scholar] [CrossRef] [PubMed]
- Fetanat, M.; Stevens, M.; Hayward, C.; Lovell, N. Adaptive sensorless control of LVAD using deep convolutional neural network. J. Heart Lung Transplant. 2021, 40, S172. [Google Scholar] [CrossRef]
- Bakouri, M.; Alassaf, A.; Alshareef, K.; Abdelsalam, S.; Ismail, H.F.; Ganoun, A.; Alomari, A.-H. An Optimal H-Infinity Controller for Left Ventricular Assist Devices Based on a Starling-like Controller: A Simulation Study. Mathematics 2022, 10, 731. [Google Scholar] [CrossRef]
- Cordeiro, T.D.; Sousa, D.L.; Cestari, I.A.; Lima, A.M.N. A physiological control system for ECG-synchronized pulsatile pediatric ventricular assist devices. Biomed. Signal Process. Control 2020, 57, 101752. [Google Scholar] [CrossRef]
- Son, J.; Du, D.; Du, Y. Modelling and control of a failing heart managed by a left ventricular assist device. Biocybern. Biomed. Eng. 2020, 40, 559–573. [Google Scholar] [CrossRef]
- Huang, F.; Ruan, X.; Fu, X. Pulse-Pressure–Enhancing Controller for Better Physiologic Perfusion of Rotary Blood Pumps Based on Speed Modulation. ASAIO J. 2014, 60, 269–279. [Google Scholar] [CrossRef]
- Choi, S.; Antaki, J.E.; Boston, R.; Thomas, D. A sensorless approach to control of a turbodynamic left ventricular assist system. IEEE Trans. Control Syst. Technol. 2001, 9, 473–482. [Google Scholar] [CrossRef]
- Wang, Y.; Koenig, S.C.; Wu, Z.J.; Slaughter, M.S.; Giridharan, G.A. Sensorless physiologic control, suction prevention, and flow balancing algorithm for rotary biventricular assist devices. IEEE Trans. Control Syst. Technol. 2017, 27, 717–729. [Google Scholar] [CrossRef]
- Lim, E.; Dokos, S.; Cloherty, S.L.; Salamonsen, R.F.; Mason, D.G.; Reizes, J.A.; Lovell, N.H. Parameter-Optimized Model of Cardiovascular–Rotary Blood Pump Interactions. IEEE Trans. Biomed. Eng. 2009, 57, 254–266. [Google Scholar] [CrossRef]
- Lim, E.; Dokos, S.; Salamonsen, R.F.; Rosenfeldt, F.L.; Ayre, P.J.; Lovell, N.H. Numerical optimization studies of cardiovascular–rotary blood pump interaction. Artif. Organs 2012, 36, E110–E124. [Google Scholar] [CrossRef] [PubMed]
- Bakouri, M.A.; Savkin, A.V.; Alomari, A.H. Nonlinear modelling and control of left ventricular assist device. Electron. Lett. 2015, 51, 613–615. [Google Scholar] [CrossRef]
- Bakouri, M.A.; Salamonsen, R.F.; Savkin, A.V.; AlOmari, A.H.; Lim, E.; Lovell, N.H. A Sliding Mode-Based Starling-Like Controller for Implantable Rotary Blood Pumps. Artif. Organs 2014, 38, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Nise, N.S. Control Systems Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Jayetileke, H.R.; de Mel, W.R.; Mukhopadhyay, S.C. Real-Time Metaheuristic Algorithm for Dynamic Fuzzification, De-Fuzzification and Fuzzy Reasoning Processes. Appl. Sci. 2022, 12, 8242. [Google Scholar] [CrossRef]
- Lim, E.; Alomari, A.H.; Savkin, A.V.; Dokos, S.; Fraser, J.F.; Timms, D.L.; Mason, D.G.; Lovell, N.H. A method for control of an implantable rotary blood pump for heart failure patients using noninvasive measurements. Artif. Organs 2011, 35, E174–E180. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S. Physiologic outcome of varying speed rotary blood pump support algorithms: A review study. Australas. Phys. Eng. Sci. Med. 2016, 39, 13–28. [Google Scholar] [CrossRef]
- Petukhov, D.; Korn, L.; Walter, M.; Telyshev, D. A novel control method for rotary blood pumps as left ventricular assist device utilizing aortic valve state detection. BioMed Res. Int. 2019, 11, 2019. [Google Scholar] [CrossRef]
- Fetanat, M.; Stevens, M.; Hayward, C.; Lovell, N.H. A physiological control system for an implantable heart pump that accommodates for interpatient and intrapatient variations. IEEE Trans. Biomed. Eng. 2019, 67, 1167–1175. [Google Scholar] [CrossRef]
- Alomari, A.H.; Savkin, A.V.; Stevens, M.; Mason, D.G.; Timms, D.L.; Salamonsen, R.F.; Lovell, N.H. Developments in control systems for rotary left ventricular assist devices for heart failure patients: A review. Physiol. Meas. 2013, 34, R1. [Google Scholar] [CrossRef]
- Peng, J.; Lu, A.; Wang, Y. Modeling of a New Sensorless Suction Detection System for the Rotary Left Ventricular Assist Device. In Proceedings of the 9th International Conference in Information Technology Medicine Education ITME 2018, Hangzhou, China, 19–21 October 2018. [Google Scholar]
- Son, J.; Du, D.; Du, Y. Feedback control of rotary blood pump for preventing left ventricular suction. In Proceedings of the America Control Conference 2019, Philadelphia, PA, USA, 9–13 March 2019; pp. 5426–5431. [Google Scholar]
- Bakouri, M.; Alassaf, A.; Alshareef, K.; Smida, A.; AlMohimeed, I.; Alqahtani, A.; Aboamer, M.A.; Alharbi, Y. A Feasible Method to Control Left Ventricular Assist Devices for Heart Failure Patients: A Numerical Study. Mathematics 2022, 10, 2251. [Google Scholar] [CrossRef]
- Salamonsen, R.F.; Lim, E.; Gaddum, E.; Alomari, A.H.; Gregory, S.D.; Stevens, M.; Mason, D.G.; Fraser, J.F.; Timms, D.; Karunanithi, M.K.; et al. Theoretical foundations of a Starling-like controller for rotary blood pumps. Artif. Organs 2012, 36, 787–796. [Google Scholar] [CrossRef]


| NG3 | NG2 | NG1 | Z | PO1 | PO2 | PO3 |
|---|---|---|---|---|---|---|
| Large negative | Medium negative | Small negative | Zero | Small positive | Medium positive | Large positive |
| NG3 | NG2 | NG1 | Z | PO1 | PO2 | PO3 | ||
|---|---|---|---|---|---|---|---|---|
| NG3 | NG3 | NG3 | NG3 | NG2 | NG1 | NG1 | Z | |
| NG2 | NG3 | NG3 | NG2 | NG1 | NG1 | Z | PO1 | |
| NG1 | NG3 | NG2 | NG1 | NG1 | Z | PO1 | PO1 | |
| Z | NG2 | NG1 | NG1 | Z | PO1 | PO1 | PO2 | |
| PO1 | NG1 | NG1 | Z | PO1 | PO1 | PO2 | PO3 | |
| PO2 | NG1 | Z | PO1 | PO1 | PO2 | PO3 | PO3 | |
| PO3 | Z | PO1 | PO1 | PO2 | PO3 | PO3 | PO3 | |
| Parameter | Healthy Case | Heart Failure Case |
|---|---|---|
| 5300 mL | 5800 mL | |
| 3.54 mm Hg/mL | 0.71 mm Hg/mL | |
| 1.75 mm Hg/mL | 0.53 mm Hg/mL | |
| 0.74 mm Hg·s/mL | 1.11 mm Hg·s/mL |
| Parameters | Healthy | HF and IRBP | |
|---|---|---|---|
| Rest | Exercise | ||
| (mmHg) | 25 | 15 | 10 |
| (mmHg) | 120 | 100 | 80 |
| (mmHg) | 120 | 95 | 80 |
| (L/min) | 5.5 | 2 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakouri, M.; Alassaf, A.; Alshareef, K.; AlMohimeed, I.; Alqahtani, A.; Aboamer, M.A.; Alonazi, K.A.; Alharbi, Y. In Silico Evaluation of a Physiological Controller for a Rotary Blood Pump Based on a Sensorless Estimator. Appl. Sci. 2022, 12, 11537. https://doi.org/10.3390/app122211537
Bakouri M, Alassaf A, Alshareef K, AlMohimeed I, Alqahtani A, Aboamer MA, Alonazi KA, Alharbi Y. In Silico Evaluation of a Physiological Controller for a Rotary Blood Pump Based on a Sensorless Estimator. Applied Sciences. 2022; 12(22):11537. https://doi.org/10.3390/app122211537
Chicago/Turabian StyleBakouri, Mohsen, Ahmad Alassaf, Khaled Alshareef, Ibrahim AlMohimeed, Abdulrahman Alqahtani, Mohamed Abdelkader Aboamer, Khalid A. Alonazi, and Yousef Alharbi. 2022. "In Silico Evaluation of a Physiological Controller for a Rotary Blood Pump Based on a Sensorless Estimator" Applied Sciences 12, no. 22: 11537. https://doi.org/10.3390/app122211537
APA StyleBakouri, M., Alassaf, A., Alshareef, K., AlMohimeed, I., Alqahtani, A., Aboamer, M. A., Alonazi, K. A., & Alharbi, Y. (2022). In Silico Evaluation of a Physiological Controller for a Rotary Blood Pump Based on a Sensorless Estimator. Applied Sciences, 12(22), 11537. https://doi.org/10.3390/app122211537






