Customer Complaints as an Evaluation Tool Assessing the Performance and Clinical Suitability of Different Implant Design
Abstract
1. Introduction
2. Materials and Methods
- KOS®
- Strategic Implant®, BCS®, GBC®, Beces®
- BOI®/TOI®
- SSO, STI with max. neck Ø4.8 mm,
- STW®, GTW®
- Bone Level Plus
- Hexacone®/GIH®
- Place®
- TPG®
- BCS®/GBC®/BECES®
- TOI®/BOI®
- TPG®
- Standard cylindrical full screw implants (neck max. 4.8 mm, SSO, STI)
- Wide neck cylindrical full screw implants (STW®, GTW®)
- Bone level implant (Bone Level Plus)
- Internal Hex standard implant (Hexacone®/GIH®)
- Tri-Lobe Implants (Place®)
- Compression screw implants (KOS®)
Exclusion Criteria
3. Results
4. Discussion
5. Limitations
6. Conclusions
- Implants with polished endosseous surfaces and cortical engagement had the lowest rate of customer complaints, with only 0.04% of such implants being returned.
- Single-piece implants intended for instant loading have substantially lower complaint rates than “two-stage systems,” particularly when endosseous parts are “rough”
- Compared to cylindrical core implants with tiny threads, conical core implants show a 3.8-times reduced complaint rate.
- Compared to other thread types and to all other implants, aggressively threaded implants created for engagement in the second cortical have the lowest percentage of complaints (0.03%).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karl, M.; Albrektsson, T. Clinical Performance of Dental Implants with a Moderately Rough (TiUnite) Surface: A Meta-Analysis of Prospective Clinical Studies. Int. J. Oral Maxillofac. Implant. 2017, 32, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term Survival of Straumann Dental Implants with TPS Surfaces: A Retrospective Study with a Follow-up of 12 to 23 Years. Clin. Implant Dent. Relat. Res. 2016, 18, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Staas, T.; Urban, I. A Retrospective Observational Study Assessing the Clinical Outcomes of a Novel Implant System with Low-Speed Site Preparation Protocol and Tri-Oval Implant Geometry. J. Clin. Med. 2022, 11, 4859. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Larjava, H.; Ofec, R. Retrospective cohort study of 4591 Straumann implants in private practice setting, with up to 10-year follow-up. Part 1: Multivariate survival analysis. Clin. Oral Implant. Res. 2015, 26, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G. Success Criteria in Implant Dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Available online: www.implants.com (accessed on 10 August 2022).
- Kullar, A.S.; Miller, C.S. Are There Contraindications for Placing Dental Implants? Dent. Clin. N. Am. 2019, 63, 345–362. [Google Scholar] [CrossRef]
- Lee, J.-H.; Frias, V.; Lee, K.-W.; Wright, R.F. Effect of implant size and shape on implant success rates: A literature review. J. Prosthet. Dent. 2005, 94, 377–381. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Szyszkowski, A.; Kozakiewicz, M. Effect of Implant-Abutment Connection Type on Bone Around Dental Implants in Long-Term Observation: Internal Cone Versus Internal Hex. Implant Dent. 2019, 28, 430–436. [Google Scholar] [CrossRef]
- Camps-Font, O.; Rubianes-Porta, L.; Valmaseda-Castellón, E.; Jung, R.E.; Gay-Escoda, C.; Figueiredo, R. Comparison of external, internal flat-to-flat, and conical implant abutment connections for implant-supported prostheses: A systematic review and network meta-analysis of randomized clinical trials. J. Prosthet. Dent. 2021, S0022-3913(21)00529-1. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29, 106–134. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef] [PubMed]
- Rocci, A.; Martignoni, M.; Gottlow, J. Immediate Loading of Brånemark System® TiUnite™ and Machined-Surface Implants in the Posterior Mandible: A Randomized Open-Ended Clinical Trial. Clin. Implant Dent. Relat. Res. 2003, 5, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Felice, P.; Barausse, C.; Pistilli, R.; Grandi, G.; Simion, M. Immediately loaded machined versus rough surface dental implants in edentulous jaws: One-year postloading results of a pilot randomised controlled trial. Eur. J. Oral Implant. 2015, 8, 387–396. [Google Scholar]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implant. Res. 2006, 17, 124–135. [Google Scholar] [CrossRef]
- De Bruyn, H.; Collaert, B. Early Loading of Machined-Surface Branemark Implants in Completely Edentulous Mandibles: Healed Bone versus Fresh Extraction Sites. Clin. Implant. Dent. Relat. Res. 2002, 4, 136–142. [Google Scholar] [CrossRef]
- Palka, L.; Lazarov, A. Immediately loaded bicortical implants inserted in fresh extraction and healed sites in patients with and without a history of periodontal disease. Ann. Maxillofac. Surg. 2019, 9, 371–378. [Google Scholar] [CrossRef]
- Esposito, M.; Murray-Curtis, L.; Grusovin, M.; Coulthard, P.; Worthington, H. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2007, 17, CD003815, Correction: Cochrane Database Syst. Rev. 2014, 7, CD003815. [Google Scholar] [CrossRef]
- Brunski, J.B. In Vivo Bone Response to Biomechanical Loading at the Bone/Dental-Implant Interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef]
- Ganeles, J.; Zöllner, A.; Jackowski, J.; Bruggenkate, C.T.; Beagle, J.; Guerra, F. Immediate and early loading of Straumann implants with a chemically modified surface (SLActive) in the posterior mandible and maxilla: 1-year results from a prospective multicenter study. Clin. Oral Implant. Res. 2008, 19, 1119–1128. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Limírio, J.P.J.; Lemos, C.; Gomes, J.M.D.L.; Minatel, L.; Rezende, M.C.R.A.; Pellizzer, E.P. A clinical comparison of 1-piece versus 2-piece implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2019, 124, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Nikolai, J.; Zarb, G.A. Immediate and early implant loading protocols: A literature review of clinical studies. J. Prosthet. Dent. 2005, 94, 242–258. [Google Scholar]
- Kopp, S.; Kopp, W. Comparison of Immediate vs. delayed basal implants. J. Maxillofac. Oral Surg. 2008, 1, 116–122. [Google Scholar]
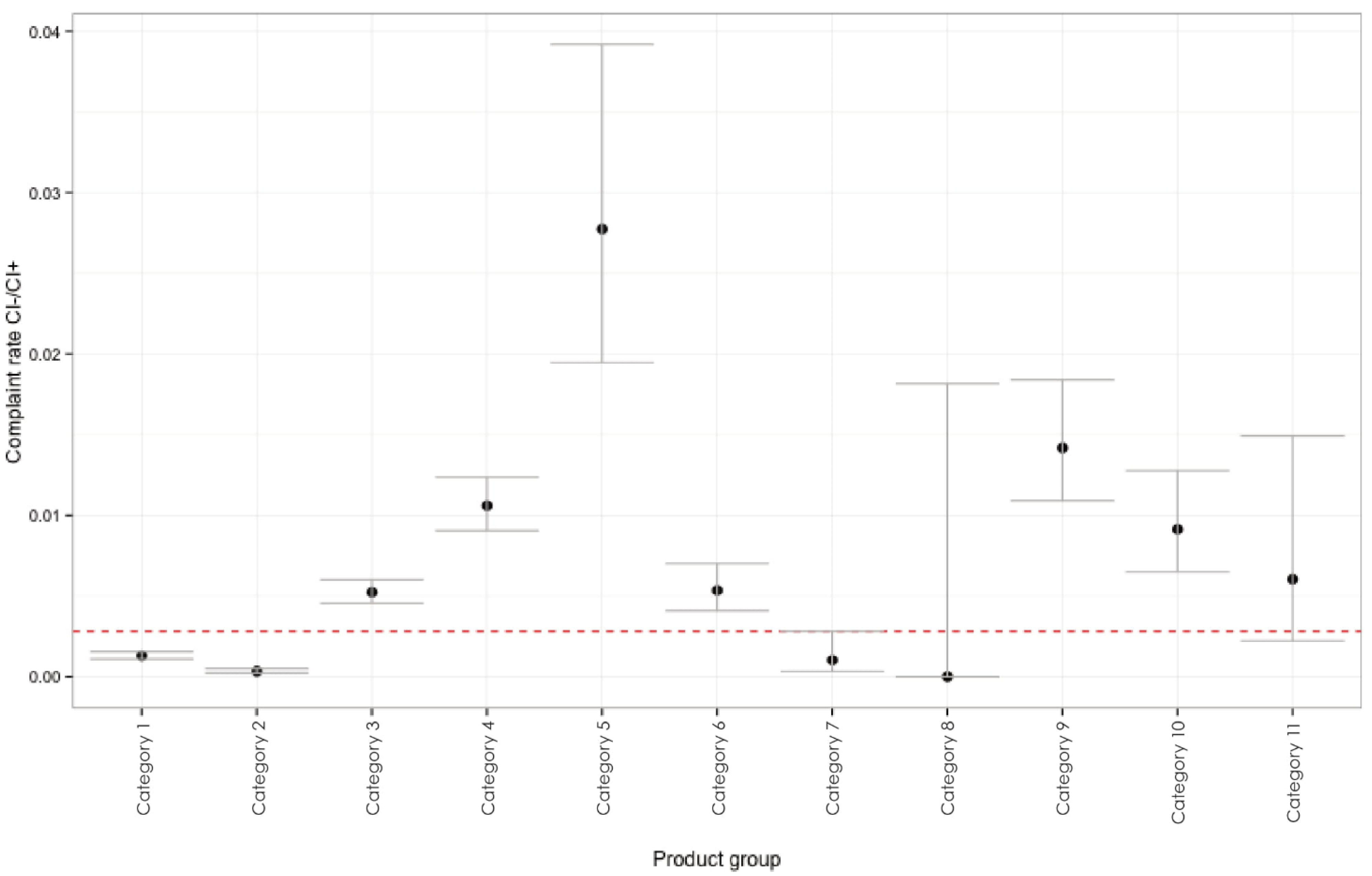
| Category | Implant Type | Returned Implants % (UCI; LCI) | Implant Design | Clinical Appearance | Endosseous Surface |
|---|---|---|---|---|---|
| 1 | KOS/GCS | 0.13 (0.109; 0.155) | 1-piece, immediate loading |  | sandblasted |
| 2 | BCS, GBC, Beces | 0.03 (0.022; 0.050) | 1-piece, immediate loading |  | Machined |
| 3 | Hexacone® | 0.52 (0.455; 0.601) | 2-piece, delayed loading |  | SA |
| 4 | SSO®, STI® | 1.06 (0.907; 1.237) | 2-piece, delayed loading |  | SA |
| 5 | STW, GIW | 2.77 (1.946; 3.917) | 2-piece, delayed loading |  | SA |
| 6 | Xign®(Xive-compatible) | 0.54 (0.407; 0.701) | 2-piece, delayed loading |  | SA |
| 7 | (disk implants); BOI®/TOI® | 0.10 (0.033; 0.282) | 1-piece, immediate loading |  | machined |
| 8 | TPG | 0 | 2-piece, immediate loading |  | machined |
| 9 | Bone Level Plus (Straumann-like) | 1.42 (1.090; 1.838) | 2-piece, delayed loading |  | SA |
| 10 | STO, STC, (Straumann- like) | 0.91 (0.650; 1.277) | 2-piece, delayed loading |  | SA |
| 11 | Place® (GPL) | 0.60 (0.223; 1.491) | 2-piece, delayed loading |  | SA |
| Group | Implant Surface and Design | Returned Number of Implants in % |
|---|---|---|
| group A | 1-piece (rough and polished) | 0.09 |
| group A, including TPG system | immediate loading systems (1-piece and 1-piece) | 0.09 |
| group B | two-stage implants with SA/etched surface | 0.73 |
| excluded from group B | conical core, sandblasted/etched surface (Hexacone, Place, Xign) | 0.91 |
| group B, including TGP system | all implants with polished endosseous surface (1-piece, 2-piece) | 0.70 |
| group C | all implants with polished endosseous surface (1-piece, 2-piece) | 0.04 |
| group D | all implants with SA/etched surface (single-piece, two-piece) | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihde, S.; Pałka, Ł. Customer Complaints as an Evaluation Tool Assessing the Performance and Clinical Suitability of Different Implant Design. Appl. Sci. 2022, 12, 11394. https://doi.org/10.3390/app122211394
Ihde S, Pałka Ł. Customer Complaints as an Evaluation Tool Assessing the Performance and Clinical Suitability of Different Implant Design. Applied Sciences. 2022; 12(22):11394. https://doi.org/10.3390/app122211394
Chicago/Turabian StyleIhde, Stefan, and Łukasz Pałka. 2022. "Customer Complaints as an Evaluation Tool Assessing the Performance and Clinical Suitability of Different Implant Design" Applied Sciences 12, no. 22: 11394. https://doi.org/10.3390/app122211394
APA StyleIhde, S., & Pałka, Ł. (2022). Customer Complaints as an Evaluation Tool Assessing the Performance and Clinical Suitability of Different Implant Design. Applied Sciences, 12(22), 11394. https://doi.org/10.3390/app122211394






