Abstract
Background: Aerobic exercise reduces pain sensitivity, a phenomenon known as exercise-induced hypoalgesia (EIH); however, little is known about EIH when the upper limbs are aerobically exercised. This study aimed to test the acute effect of a single aerobic upper-limb exercise on pain threshold and pain intensity in healthy participants, with two different protocols for controlling intensity. Methods: 31 participants performed two 20 min exercise sessions a week apart. In each session, the intensity was controlled by a target heart rate (THR) of 60% of heart rate reserve or by a rate of perceived exertion (RPE) of 7/10 on the Borg scale. Pain threshold for pressure (PPT) heat (HPT) and pain intensity in response to Tonic Heat Pain (THP) were measured pre- and post-exercise. To examine the effect of exercise in each protocol on pain sensitivity, rmANOVA was conducted. Results: Pain sensitivity remained unchanged following arm exercise in both protocols (PPT, p = 0.67; HPT, p = 0.56; and THP p = 0.39). Higher HR in the THR protocol was demonstrated with a significant protocol X time, interaction effect (F(3) = 11.194 p < 0.004). Conclusions: Moderate–high-intensity upper-limb aerobic exercise did not affect pain sensitivity in healthy individuals. Exercise intensity when controlled by THR showed a higher mean heart rate compared to exercise intensity based on RPE.
1. Introduction
Acute dynamic and isometric resistance exercise and aerobic exercise reduce pain sensitivity in healthy [1,2] and chronic pain [3,4] subjects. This is manifested experimentally as a decrease in pain ratings and an increase in pain thresholds and tolerance; a phenomenon termed exercise-induced hypoalgesia (EIH) [5]. The hypoalgesic effects of EIH have been found to be associated with the intensity and duration of aerobic exercise [5,6].
To date, almost all studies investigating the EIH effect of aerobic exercise have examined the large muscle groups of the lower extremities [2,5,7,8,9]. However, in some clinical conditions (e.g., paraplegia [10], lower-limb amputation [11], post-total knee replacement surgery or injuries), when exercise with lower extremities is limited, upper-limb exercise is recommended in order to improve functioning or fitness. However, to our knowledge, only two studies have examined the effect of aerobic exercise of the upper limbs on experimental pain sensitivity. These studies found a decrease in pain sensitivity manifested as an increase in pain thresholds [12,13]. However, in order to investigate the hypoalgesic effects, it is necessary to evaluate supra-threshold processing by estimating the pain magnitude rating [14].
Different physiological and metabolic responses have been reported from conducting upper-limb versus lower-limb aerobic exercise. Specifically, arm exercise has elicited a lower maximum rate of oxygen consumption (VO2) (34% less VO2) [15], a lower anaerobic threshold (AT) [15,16], and a lower maximal heart rate (HR) [17] compared to leg exercise. In order to maintain the same power output with a smaller muscle mass, one needs to work harder, and the rate of perceived exertion (RPE) evaluated by the Borg scale is higher. Accordingly, during upper-limb exercise, a higher RPE has been reported at a lower HR compared to leg exercise [18] The use of RPE to control for a given exercise intensity across different exercise modalities is useful. However, the physiological responses as related to the RPE vary according to the exercised muscle size [19].
Focusing on HR, Bhambhani et al. [17] found that the maximal HR in an upper-limb cranking exercise was about 90% of the maximal HR of lower-limb pedaling. Similarly, the maximal VO2 during the upper-limb exercise only reached about 70% of the VO2 achieved with the lower-limb exercise. These differences challenge the determination of the target HR for aerobic exercise based on the maximal HR formulas of Tanaka [20] or the formula of 220-age [21]. Indeed, it has been reported that the prediction equations for maximal HR significantly overestimate the true maximal HR when performing upper-limb exercise [22]. Furthermore, the lactate accumulation point was found to occur at a lower maximal HR with upper-limb versus lower-limb aerobic exercise (61% vs. 76%, respectively) [16]. Based on these physiological differences between aerobic exercise conducted by the lower vs. upper limbs, the question arises as to whether the EIH effects previously reported with lower-limb exercise will also be achieved during upper-limb aerobic exercise of moderate to high intensity.
This study aimed (1) to experimentally test the acute effects of a single aerobic exercise of the upper limbs on pain threshold and pain intensity in healthy subjects, and (2) to compare the HR, RPE, workload (power) and the hypoalgesic effects of two protocols controlling for intensity: one adjusted to the target HR (THR) of 60% of heart rate reserve and the other based on a rating of 7/10 on Borg’s scale.
Considering the differences between aerobic activity in the upper limbs compared to the lower limbs, we aimed to explore if EIH is observed following upper extremity exercise, which may serve as a tool to improve physical function and to reduce pain in those who are limited to perform an exercise with the lower limbs.
In addition, we assumed that intensity adjusted to RPE of 7/10 on the Borg scale would result in the same HR as the target heart rate of 60% of heart rate reserve.
2. Materials and Methods
2.1. Participants
Healthy non-smoking subjects aged 18–55 years were recruited for the study. Exclusion criteria were those with acute or chronic illness, individuals who completed the PAR-Q (Physical Activity Readiness Questionnaire) and answered “yes” to one or more questions, acute and chronic pain conditions, onset pain, or any impairment which may restrict intensive upper-limb effort. Approval for the study was obtained from the institutional review board of the University of Haifa. Informed consent was obtained before participation (# 335/20).
2.2. Measurement Tools
2.2.1. Quantitative Sensory Testing (QST)
The QST was conducted on the Tibialis anterior muscle belly of the participants’ dominant side.
Pressure Pain Threshold (PPT)
The PPT was measured with a pressure algometer (Somedic, Algometer, Sweden). The stimulation area was 1 cm² and the pressure increase rate was 30 kPa/s. The participants were asked to push a button as soon as the pressure sensation became painful and that was defined as the PPT. Five pressure stimuli were applied at 5 locations close to each other, and the mean of the 3 closest PPT ratings was used to define the mean PPT. The locations of the selected 3 stimuli were marked for further measurements [23,24].
Heat Pain Threshold (HPT)
The HPT was measured by the Thermal Sensory Analyzer (TSA, Medoc, Ramat-Yishay, Israel) using the 30 × 30 mm thermode. The baseline temperature was 32 °C, and the temperature was increased at a rate of 1 °C, with a maximal temperature of 50.5 °C. The participants were asked to press a computer mouse button when the warm sensation became painful. Five different stimuli were applied with 15 s inter-stimulus intervals, and the mean of the 3 closest readings was defined as the mean HPT [23,24].
Tonic Heat Pain (THP)
THP was also measured by the TSA with the 30 × 30 mm thermode. A constant stimulus of 46.5° was delivered for 2 min. Every 10 s, the participants were asked to grade the pain intensity using the 0–100 numerical pain scale (NPS) where ‘0′ denoted “no pain” and ‘100′ denoted “maximum pain imaginable”. The mean of the THP ratings was calculated [25].
The order of the pain threshold measures was randomly assigned for each participant and was repeated in the same order each time. The THP was always delivered immediately after the HPT.
2.2.2. Questionnaires
Physical Activity Readiness Questionnaire (PARQ)
The PARQ is a screening tool that is used to estimate risk factors and readiness to engage in physical activity. The questionnaire includes 7 yes/no questions about the participants’ medical status [26].
The International Physical Activity Questionnaire (IPAQ)
The IPAQ [27,28] was used to examine the level of daily activity in a representative week. The weekly physical activity is transferred to a metabolic equivalent (MET) to create 4 levels: vigorous (8 MET), moderate (4 MET), walking (3.3 MET), and sitting (0 MET). The overall metabolic value is calculated as MET level x minutes of activityx events per week. The IPAQ was validated to Hebrew [29].
Enjoyment Scale
Each participant was asked to rate his or her enjoyment of the exercise on a 1–10 scale, where “10” indicates “I enjoyed it very much”, and “1” indicates “I did not enjoy it at all”.
2.2.3. Exercise Intensity Measurements
Heart Rate
HR was monitored at rest and throughout the exercise by using an HR chest band (polar H7).
The Borg Rating of Perceived Exertion Scale
Participants were asked to rate their exertion on the Borg 0–10 scale (from, “0”, “No exertion at all”, to “10”, “maximal exertion”) during the study exercise, which combines all sensations and feelings of physical stress and fatigue [30]. The scale was displayed in front of the participants during the exercise, and they were asked to point to a number that best described how hard the exercise felt based on the physical sensations that they experienced.
Blood Lactate Measurement
Capillary whole blood lactate was measured from the index finger using a lactometer (Lactate scout, SensLab, Leipzig, GM) before and immediately after the exercise. A value above 4 mmol/L corresponded to the onset of blood lactate accumulation (OBLA) indicating an intensity in the range of the anaerobic threshold [31].
2.2.4. Aerobic Exercise Protocols
Each participant underwent two exercise protocols (based on THR or RPE 7 out of 10 on the Borg scale, as described below) on the upper body ergometer (Excite Live Top, Technogym, Cesena, Italy) while sitting. The exercise protocols were given in random order and conducted on different days. Each exercise was performed for 20 min after a 5 min warm-up in which the resistance was gradually increased until the target intensity was reached.
THR Intensity Protocol
The intensity of the exercise was targeted to 60% of reserve HR. The THR was calculated using the Karvonen formula for age-predicted maximal HR (MHR) [32] as follows: THR = resting HR + [(0.6 (220-age)) − resting HR], ±5 beats per minute. Participants maintained their pedaling rate (50–60 revolutions per minute) while the load was increased gradually until THR was reached in the first 5 min. Then, the load was changed to keep the THR.
BORG Intensity Protocol
Exercise intensity was controlled according to the participants’ subjective effort (a rating of 7 out of 10 on the Borg scale). Participants maintained their pedaling rate (50–60 revolutions per minute) while the resistance was increased gradually until a Borg raring of 7, corresponding to “very severe”, was reached in the first 5 min. Then, load was adjusted to keep the perceived exertion rating of 7 out of 10. Throughout the exercise, the researcher verified that the participants’ ratings of the exercise intensity matched their 7/10 score, and if not, the load was changed accordingly.
2.3. Study Procedure
After a detailed explanation of the study requirements, participants who were interested in participating in the study were asked to sign an informed consent form and complete a self-report health declaration using the PARQ on the first session. Their blood pressure and resting HR were then measured. Participants were asked to attend 2 sessions one week apart. In the first session, they were asked to complete the IPAQ and were randomly assigned one of the 2 protocols (the other protocol was completed in the second session). This was followed by familiarization with the QST.
Participants were excluded if: (i) their resting blood pressure was >140/90 mmHg or their resting HR was >100; (ii) they graded the suprathreshold THP < 25 on the 0–100 NPS in order to avoid a floor effect; and (iii) they provided a positive response on one of the questions in the PARQ.
During the first exercise session, PPT, HPT, and THP were measured before and after 20 min of rest in a sitting position (control condition). The measures taken at the end of the 20 min rest served as baseline measures for the exercise that was performed immediately after. During the 20 min of exercise, RPE, HR, and power (Watt) were recorded every 5 min. Immediately following exercise completion, participants were asked about their level of fatigue and the degree of their enjoyment during the exercise using a 0–10 NRS, and the QST measures were repeated. To assess lactate concentration a capillary blood sample was taken from the finger before and immediately after the exercise.
In the second session, HR and blood pressure were measured at rest to ensure inclusion criteria were still being met. All the measurements (QST, HR, RPE. Watt, blood lactate, enjoyment and fatigue ratings) were repeated in the same way as during the first session.
2.4. Data Analysis
Statistical analyses were performed using SAS software version 9.4 for Windows. Determination of a normal distribution of variables was examined using the Shapiro–Wilk normality test. The data are presented as mean and standard deviation, or median and interquartile range (IQR), as appropriate. The descriptive statistics include demographic anthropometric and level of physical activity parameters.
Paired t-tests were performed for comparing the following variables between the 2 exercise intensity protocols: mean HR during the 20 min of exercise, RPE, power, lactic acid concentration delta (pre- and post-exercise), enjoyment, and the level of fatigue from the exercise.
A two-way mixed-model repeated-measures analysis of variance (rmANOVA) was applied to examine the effect of the protocol (BORG, THR) and time (5, 10, 15, and 20 min) on HR, RPE, and Watt and to examine the effect of condition (exercise vs. rest) and time (pre/post) in each protocol (BORG/THR) on the pain measurements (PPT, HPT and THP).
Results were considered significant at the p < 0.05 level.
3. Results
Thirty-six subjects volunteered to participate in the study; three subjects were excluded because they did not meet the admission criteria (one due to high blood pressure at rest and two because they rated a pain intensity lower than the inclusion criteria for the trial). In addition, two subjects completed only one session of the study (one due to a headache that appeared during the test and one for personal reasons) and therefore were excluded from the analyses. Accordingly, 31 participants (17 women) completed the two protocols and were included in the data analysis. Their median age was 40 years old (IQR 22–50), with a mean weight 73.0 ± 13.7 kg, mean height 1.69 ± 0.1 m, and median BMI 25.7 m/kg2 (IQR 22.19–27.31). Overall, 15 participants had a normal BMI, 13 were overweight and 3 were defined as obese. The median IPAQ score was 906 MET/week (IQR 268–1362), indicating that most participants were minimally active.
3.1. Comparison between the Protocols for Physiological Responses and Subjective Perception
Significant differences were found for the participants’ HR and power between the 2 protocols. Specifically, higher HR and power were found during the THR protocol (Table 1). Participants’ lactate concentration was higher than the onset of blood lactate accumulation (OBLA) point of 4 mmol/L in both protocols (THR 6.2 ±2.1; BORG 5.7 ± 2.2 mmol/L), with no difference between the protocols. In addition, no differences were found in the participants’ RPE, degree of enjoyment and fatigue between the two protocols.

Table 1.
Exercise variables and statistical differences between THR and BORG protocols.
HR, RPE, and power were measured every 5 min during the exercise and are presented in Figure 1. The analysis of a two-way ANOVA (2 protocols THR/BORG) and time (four time points: 5, 10, 15, 20 min) demonstrated a main effect for time (F (3) = 21.4, p > 0.001), for the protocol (F (1) = 11.19 p > 0.002), and for an interaction effect (protocol X time) (F (3) = 11.194.83 p > 0.004). Participants’ heart rate in the THR protocol was higher compared to the BORG protocol. For the RPE variable, no significant main effects were noticed. Lastly, the power variable demonstrated a significant main effect for time (F (3) = 2.97, p > 0.049) and the protocol (F (1) = 5.24 p > 0.03) with no interaction. The THR was conducted at a power of 5–10 watts more compared to the BORG protocol.
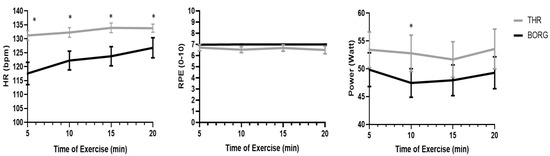
Figure 1.
HR, RPE, and Power during the 20 min of exercise for both protocols. HR (left panel), RPE (middle), and power (right panel) variables during the exercise as recorded every 5 min on THR (gray line) and BORG (black line) protocols. * indicated significant differences over time between the protocols p < 0.05.
3.2. Pain Measurements
Pain measurements including PPT, HPT, and THP pain ratings at rest (BL and after 20 min of sitting (pre-exercise), and immediately after the exercise are presented in Table 2. No time effect was found for the PPT [F (90) = 0, p = 1], HPT [F (90) = 0, p = 0.94] or THP [F (116) = 0, p = 0.76] for both protocols. Similarly, no interactions between time and protocol were found for PPT [F (90) = 0, p = 0.67], HPT [F (90) = 0, p = 0.56] and THP [F (116) = 0, p = 0.39].

Table 2.
Pain sensitivity measures pre- and post-rest session and pre- and post-exercise in both control intensity protocols.
4. Discussion
In this within-subject study design, we examined the effects of upper-limb exercise on experimental pain sensitivity in healthy individuals. We compared the changes in pain sensitivity between two different protocols to control for exercise intensity: one targeted HR (THR protocol), and the other was based on a subjective rating of perceived exertion (BORG protocol). We found that arm aerobic exercise at a moderate–high intensity of 60% of reserve HR, or of 7 on the 1–10 Borg scale, did not lead to the expected hypoalgesic effects. Specifically, no changes in the PPT, HPT, and pain ratings of THP were identified compared to the resting condition in both protocols. A higher mean HR was found when the THR protocol was applied compared to the BORG protocol, while the RPE was the same.
To the best of our knowledge, only a few previous studies have examined the effects of upper-limb aerobic exercise on pain sensitivity. In the study of Staude et al. [13], healthy female participants and fibromyalgia patients performed arm aerobic exercise in two sessions with a 15 min pause between, at a constant power of 60 watt until fatigue. PPT increased in both groups following the exercise, indicating an EIH effect, yet no differences were found in the THP ratings. Staude et al. [13] determined the exercise protocol based on the power, while HR was not monitored. This eliminates comparability to the current study results. However, the fact that the participants continued until exhaustion indicates that a higher effort for a short duration was achieved. This may explain the hypoalgesic effects identified in the Satude study, but not in our study.
Another study that examined the hypoalgesic effects of upper-limb aerobic exercise was carried out by Olausson et al. [12]. Specifically, they examined the effect of both lower and upper-limb aerobic exercise on toothache threshold induced by electrical stimulation. The 20 min exercise intensity was determined by a HR of 150 beats per minute for all participants. The results revealed that both lower-limb pedaling and arm cranking induced EIH, as expressed by an increase in pain threshold with no differences between the muscles involved. The study defined the pain threshold as an “unpleasant but not painful sensation”. The different definition of pain threshold that was used in our study, where participants were asked to indicate the onset of pain sensation, may explain the different results between the Olausson et al. findings and our study. In addition, the mean HR in our study was 130 bpm, lower than that of the Olausson et al. study, which may suggest that exercise intensity in our study was not sufficiently challenging to induce EIH. However, the fact that our participants were not accustomed to arm aerobic exercise, rating the exercise as “very severe” (i.e., 7/10 on the Borg scale), with lactate accumulation above the OBLA point at the end of the exercise, may imply that a higher intensity would be hard to maintain and may lead to exercise termination in a short time. The evidence from these two previous studies may suggest that intensity and not duration is more important for inducing EIH following upper-limb aerobic exercise, but this needs further examination.
The aerobic exercise intensity required to induce hypoalgesia has been investigated in many studies [6,23,33,34,35], and various intensity methods were tested on the lower limbs. In detail, Naugle et al. [6] found that 20 min of lower-limb pedaling at an intensity of 70% of the predicted maximal HR led to greater increase in PPT compared to 50% of the predicted maximal HR. However, the same degree of decrease in THP ratings was observed at both intensities. Kodesh and Weissman-Fogel [23] found a decrease in THP ratings only after interval training at an intensity of 85% of the reserved HR, which was not demonstrated from aerobic exercise at an intensity of 70% of the HR reserve (HRR). Moreover, at both intensities, no change in PPT measures were reported. Another study by Schmitt et al. [35] determined exercise intensity by the HR measured at the anaerobic threshold. They demonstrated that aerobic exercise for 20 min at an intensity 20% higher than the anaerobic threshold HR resulted in a significant increase in HPT and pain tolerance, compared to HR lower than the anaerobic threshold. Similarly, Micalos and Arendt-Nielsen [34] reported an increase in PPT pain threshold after an effort of 70% of maximum oxygen consumption but not at 30%. Hoffman et al. [33] examined the change in pressure pain intensity after aerobic exercise on a treadmill and found a decrease in pain intensity after exercise at an intensity of 75% of the maximal oxygen consumption, but no significant change was identified in response to exertion at 50% of maximal oxygen consumption. In addition, a local increase in PPT at 70% HRR but not at 50% was reported [36].
In contrast to these studies which indicate that EIH is related to higher intensities (70% of aerobic capacities), there are studies which indicate that lower intensities have a beneficial effect on pain sensitivity. For example, a recent meta-analysis which examined the effect of exercise on pain thresholds in healthy individuals found that moderate aerobic exertion, defined as 11-14/20 on the Borg scale (fairly light to somewhat hard), had a greater effect on the pain threshold compared to other intensities (less or more intense) [7]. Taken together, the inconsistency seen in various studies of the hypoalgesic effects following aerobic exercise, i.e., with different intensities and durations required to achieve EIH effects, explains the fact that there is no optimal recommended protocol and emphasizes the need for further research. In the present study, the target HR was determined at the intensity of 60% of maximal HR based on previous studies, indicating that maximum effort in the upper limbs is obtained at a lower HR than in the lower limbs [17,37]. We therefore assumed that this intensity would allow untrained individuals to maintain arm aerobic exercise for 20 min. At this intensity, lactate was higher than the OBLA point, and the exercise was rated as hard. Nevertheless, this intensity may not be sufficient to induce hypoalgesic effects, even when it is rated as severe, and the effort involves exertion of a smaller muscle mass.
EIH has been tested in different body locations in order to test the involvement of local and central mechanisms. Local hypoalgesic effects have been found near the exercised muscles [38,39], and a systematic effect has been observed in remote areas from the exercised muscles, although the local hypoalgesic effects are stronger [40,41]. Studies that examined the hypoalgesic effects accompanying lower extremities aerobic exercise have also shown inconsistent findings regarding pain thresholds in remote areas [34,35,42,43,44,45,46]. Decreased sensitivity to pain in distant regions may indicate systemic modulation mediated by endogenous inhibition mechanisms involving the descending inhibitory pathways [34]. In the present study, we chose to perform the pain measurements in a remote area (lower limb) to learn about the systemic effects of upper-limb exercise. The rationale was that we aimed to test whether the upper-limb exercise will reduce pain sensitivity in the lower limbs and thus may be suitable as a therapeutic tool for people suffering from leg pain of various types. The lack of hypoalgesic effects that we found in a healthy population may suggest that arm exercise is not applicable for clinical pain; however, further investigation of patient populations with lower-limb pain is needed.
Various mechanisms have been suggested to explain EIH and the differences between lower and upper-limb exercise. However, the underlying mechanism is not yet fully understood [47]. The most widely discussed hypothesis for EIH is that of the endocrine system response to stress [47,48]. There is growing evidence of an increase in stress hormones and hormones associated with the hypothalamic–pituitary–adrenal axis (HPA) following exercise, and their effect on pain sensitivity through a cascade of responses in the endocrine system [48,49]. Considering this hypothesis, Maresh et al. compared pituitary-adrenal hormone levels (adrenocorticotropic hormone-ACTH, cortisol and beta-endorphin) in untrained young men after 30 min of upper-limb or lower-limb aerobic exercise. They found an increase in the hormonal concentrations after exertion of 80% of maximal oxygen consumption (rather than 60%) and no difference in hormonal concentrations between exercise in the upper limbs compared to the lower limbs [50]. This finding is consistent with a previous study by Goldfarb et al., who found an increase in beta endorphin only following aerobic exercise of at least 70% of maximal oxygen consumption [51]. We did not measure the concentration of such hormones directly in the current study; however, since the exercise carried out resulted in lactate accumulation above the OBLA point, it can be assumed that the exercise intensity was sufficient to activate the opioid mechanism. Nevertheless, this does not fully explain the EIH effects observed in studies at more moderate intensities.
Another proposed mechanism for the EIH effect is related to increased blood pressure during exercise, which activates the endogenous-opioid system to reduce pain. The mechanism is not entirely clear, but one hypothesis is that the increase in blood pressure activates the baroreceptors in the carotid and the aortic arch that are innervated by vagal afferents, which transmit the neural response to structures responsible not only for cardiovascular activity but also for pain regulation [52,53,54]. Aerobic exercise of large muscle groups in the lower extremities elevate the cardiac output more than the upper extremities [55], which may explain the lack of hypoalgesic effects in our exercise protocol.
PPT is the most common outcome measure in EIH studies [2,56] demonstrating a very high test–retest reliability [44,57] Conversely, HPT has moderate–good reliability [58,59], and it is considered a less reliable outcome measure [59] with no consistent results following vigorous aerobic exercise [23,35,60,61]. While THP has a good test–retest reproducibility, it has a high inter-subject variability [14]. Several studies have examined EIH using THP as an outcome measure [6,23,60,62] and found a decrease in pain ratings after aerobic exercise, yet no change in PPT [6,23]. Overall, the use of THP as an outcome measure is less prevalent in EIH studies [60]. In the present study, conventional pain measures were used, yet due to great variability in the measurement tools used in EIH studies, it is hard to decide which of the measures is most sensitive to detect changes in pain sensitivity following exercise. Therefore, it seems that a number of outcome measures should be chosen to identify the effect of exertion on pain sensitivity. The lack of a hypoalgesic effect in any of the outcome measures in our study strengthens the findings that there was indeed no hypoalgesic effect in both exercise sessions.
Since we aimed to examine the feasibility of using arm aerobic exercise to induce EIH, and the existing literature lacks information about the optimal protocol for arm aerobic exercise intensities, we decided to test two different methods for controlling intensity that was targeted to a moderate–high level. We found that while the two protocols were similar in terms of the subjects’ perception of exertion, in the THR protocol the participants maintained a higher power and a higher HR. This finding is important for future studies that are required to further test the optimal protocol for inducing EIH following upper-limb exercise, while also suggesting that the target HR above 60% needs to be investigated in this kind of exercise modality.
This study has some limitations. In detail, we examined changes in pain sensitivity only in a remote area in order to test the EIH which is known to be mediated by central mechanisms that affect systemic pain sensitivity [8]. However, it is possible that testing local changes in pain sensitivity would have given us a broader picture of the effect of the different protocols. In addition, no maximal exercise test was performed for the arm aerobic test in order to determine maximal HR and its derivatives. If this is done, it may help determine a more accurate exercise intensity and avoid the use of predictable maximal HR, which may not be suitable for upper-limb exercise. Finally, it is suggested that future studies would compare the EIH of the upper limbs vs. lower limbs, in the same cohort of participants, in order to better understand physiological and metabolic differences.
5. Conclusions
Based on our protocols, EIH was not demonstrated following upper-limb aerobic exercise. Therefore, these protocols may not be suitable as a therapeutic pain-reducing intervention. Further research is needed to find the appropriate protocol to induce hypoalgesia from exercise executed using a small muscle mass.
Author Contributions
Conceptualization, N.K.-B., I.W.-F. and E.K.; methodology, N.K.-B., I.W.-F. and E.K.; formal analysis, I.W.-F. and E.K.; investigation, N.K.-B., I.W.-F. and E.K.; resources I.W.-F. and E.K.; data curation, N.K.-B.; writing—original draft preparation, N.K.-B.; writing—review and editing, I.W.-F. and E.K.; supervision, I.W.-F. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Haifa (protocol code # 335/20 approved on 29 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Koltyn, K.F. Analgesia following exercise: A review. Sport. Med. 2000, 29, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L., 3rd. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Nasri-Heir, C.; Patil, A.G.; Korczeniewska, O.A.; Zusman, T.; Khan, J.; Heir, G.; Benoliel, R.; Eliav, E. The Effect of Nonstrenuous Aerobic Exercise in Patients with Chronic Masticatory Myalgia. J. Oral Facial Pain Headache 2019, 33, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, L.W.; Koltyn, K.F.; Morgan, W.P.; Cook, D.B. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med. Sci. Sport. Exerc. 2011, 43, 1106–1113. [Google Scholar] [CrossRef]
- Koltyn, K.F. Exercise-induced hypoalgesia and intensity of exercise. Sports Med. 2002, 32, 477–487. [Google Scholar] [CrossRef]
- Naugle, K.M.; Naugle, K.E.; Fillingim, R.B.; Samuels, B.; Riley, J.L., 3rd. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med. Sci. Sport. Exerc. 2014, 46, 817–825. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Carolyna Gianlorenco, A.; Machado, R.; Queiroga, M.; Zeng, H.; Shaikh, E.; Yang, Y.; Nogueira, B.; Castelo-Branco, L.; Fregni, F. Exercise-induced pain threshold modulation in healthy subjects: A systematic review and meta-analysis. Princ. Pract. Clin. Res. 2020, 6, 11–28. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Fehrmann, E.; Gajsar, H.; Kreddig, N. Endogenous Modulation of Pain: The Role of Exercise, Stress, and Cognitions in Humans. Clin. J. Pain 2020, 36, 150–161. [Google Scholar] [CrossRef]
- Kesiktas, F.N.; Kasikcioglu, E.; Paker, N.; Bayraktar, B.; Karan, A.; Ketenci, A.; Muslumanoglu, L. Comparison of the functional and cardiovascular effects of home-based versus supervised hospital circuit training exercises in male wheelchair users with chronic paraplegia. Turk. J. Phys. Med. Rehabil. 2021, 67, 275–282. [Google Scholar] [CrossRef]
- Christensen, J.; Tang, L.; Doherty, P.; Langhorn, C.; Langberg, H. Test-retest reliability of a maximal arm cycle exercise test for younger individuals with traumatic lower limb amputations. Eur. J. Physiother. 2020, 22, 115–120. [Google Scholar] [CrossRef]
- Olausson, B.; Eriksson, E.; Ellmarker, L.; Rydenhag, B.; Shyu, B.C.; Andersson, S.A. Effects of naloxone on dental pain threshold following muscle exercise and low frequency transcutaneous nerve stimulation: A comparative study in man. Acta Physiol. Scand. 1986, 126, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Robinson, M.E.; Weyl, E.E.; Price, D.D. Pain variability in fibromyalgia is related to activity and rest: Role of peripheral tissue impulse input. J. Pain 2010, 11, 1376–1383. [Google Scholar] [CrossRef]
- Naert, A.L.G.; Kehlet, H.; Kupers, R. Characterization of a novel model of tonic heat pain stimulation in healthy volunteers. Pain 2008, 138, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.L.; Williamson, P.; Anderson, W.; Ross, R.; McCafferty, S.; Fettes, P. Cardiopulmonary exercise testing: Arm crank vs cycle ergometry. Anaesthesia 2013, 68, 497–501. [Google Scholar] [CrossRef]
- Keyser, R.E.; Mor, D.; Andres, F.F. Cardiovascular responses and anaerobic threshold for bicycle and arm ergometer exercise. Arch. Phys. Med. Rehabil. 1989, 70, 687–691. [Google Scholar]
- Bhambhani, Y.; Maikala, R.; Buckley, S. Muscle oxygenation during incremental arm and leg exercise in men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 422–431. [Google Scholar] [CrossRef]
- Borg, G.; Hassmen, P.; Lagerstrom, M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 679–685. [Google Scholar] [CrossRef]
- Hill, M.; Puddiford, M.; Talbot, C.; Price, M. The validity and reproducibility of perceptually regulated exercise responses during combined arm + leg cycling. Eur. J. Appl. Physiol. 2020, 120, 2203–2212. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Fox, S.M., 3rd; Naughton, J.P. Physical activity and the prevention of coronary heart disease. Prev. Med. 1972, 1, 92–120. [Google Scholar] [CrossRef]
- Hill, M.; Talbot, C.; Price, M. Predicted maximal heart rate for upper body exercise testing. Clin. Physiol. Funct. Imaging 2016, 36, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kodesh, E.; Weissman-Fogel, I. Exercise-induced hypoalgesia - interval versus continuous mode. Appl. Physiol. Nutr. Metab. 2014, 39, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D. Quantitative sensory testing. Muscle Nerve 1997, 20, 198–204. [Google Scholar] [CrossRef]
- Tousignant-Laflamme, Y.; Page, S.; Goffaux, P.; Marchand, S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008, 1230, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Qualified Fitness and Exercise as Professionals and Exercise Prescription: Evolution of the PAR-Q and Canadian Aerobic Fitness Test. J. Phys. Act. Health 2015, 12, 454–461. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Fogelholm, M.; Malmberg, J.; Suni, J.; Santtila, M.; Kyrolainen, H.; Mantysaari, M.; Oja, P. International Physical Activity Questionnaire: Validity against fitness. Med. Sci. Sport. Exerc. 2006, 38, 753–760. [Google Scholar] [CrossRef]
- Weissblueth, E. Short Hebrew International Physical Activity Questionnaire: Reliability and Validity. Balt. J. Health Phys. Act. 2015, 7, 7–13. [Google Scholar] [CrossRef]
- Noble, B.J.; Borg, G.A.; Jacobs, I.; Ceci, R.; Kaiser, P. A category-ratio perceived exertion scale: Relationship to blood and muscle lactates and heart rate. Med. Sci. Sport. Exerc. 1983, 15, 523–528. [Google Scholar] [CrossRef]
- Tesch, P.A.; Daniels, W.L.; Sharp, D.S. Lactate accumulation in muscle and blood during submaximal exercise. Acta Physiol. Scand. 1982, 114, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D. The Complete Book of Personal Training; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Hoffman, M.D.; Shepanski, M.A.; Ruble, S.B.; Valic, Z.; Buckwalter, J.B.; Clifford, P.S. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch. Phys. Med. Rehabil. 2004, 85, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Micalos, P.S.; Arendt-Nielsen, L. Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus 2016, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Wallat, D.; Stangier, C.; Martin, J.A.; Schlesinger-Irsch, U.; Boecker, H. Effects of fitness level and exercise intensity on pain and mood responses. Eur. J. Pain 2020, 24, 568–579. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, C.; Yang, S.; Wang, X. Aerobic Exercise Attenuates Pain Sensitivity: An Event-Related Potential Study. Front. Neurosci. 2021, 15, 735470. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Barnes, M.J.; Hodges, L.D. Comparison study of treadmill versus arm ergometry. Clin. Physiol. Funct. Imaging 2011, 31, 326–331. [Google Scholar] [CrossRef]
- Ge, H.Y.; Nie, H.; Graven-Nielsen, T.; Danneskiold-Samsoe, B.; Arendt-Nielsen, L. Descending pain modulation and its interaction with peripheral sensitization following sustained isometric muscle contraction in fibromyalgia. Eur. J. Pain 2012, 16, 196–203. [Google Scholar] [CrossRef]
- Lanefelt, S.V.; Melo-Gomez, M.; Chizari, M.; Krsek, M.; Christidis, N.; Kosek, E.; Ernberg, M. Tooth Clenching Until Exhaustion Evokes Exercise-Induced Hypoalgesia in Healthy Persons and in Patients with Temporomandibular Disorders. J. Oral Facial Pain Headache 2019, 33, 14–24. [Google Scholar] [CrossRef]
- Kuppens, K.; Struyf, F.; Nijs, J.; Cras, P.; Fransen, E.; Hermans, L.; Meeus, M.; Roussel, N. Exercise-and stress-induced hypoalgesia in musicians with and without shoulder pain: A randomized controlled crossover study. Pain Physician 2016, 19, 59–68. [Google Scholar]
- Kosek, E.; Lundberg, L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur. J. Pain 2003, 7, 251–258. [Google Scholar] [CrossRef]
- Wassinger, C.A.; Lumpkins, L.; Sole, G. Lower Extremity Aerobic Exercise as a Treatment for Shoulder Pain. Int. J. Sport. Phys. Ther. 2020, 15, 74–80. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Handberg, G.; Graven-Nielsen, T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014, 155, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Bjerregaard, L.K.; Redin, M.M.; Rasmussen, S.H.; Graven-Nielsen, T. Hypoalgesia after bicycling at lactate threshold is reliable between sessions. Eur. J. Appl. Physiol. 2019, 119, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F.; Garvin, A.W.; Gardiner, R.L.; Nelson, T.F. Perception of pain following aerobic exercise. Med. Sci. Sport. Exerc. 1996, 28, 1418–1421. [Google Scholar] [CrossRef]
- Gomolka, S.; Vaegter, H.B.; Nijs, J.; Meeus, M.; Gajsar, H.; Hasenbring, M.I.; Titze, C. Assessing Endogenous Pain Inhibition: Test-Retest Reliability of Exercise-Induced Hypoalgesia in Local and Remote Body Parts After Aerobic Cycling. Pain Med. 2019, 20, 2272–2282. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 3–13. [Google Scholar]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef]
- Nijs, J.; Kosek, E.; Van Oosterwijck, J.; Meeus, M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: To exercise or not to exercise? Pain Physician 2012, 15, ES205–ES213. [Google Scholar] [CrossRef] [PubMed]
- Maresh, C.M.; Sokmen, B.; Kraemer, W.J.; Hoffman, J.R.; Watson, G.; Judelson, D.A.; Gabaree-Boulant, C.L.; Deschenes, M.R.; Vanheest, J.L.; Armstrong, L.E. Pituitary-adrenal responses to arm versus leg exercise in untrained man. Eur. J. Appl. Physiol. 2006, 97, 471–477. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Hatfield, B.D.; Potts, J.; Armstrong, D. Beta-endorphin time course response to intensity of exercise: Effect of training status. Int. J. Sport. Med. 1991, 12, 264–268. [Google Scholar] [CrossRef]
- Koltyn, K.F.; Umeda, M. Exercise, hypoalgesia and blood pressure. Sport. Med. 2006, 36, 207–214. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Song, J.S.; Spitz, R.W.; Yamada, Y.; Bell, Z.W.; Wong, V.; Abe, T.; Loenneke, J.P. Exercise-induced hypoalgesia and pain reduction following blood flow restriction: A brief review. Phys. Ther. Sport 2021, 50, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, S.; Goomar, M.; Nikbakht, M. Comparison of Submaximal aerobic exercise effects in different intensities on heart rate and oxygen consumption during arm and leg exercise. Ann. Biol. Res. 2012, 3, 3287–3291. [Google Scholar]
- Wewege, M.A.; Jones, M.D. Exercise-induced hypoalgesia in healthy individuals and people with chronic musculoskeletal pain: A systematic review and meta-analysis. J. Pain 2021, 22, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Dørge, D.B.; Schmidt, K.S.; Jensen, A.H.; Graven-Nielsen, T. Test-retest reliabilty of exercise-induced hypoalgesia after aerobic exercise. Pain Med. 2018, 19, 2212–2222. [Google Scholar] [CrossRef]
- Moloney, N.A.; Hall, T.M.; Doody, C.M. Reliability of thermal quantitative sensory testing: A systematic review. J. Rehabil. Res. Dev. 2012, 49, 191. [Google Scholar] [CrossRef]
- Vaegter, H.; Hoeger Bement, M.; Madsen, A.; Fridriksson, J.; Dasa, M.; Graven-Nielsen, T. Exercise increases pressure pain tolerance but not pressure and heat pain thresholds in healthy young men. Eur. J. Pain 2017, 21, 73–81. [Google Scholar] [CrossRef]
- Ruble, S.B.; Hoffman, M.D.; Shepanski, M.A.; Valic, Z.; Buckwalter, J.B.; Clifford, P.S. Thermal pain perception after aerobic exercise. Arch. Phys. Med. Rehabil. 2005, 86, 1019–1023. [Google Scholar] [CrossRef]
- Samuelly-Leichtag, G.; Kodesh, E.; Meckel, Y.; Weissman-Fogel, I. A fast track to hypoalgesia–the anaerobic exercise effect on pain sensitivity. Int. J. Sport. Med. 2018, 39, 473–481. [Google Scholar] [CrossRef]
- Naugle, K.M.; Naugle, K.E.; Riley, J.L., III. Reduced modulation of pain in older adults after isometric and aerobic exercise. J. Pain 2016, 17, 719–728. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).