1. Background
Neurological and ocular manifestations of COVID-19 infection and vaccine have been widely reported in the literature [
1,
2,
3,
4], with infective, vascular, and immunological pathogenic mechanisms hypothesized [
1,
5]. Central nervous system (CNS) and peripheral nervous system (PNS) involvement has been described [
5]. Of the cases, the CNS and PNS were involved up to 25% and 10%, respectively [
1], with significant variation in presentation, severity, and prognosis [
6]. Cases ranged from simple headache, one of most common and mild symptoms, to fatal cerebrovascular strokes [
6].
Focusing on the relationship between COVID-19 and inflammatory CNS disorders, in particular multiple sclerosis (MS), the question arises as to whether COVID-19 infection may act as an immunogenic CNS inflammatory trigger in genetically predisposed individuals, thus acting as a precipitating event rather than the direct cause [
7]. However, to date, just six case reports have been reported in the literature [
7,
8,
9,
10,
11,
12].
In our case, we present a young woman presenting with a typical inflammatory event, suggestive of a first demyelinating event, on day 4 of her symptomatic COVID-19 infection. The presentation in this case was different from the other case reports.
2. Case Presentation
A 35-year-old female teacher presented at the Emergency Department with a 7-day history of headache, tingling and numbness involving the right lower limb, and visual disturbance on the right side of her vision. These symptoms had started 4 days after COVID-19 was confirmed by PCR testing. She had experienced coughing as her only symptom of COVID-19 infection.
Prior to this admission, there were no historical episodes of neurological dysfunction to suggest a relapsing remitting disorder.
She had a past medical history of bronchial asthma and migraine. No recent trauma was reported. She was on the combined oral contraceptive pill and inhalers for her asthma. Her family history was notable for cancer; breast cancer affecting her mother and lung cancer affecting her father. She was drinking alcohol occasionally and had never been a smoker.
On admission, her vitals were normal. Her blood pressure (BP) was 114/82 mmHg, heart rate (HR) was 83 pulses per minute, respiratory rate (RR) was 19 breaths per minute, and peripheral capillary oxygen saturation (SpO2) was 98% on room air.
3. Investigations
On examination, she was alert and orientated. She had not noticed any change in her hearing or sense of smell. There was no evidence of a relative afferent pupillary defect (RAPD). She had a full range of eye movements. There was no facial asymmetry or weakness. Fundoscopy was normal. No evidence of red colour desaturation was detected. She was found to have a right inferior quadrantanopia. There was diminished pinprick and light touch sensation of the right side of face. The remainder of the cranial nerves were intact.
She had normal tone and reflexes. Power was 5/5 in both the upper and lower limbs. Diminished pinprick and light touch sensation of upper and lower right limbs was present on the right. There were no extrapyramidal or cerebellar signs.
The patient was hospitalised for further investigation. She initially underwent a computerised tomography (CT) scan of the head, which was reported as normal with no evidence of an intracranial haemorrhage, ischaemic stroke, or a space-occupying lesion.
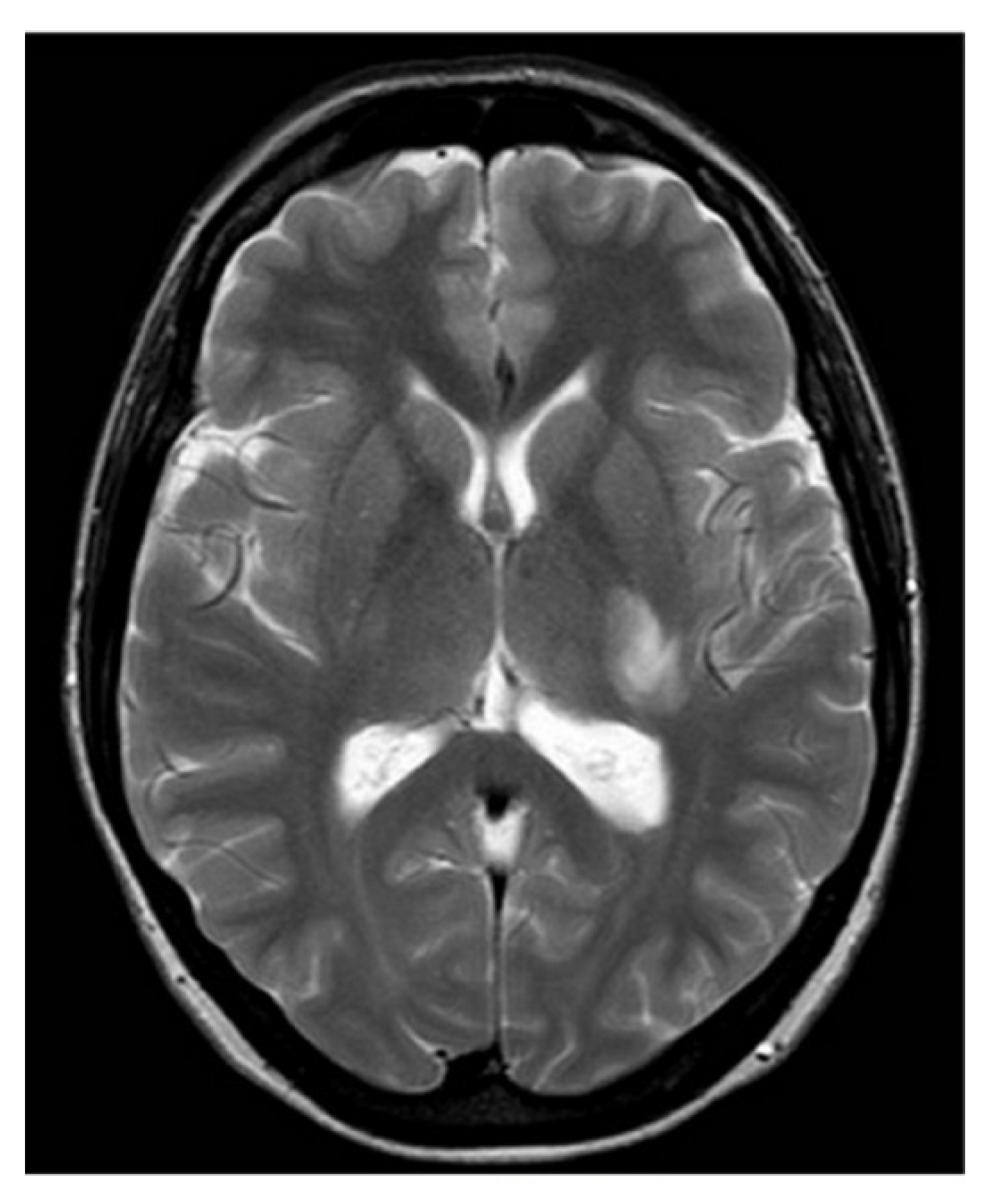
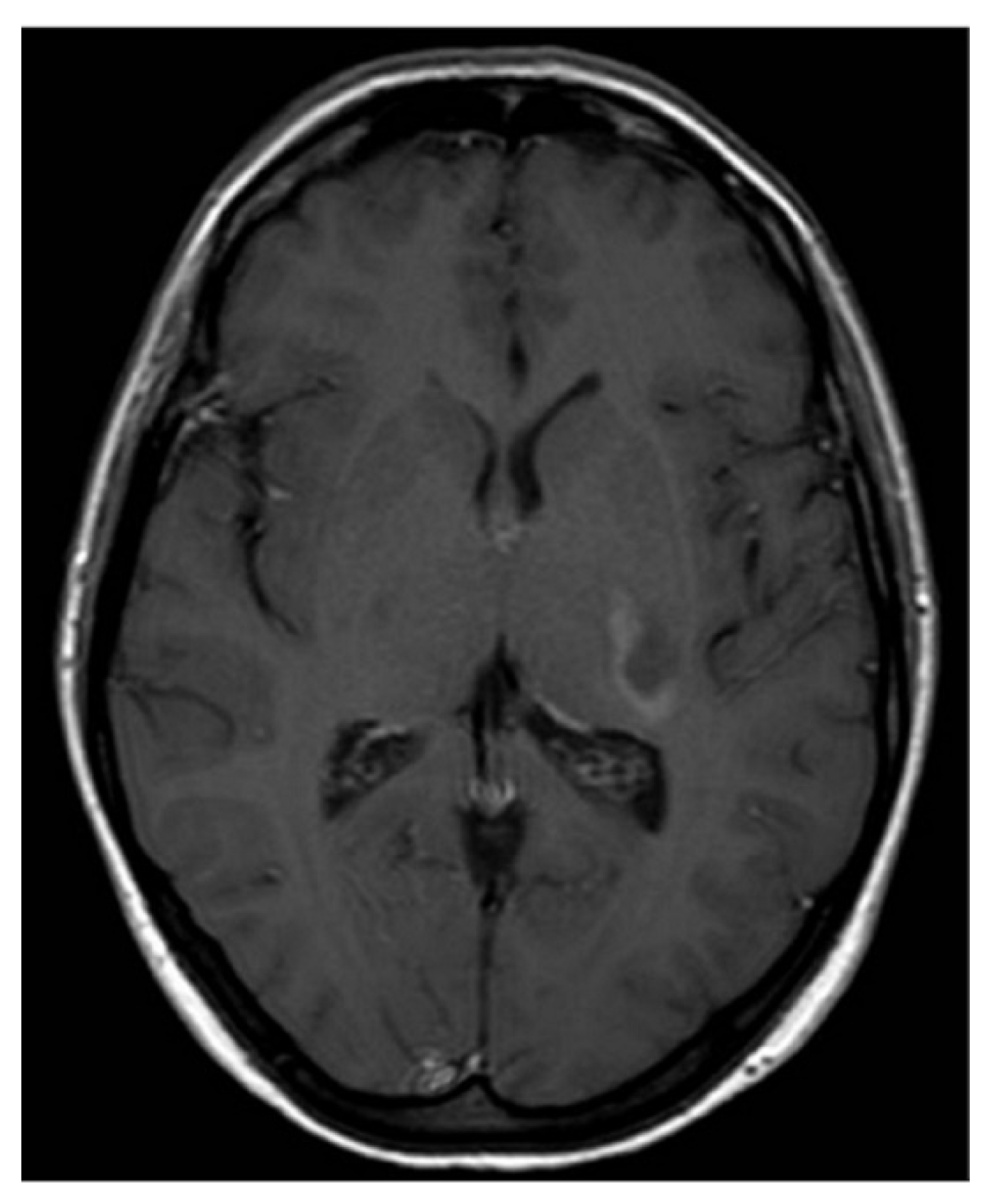
Subsequent magnetic resonance imaging (MRI) of the brain and C-spine revealed an area of hyperintense lesion on T2-weighted imaging involving the posterior limb of the internal capsule, adjacent to the left posterior thalamus with perilesional oedema (
Figure 1 and
Figure 2). Further hyperintensities were noted in the left frontotemporal region. These lesions demonstrated incomplete ring enhancement with gadolinium and facilitated diffusion restriction, consistent with acute demyelinating lesions. The lesions on the scan did not meet the MAGNIMS criteria for a multiple sclerosis diagnosis.
A lumbar puncture (LP) was performed. Oligoclonal bands were detected in the cerebrospinal fluid (CSF) and serum, with additional bands in the CSF which were not present in the serum. This implied a systemic inflammatory response with an additional CNS only response.
Full blood count, urea and electrolytes, liver function tests, bone profile, and erythrocyte sedimentation rate were all normal. Syphilis, human immunodeficiency virus (HIV), and hepatitis B and C serologies were negative. The autoimmune profile was normal: “Anti-AQP-Ab negative, anti-MOG-Ab negative”.
We also performed a lupus panel: anti-ANA, anti-native DNA, anti-U1RNP, anti-SnRNP, SSA, SSB, anti-Histone, and anti-phospholipids were all negative.
An additional antibody panel was performed on CSF and blood serum to indirectly evaluate the haematoencephalic barrier permeability. In particular, measles antibodies showed a CSF/serum ratio of 0.2, not indicative of an intrathecal antibody synthesis. Blood levels were IgG 420 U/mL and IgM 3 U/mL. These values were compatible with the patient-referred puerile infection. Varicella-zoster virus (VZV) antibodies had a CSF/serum ratio of 0.4. The patient has undergone vaccine two years before and she had never developed clinical infection.
ELISA test did not detect HSV-1 and HSV-2 IgM or IgG in CSF. Since the patient lived near the countryside, we performed an ELISA test for Borrelia burgdorferi, which resulted negative. CXCL13 chemochine on CSF sample was undetectable. Moreover, the patient did not remember any tick bite episode. Finally, we tested SARS-CoV-2 Ab, which was at an undetectable level in CSF, while serum IgG level was high for anti-nucleocapside (anti-N) and anti-spke (anti-S) (IgM negative). These values might be due to the previously executed three-dose COVID vaccine. Solely rubeola antibodies were not analysed. In consideration of the patient’s age and work, she had been vaccinated for rubeola, and her last antibody dosage was 37 U/mL four years before.
4. Differential Diagnosis
The main differential diagnosis was between acute disseminated encephalomyelitis (ADEM) and a clinically isolated syndrome (CIS). The age of the patient, absence of an encephalopathy, and presence of oligoclonal bands were against the diagnosis of ADEM. The MRI findings were insufficient to provide evidence of dissemination in space in order to make a diagnosis of multiple sclerosis at this time. Thus, a diagnosis of CIS was initially made.
5. Treatment, Outcome, and Follow-Up
The patient commenced on intravenous (IV) methylprednisolone 1 g once daily for 5 days.
After the treatment, the patient was discharged with improving symptoms, although she estimated it took nearly 8 months to return to her baseline function.
She had follow-up imaging at 12 months, which showed new inflammatory lesions in the right frontal lobe and at the septocallosal interface and a lesion of the right hemi-cord at C3. These were not accompanied by new symptoms. However, shortly afterwards, she developed symptoms of vertigo and unsteadiness and signs consistent with a brainstem/cerebellar relapse. On the basis of clinical and radiological criteria using the 2017 McDonald, criteria a diagnosis of relapsing remitting multiple sclerosis was made, and plans for first line disease modifying therapy are underway.
6. Discussion
Multiple sclerosis is an inflammatory, immune-mediated, demyelinazing, and neurodegenerative disease of the central nervous system. The disease’s onset results from a combination of genetic and environmental factors, especially infections. Currently, many theories accept that immune system activation may be triggered by multiple mechanisms associated with some viral agents. For some time, the candidate role of the human herpes virus (HHV) group as a main trigger has been accepted. The blood–brain barrier may become permeable to T cells as a consequence of an infection, and T cells remain trapped and activated in situ even after the infection has been solved.
The neuroinvasive potential of SARS-CoV-2 has been already described. SARS-CoV-2 may cross the blood–brain barrier through particular cell types: pericytes or astrocytes. The virus stimulates the local activation of the immune system, and therefore, consequent production of locally recruited IL-1 beta and IL6 by T cells may occur. Another theory identifies the role of IFN-I response. Its deficiency in some individuals promotes the development of severe COVID-19. The corresponding clinical lapel shows that MS patients lacking IFN-I may exhibit decreased COVID-19 risk after IFN-I administration.
Similarly, Th-17 axis is also highly involved in MS pathogenesis. TH-17 axis is highly expressed in individuals with aggressive MS, and it is further stimulated by SARS-CoV-2, suggesting a synergy between these two diseases.
Ultimately, SARS-CoV-2 may activate inflammasome complexes, which represent a molecular drivers of the innate immune system’s inflammatory response. These cellular complexes are highly expressed in people with MS and play a significant role in supporting MS inflammation. [
13,
14]
Since the outbreak of the COVID-19 pandemic, the number of cases reporting a temporal association between the infection and the onset of demyelinating diseases is increasing [
5]. Either the central nervous system and/or the peripheral nervous system seem to be involved, as the manifestation of COVID-19-related demyelinating diseases included inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN), and acute disseminated encephalomyelitis (ADEM) [
5]. A relationship between COVID-19 and onset of multiple sclerosis (MS) are reported in just six case reports [
6,
7,
8,
9,
10,
11].
The question arises as to whether COVID-19 infection may trigger an immunogenic central nervous system (CNS) inflammatory response in genetically predisposed individuals, acting as a precipitating event rather than as a direct cause [
7]. Despite the complete aetiology of MS still being unknown, the importance of viral infections as a possible trigger factor is widely suspected [
15,
16].
This idea has been supported by a recent observational study on the role of EBV in the pathogenesis of multiple sclerosis points towards the likelihood of a viral trigger whether by stimulation of the immune responses in genetically predisposed individuals or as a result of molecular mimicry between viral antigens and CNS cell structures [
17].
Other findings are that that in up to 90% of MS cases, patients manifest oligoclonal bands in the CSF, and that chronic inflammatory disorders of CNS are especially infectious. Amongst the viruses, Epstein–Barr (EBV) infection may be a risk factor for the development of the disease [
18], while human herpesvirus 6 (HHV-6) may be a risk factor for its exacerbation [
19]. Indeed, influenza virus, adenoviruses, and herpes simplex virus have been associated with more frequent and severe relapses of MS [
16].
Considering instead the relationship between coronaviruses (CoV) and MS, it has been experimentally proved that some strains of CoV have neuroinvasive and neurotropic potentials. The virus may enter through hematogenous spread or intranasal inoculation, and from there trans-synaptically transfer across infected neurons [
2].
The pathogenic effect of the infection seems to be more related to the overreacting of the innate immune system to the infection, which results in an abnormal response and the uncontrolled release of cytokines and chemokines and subsequent inflammation and damage rather than a direct cytopathic effect of the virus [
5,
20].
Mice infected by the murine coronavirus mouse hepatitis virus (MHV) developed a wide spectrum of neurological diseases ranging from fatal encephalomyelitis to demyelinating disorders, including MS [
21]. This ability to experimentally produce demyelination lesions in infected mice has led to few searches for human coronaviruses in MS-afflicted brains.
Murray et al. [
22] were the first to detect coronavirus RNA in the brains of 12 of 22 patients with MS, including coronavirus antigen in two patients with rapidly progressive disease, while Stewart et al. [
23] detected the RNA of human coronavirus (HCoV)—229E in nervous system tissue of 4 of 11 MS patients and in none of 6 neurological and 5 normal controls.
However, not all of the seven human coronaviruses (HCoV) can infect neurons. Among them, this characteristic is appears only in HCoV-229E, HCoV-OC43, and the severe acute respiratory syndrome coronavirus (SARS-CoV-1) [
20].
Subsequently, neurotropism of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible of COVID-19 pandemic, has been postulated. This is not only due to the reported neurological presentations in patients, but also to its structure and model of replication.
Indeed, these are similar to the other neuroinvasive and neurotropic coronaviruses—both human, such as the SARS-CoV-1 [
24,
25], and animal, such as the murine coronavirus mouse hepatitis virus [
26]—which, as aforementioned [
21], induced in mice an acute infection of the CNS with an immune-mediated chronic demyelinating disease, similar to multiple sclerosis in humans.
Comparing our case of COVID-19’s possible relation to MS to the other six [
6,
7,
8,
9,
10,
11], it is unique; in none of them was COVID-19 swab negative, were there no features of optical neuritis, or were there no previously reported anosmia and/or dysgeusia that related to typical human coronavirus infection at the same time. This highlights how varied and subtle the manifestations of this potential relationship can be. In this case, there appears to be a temporal link to her COVID-19 infection.
In conclusion, as for the other infective diseases, is still not possible to prove a causal relationship between COVID-19 and multiple sclerosis.
7. Conclusions
Paucisymptomatic COVID-19 infection can lead to neurological complications similar to those described in more severe COVID-19 infection. COVID-19 neurological manifestations may be ambiguous, mimicking other neuroinflammatory disorders. In patients with a possible related COVID-19 demyelinating disease, prolonged follow-up is needed to evaluate if the clinical course is monophasic or a precipitating event for a relapsing and remitting disorder.
In conclusion, is not possible to prove a causal relationship between the COVID-19 with multiple sclerosis. Although few cases are reported, we would encourage others to be aware of this neurological manifestation in patients with recent or concurrent COVID-19 infection.
Author Contributions
Conceptualization, D.R.,V.R., A.M. and R.E.; methodology, D.R., A.M., G.G., D.M., A.B., V.R., F.S. and R.E.; software, D.R. and G.G.; validation, V.R., A.M., G.G. and R.E.; formal analysis, R.E.; investigation, A.M. and R.E.; resources, D.R., A.M., G.G., D.M., A.B., V.R., F.S. and R.E.; data curation, G.G. and D.M.; writing—original draft preparation, D.R., A.M., V.R. and R.E.; writing—review and editing, D.R., A.M., G.G., D.M., A.B., V.R., F.S. and R.E.; visualization, D.R., A.M., G.G., D.M., A.B., V.R., F.S. and R.E.; supervision, G.G. and R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desai, I.; Manchanda, R.; Kumar, N.; Tiwari, A.; Kumar, M. Neurological manifestations of coronavirus disease 2019: Exploring past to understand present. Neurol. Sci. 2021, 42, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Romano, D.; Morescalchi, F.; Romano, V.; Semeraro, F. COVID-19 AdenoviralVector Vaccine and Central Retinal Vein Occlusion. Ocul. Immunol. Inflamm. 2022, 30, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Miar, P.; Norouzi, S.; Nikpour, P. Nervous System Involvement in COVID-19: A Review of the Current Knowledge. Mol. Neurobiol. 2021, 58, 3561–3574. [Google Scholar] [CrossRef]
- Collantes, M.E.V.; Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. Neurological Manifestations in COVID-19 Infection: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2021, 48, 66–76. [Google Scholar] [CrossRef]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.M.; Díaz-Maroto, I.; Segura, T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef]
- Moore, L.; Ghannam, M.; Manousakis, G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci 2021, 22, 100299. [Google Scholar] [CrossRef]
- Yavari, F.; Raji, S.; Moradi, F.; Saeidi, M. Demyelinating Changes Alike to Multiple Sclerosis: A Case Report of Rare Manifestations of COVID-19. Case Rep. Neurol. Med. 2020, 2020, 6682251. [Google Scholar] [CrossRef]
- Naser Moghadasi, A. A 31-year-old female patient with concurrent clinical onset of multiple sclerosis and COVID-19: Possible role of SARS-CoV-2 in the pathogenesis of multiple sclerosis. Autoimmun. Rev. 2021, 20, 102803. [Google Scholar] [CrossRef]
- Ismail, I.I.; Al-Hashel, J.; Alroughani, R.; Ahmed, S.F. A case report of multiple sclerosis after COVID-19 infection: Causality or coincidence? Neuroimmunol. Rep. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Bellucci, G.; Rinaldi, V.; Buscarinu, M.C.; Reniè, R.; Bigi, R.; Pellicciari, G.; Morena, E.; Romano, C.; Marrone, A.; Mechelli, R.; et al. Multiple Sclerosis and SARS-CoV-2: Has the Interplay Started? Front. Immunol. 2021, 12, 755333. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Kar, I.; Chen, C.K.; Sau, C.; Woodson, S.; Serra, A.; Abboud, H. Multiple Sclerosis Disease-Modifying Therapy and the COVID-19 Pandemic: Implications on the Risk of Infection and Future Vaccination. CNS Drugs 2020, 34, 879–896. [Google Scholar] [CrossRef]
- Sarwar, S.; Rogers, S.; Mohamed, A.S.; Ogula, E.; Ayantayo, R.A.; Ahmed, A.; Shahzadi, I.; Kataria, S.; Singh, R. Multiple Sclerosis Following SARS-CoV-2 Infection: A Case Report and Literature Review. Cureus 2021, 13, e19036. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 2005, 4, 195–202. [Google Scholar] [CrossRef]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J. The role of infections in multiple sclerosis. Mult. Scler. 2019, 25, 891–901. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Houen, G.; Trier, N.H.; Frederiksen, J.L. Epstein-Barr Virus and Multiple Sclerosis. Front. Immunol. 2020, 11, 3315. [Google Scholar] [CrossRef]
- Leibovitch, E.C.; Jacobson, S. Evidence linking HHV-6 with multiple sclerosis: An update. Curr. Opin. Virol. 2014, 9, 127–133. [Google Scholar] [CrossRef]
- Pezzini, A.; Padovani, A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020, 16, 636–644. [Google Scholar] [CrossRef]
- Hosking, M.P.; Lane, T.E. The pathogenesis of murine coronavirus infection of the central nervous system. Crit. Rev. Immunol. 2010, 30, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.S.; Brown, B.; Brian, D.; Cabirac, G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992, 31, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.N.; Mounir, S.; Talbot, P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology 1992, 191, 502–505. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Brison, E.; Meessen-Pinard, M.; Talbot, P.J. Neuroinvasive and neurotropic human respiratory coronaviruses: Potential neurovirulent agents in humans. Adv. Exp. Med. Biol. 2014, 807, 75–96. [Google Scholar] [CrossRef]
- Körner, R.W.; Majjouti, M.; Alcazar, M.A.A.; Mahabir, E. Of mice and men: The coronavirus mhv and mouse models as a translational approach to understand SARS-CoV-2. Viruses 2020, 12, 880. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).