Abstract
Holarrhena pubescens Wall. ex G. Don (H. pubescens), belonging to the Apocynaceae family, is distributed in deciduous forests of the tropical Himalayas. H. pubescens is an important traditional medicinal plant, especially its seeds and barks. Therefore, we assessed the antioxidant capacity of H. pubescens extracts in Lipopolysaccharide (LPS)-induced dendritic cells (DCs) for sepsis treatment. Our results indicated that H. pubescens extracts with different doses (25 μg/mL, 50 μg/mL, 100 μg/mL) reduced the reactive oxygen species (ROS) level, and weakened the nitric oxide synthases (NOS) activity and nitric oxide (NO) level in LPS (100 ng/mL)-irritated DCs. In addition, H. pubescens extracts decreased the oxidized glutathione (GSSG) production but increased the reduced glutathione (GSH) production, thereby preserving the cellular reductive status owing to the raised GSH/GSSG ratio. Furthermore, H. pubescens extracts strengthened the antioxidant enzymes activity in LPS-induced DCs, such as glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD). Finally, we found that H. pubescens extracts significantly improved the expression of the nuclear factor erythroid 2-related factor 2 (Nrf2) and the heme oxygenase 1 (HO–1) in LPS-irritated DCs. These results indicated that H. pubescens extracts suppressed the LPS-irritated oxidative stress in DCs via Nrf2/HO–1 signaling pathway, providing a potential strategy for sepsis therapy.
1. Introduction
As antigen-presenting cells (APCs), dendritic cells (DCs) act as a bridge between innate and adaptive immunity [1]. During antigen recognition and processing, DCs are activated with phenotypic changes, and then mature DCs activate naïve T cells and promote the adaptive immune response, following a large increase production of cytokines and chemokines [2]. DCs play a crucial role not only in initiating protective immunity, but also in inducing immune tolerance. Therefore, DCs are critical for maintaining immune homeostasis [3]. Currently, DCs play a role in the therapy of some illnesses, such as sepsis, multiple sclerosis (MS), psoriasis, and rheumatoid arthritis [4,5,6,7]. All sepsis patients exhibit immune imbalance, and the joint detection of DCs phenotypes has the high prognostic value for the prognosis of patients [8]. Therefore, DCs may be a key target in various treatment approaches.
Oxidative stress refers to a state of imbalance between oxidation and antioxidation, which means that redox homeostasis tends to oxidation [9]. When excess production of reactive oxygen species (ROS) or inadequate antioxidant defense occurs, oxidative stress takes place, causing cytotoxicity and tissue damage, DNA repair system disorder, and mitochondrial impairment [10,11,12]. Under the inflammatory conditions induced by sepsis, the redox homeostasis is destroyed and excess ROS and RNS is produced, in turn enhancing dysfunction caused by sepsis, leading to a vicious cycle [13,14]. In addition, previous studies have reported that many sepsis patients exhibit features of ROS overproduction and antioxidant system dysfunction, implying that oxidative stress is closely related to sepsis [15,16]. Moreover, the occurrence and development of many illnesses are related to oxidative stress, including traumatic brain injury (TBI), osteoporosis, and atherosclerosis [17,18,19]. Some studies have found that lipopolysaccharide (LPS) can destroy the immune function of DCs during the development of sepsis [20,21]. ROS, acting as a mediator of oxidative stress, can also destroy the immunomodulatory function of DCs. For instance, ROS-triggered endoplasmic reticulum stress disrupts the antitumor immune function of DCs in ovarian cancer [22]. This may be linked to the immune dysfunction of DCs caused by oxidative stress. Our previous studies indicated that the inhibition of oxidative stress protected LPS-stimulated DCs from immunosuppression and reduced inflammatory reaction in an LPS-induced sepsis murine model [23,24,25]. Therefore, a DC-targeting strategy could be a significant and potential sepsis treatment.
Herbal medicines play a vital role in global medical development. In developing Asian countries, 70–80% of rural people conduct primary health care mainly using traditional medicine [26]. Holarrhena pubescens Wall. ex G. Don (H. pubescens), belonging to the Apocyanaceae family, is an important natural medical material. H. pubescens is usually distributed in the tropics and subtropics of Asia, especially in the deciduous forests of the tropical Himalayas, at altitudes ranging from 900 to 1250 m [27]. H. pubescens is usually used as a conventional medicine for parasitosis, such as malaria, amoebic dysentery, and intestinal helminthiasis treatment, further studies found that H. pubescens has multiple functions, including anti-microbial, anti-inflammatory, analgesic, anti-amnesic, and neuroprotective functions [28]. In addition, H. pubescens can be developed as a potential drug against diabetes and various enteric skin diseases. Each part of H. pubescens has different functions, for example, its barks and seeds are mostly used to treat diarrhea and dysentery, its roots can resist malaria, and its leaves can control stomachache and vomiting [29,30,31,32]. Most of the known chemical constituents are found in the stem, bark, leaves, and seeds of H. pubescens, and 68 alkaloids have been reported, including steroidal alkaloids and steroid alkaloids [27]. These alkaloids have many functions, such as antidiarrheal properties, antiplasmodial activity, and antibacterial function [33,34]. Of note, we previously evaluated some features of 100 traditional medicinal plants methanol extracts, and found that H. pubescens extracts can remove free radicals, implying a potential antioxidant capacity [35].
Here, we attempt to characterize the antioxidant capacity of H. pubescens extracts in the LPS-induced DCs, providing a DC-targeting strategy based on antioxidant properties for potential sepsis treatment.
2. Materials and Methods
2.1. Materials
Methanol extracts of H. pubescens seeds and barks were from Tibet and stored at the Korea Plant Extract Bank (Daejeon, Korea) [35]. Escherichia coli 026: B6-derived Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI 1640 basal medium was from Thermo Fisher Scientific’s brand Gbico (Waltham, MA, USA). Fetal bovine serum (FBS) was from YLESA (Shanghai, China). Penicillin and streptomycin (Pen Strep) were from Invitrogen (Grand Island, NY, USA). Interleukin-4 (IL-4) and recombinant mouse granulocyte macrophage colony stimulating factor (GM-CSF) were from Peprotech (Rocky Hill, NJ, USA). Erythrocyte lysis solution, Radio Immunoprecipitation Assay (RIPA) lysis buffer, phenylmethanesulfonyl fluoride (PMSF), NO assay kit, ROS assay kit, and CAT assay kit were from Beyotime (Shanghai, China). NOS assay kit, T-GSH/GSSG assay kit, GPx assay kit, and SOD assay kit were from JianCheng Bioengineering Institute (Nanjing, China). Fixation buffer and permeabilization wash buffer were from BD Biosciences (Franklin Lakes, NJ, USA). PE-nuclear factor erythroid 2-related factor 2 (Nrf2) and respective isotype were from Cell Signaling Technology (Danvers, MA, USA). Alexa Fluor 647-heme oxygenase 1 (HO–1) and respective isotype were from Abcam (Cambridge Science Park, UK).
2.2. Generation and Culture of Murine Bone Marrow-Derived DCs (BMDCs)
BMDCs were obtained and cultured by means of our improved approach [36]. Briefly, 4–6-week-old C57BL/6 mice were euthanized by cervical dislocation, the tibias and femurs were isolated and washed, marrows were collected and treated by erythrocyte lysis solution, and then bone marrow cells were cultured with complete medium (10% FBS, 1% Pen Strep, 10 ng/mL IL-4 and 10 ng/mL GM-CSF in RPMI 1640) at 37 °C in 5% CO2 atmosphere. The stale culture medium was changed lightly at 60 h. Loosely adherent cells and suspended cells were collected and then cultured in plate overnight on day 6. Cells with purity over 90% (CD11c level tested by flow cytometry (FCM)) will be chose for later experiments.
2.3. Determination of Reactive Oxygen Species (ROS)
Endogenous ROS level was tested by ROS assay kit according to the user’s manual. Under certain conditions, 2′,7′ dichlorofluorescein diacetate (DCFH-DA) can be oxidized. In short, after stimulation with H. pubescens extracts or plus LPS (100 ng/mL) for 24 h, DCs were collected from different groups, then the medium was removed, and the cells were washed with PBS and incubated with DCFH-DA (10 μM) at 37 °C for 20–30 min. DCs were washed with PBS three times and then tested by FCM. The data were analyzed using FlowJo software V10.0 (Stanford, CA, USA).
2.4. Determination of Nitric Oxide Synthases (NOS) Activity and Nitric Oxide (NO) Production
For the detection of NOS activity, DCs were collected, and then lysed using RIPA buffer including 1 mM PMSF at 4 °C for 10–20 min. Supernatant was obtained from lysate by centrifuging at 10,000× g for 20 min. The intracellular NOS activity was examined by the NOS assay kit in accordance with the user’s instruction. NOS catalyzed L-Arg and molecular oxygen to generate NO, and NO can react with nucleophile to form colored compounds which could be detected at 530 nm. As a significant index of NO synthesis, nitrite (NO2−) in the supernatant of cell cultures was tested by using the Griess reagent. Following the instructions of the NO assay kit, 50 μL DC culture supernatant, 50 μL Griess reagent I, and 50 μL Griess reagent II were added in 96-well plates in succession at room temperature. The OD540 of the samples and the standard model sodium nitrite (NaNO2) were detected quickly, and the NO content in the DC supernatant was computed via the standard curve formula.
2.5. Determination of the Reduced Glutathione (GSH) and the Oxidized Glutathione (GSSG)
DCs were cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h in vitro. Then, DCs were collected and washed with PBS, and then lysed in pre-cooled reagent 4 of T-GSH/GSSG assay kit by sonication. In addition, lysate was then centrifuged at 10,000× g at 4 °C for 10–20 min to obtain supernatant. Following the user’s manual, the T-GSH/GSSG assay kit was taken to test the total glutathione (T-GSH) content and GSSG content of lysate supernatant. The OD405 of the yellow compound was tested instantly. GSH content was calculated according to the formula GSH = T-GSH–2 × GSSG (value), and then the GSH/GSSG ratio was determined.
2.6. Determination of the Antioxidant Enzymes Activity
DCs were cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h in vitro. Then, DCs were collected and lysed using RIPA buffer including 1 mM PMSF. In addition, lysate was then centrifuged at 10,000× g for 10–20 min to obtain supernatant. The GPx assay kit was used to detect the endogenous glutathione peroxidase (GPx) activity in the cell lysate supernatant. The OD412 of the polymerized products was measured instantly. The intracellular Superoxide dismutase (SOD) level in the cell lysate supernatant was tested using the SOD assay kit according to the user’s instructions. The OD450 of the polymerized products was detected instantly. Following the user’s instructions, the catalase (CAT) level of the cell lysate supernatant was measured using the CAT assay kit. The OD520 of the polymerized products was detected instantly.
2.7. Determination of Nrf2 and HO–1
In short, DCs were cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h in vitro. In addition, then DCs were collected from different groups, washed with PBS, and then fixed and permeabilized by fixation buffer and permeabilization wash buffer by the user’s instruction. PE-Nrf2 antibody and Alexa Fluor 647–HO–1 antibody or respective isotypes were incubated with DCs at 4 °C for 20–30 min. After being washed with PBS, DCs were tested by FCM.
2.8. Statistical Analysis
All results were analyzed and processed by GraphPad Prism 8 software (San Diego, CA, USA) and the results expressed as means ± SD. One-way analysis of variance (ANOVA) was adopted to analyze and contrast the variance of each group. Statistically significant differences in groups are shown as asterisks in the figures. ** p < 0.01, * p < 0.05.
2.9. Ethics Statement
The study was performed in compliance with the guidelines of the Ethical Committee and Jiangsu Laboratory Animal Welfare, and agreed by the Jiangsu Administrative Committee of Laboratory Animals (permission number: SYXK (SU) 2017-0044). The Yangzhou University Animal Research Center (Yangzhou, China) provided us with C57BL/6 mice (4–6 weeks old). Mice were euthanized by cervical dislocation. We tried our best to minimize the number of mice used and to relieve their suffering.
3. Results
3.1. H. pubescens Extracts Inhibited the ROS Level in LPS-Irritated DCs
As a signaling molecule, ROS at low concentration plays physiological roles in various cellular processes. Pathogen infection can induce ROS production to kill invading pathogens, including bacteria and fungi [12,37]. However, excess production of ROS can damage cells and tissues [38]. As shown in Figure 1, the ROS level of LPS-induced DCs increased significantly, which was effectively inhibited by the extract of H. pubescens extracts in a dose-dependent manner.
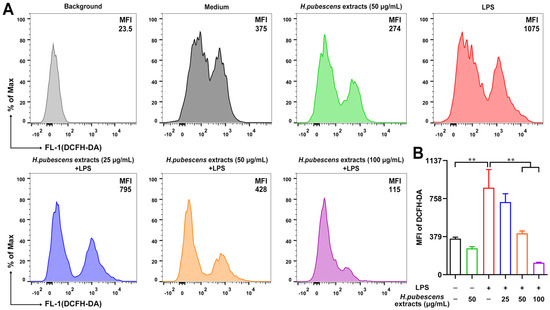
Figure 1.
H. pubescens extracts inhibited the ROS level in LPS-irritated DCs. (A,B) DCs were cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h, and then were collected and incubated with 2′,7′ dichlorofluorescein diacetate (DCFH-DA). ROS production was tested by FCM. All of the data are shown as means ± SD from three experiments. Analysis of variance (ANOVA) was employed. ** p < 0.01.
3.2. H. pubescens Extracts Reduced the NOS Activity and NO Level in LPS-Irritated DCs
Oxidative stress is always accompanied by excessive generation of reactive nitrogen species (RNS), such as NO. NO is synthetized enzymatically from L-arginine (L-Arg) under the action of NOS [39]. Therefore, the NOS activity was first tested (Figure 2A). H. pubescens extracts were shown to significantly weaken the NOS activity in LPS-irritated DCs. Then, the NO level was detected. As shown in Figure 2B, LPS remarkably raised the NO level in DCs relative to the control group. As expected, H. pubescens extracts were able to effectively lower the NO level in LPS-irritated DCs.
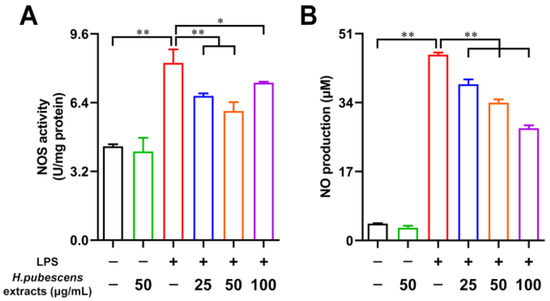
Figure 2.
H. pubescens extracts reduced the NOS activity and NO level in LPS-irritated DCs. (A,B) DCs were cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h. (A) DCs were lysed, and the intracellular NOS activity was measured using a commercial kit. (B) NO content in the DC supernatant was tested using Griess reagent I and II. All of the data are shown as means ± SD from three experiments. Analysis of variance (ANOVA) was employed. ** p < 0.01, * p < 0.05.
3.3. H. pubescens Extracts Regulated the Intracellular GSH, GSSG, and GSH/GSSG Ratio in LPS-Irritated DCs
As one of the most significant intracellular redox regulator, glutathione consists of the reduced form glutathione (GSH) and the oxidized form glutathione (GSSG) [40]. Many studies have declared that one of the critical indices of measuring the redox status is the GSH/GSSG ratio [41,42]. Therefore, we further investigated the effects of H. pubescens extracts on intracellular GSH and GSSG in LPS-irritated DCs. As shown in Figure 3, compared with the control group, LPS obviously reduced the intracellular GSH (A) content and increased the intracellular GSSG (B) content. Correspondingly, the intracellular GSH/GSSG ratio fell noticeably. Furthermore, H. pubescens extracts effectively reversed those effects in a dose-dependent manner.
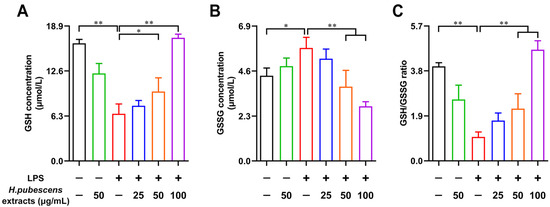
Figure 3.
H. pubescens extracts regulated the intracellular GSH, GSSG, and GSH/GSSG ratio in LPS-irritated DCs. DCs were collected and washed with PBS after being cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h. Then, DCs were lysed to detect the GSH content (A), the GSSG content (B), and the GSH/GSSG ratio (C) using a commercial kit. All of the data are shown as means ± SD from three experiments. Analysis of variance (ANOVA) was employed. ** p < 0.01, * p < 0.05.
3.4. H. pubescens Extracts Strengthened the Capacities of Antioxidant Enzymes in LPS-Irritated DCs
Antioxidant system has been evolved by cells against the excess ROS, which consists of some enzymatic antioxidants and some non-enzymatic antioxidants to reduce oxidative stress [43]. Therefore, we tested the effects of H. pubescens extracts on the capacities of antioxidant enzymes in LPS-irritated DCs. As shown in Figure 4, LPS significantly weakened the GPx (A), CAT (B), and SOD (C) activity of DCs compared with the control group. As expected, H. pubescens extracts were able to strengthen the levels of these antioxidant enzymes in LPS-irritated DCs.
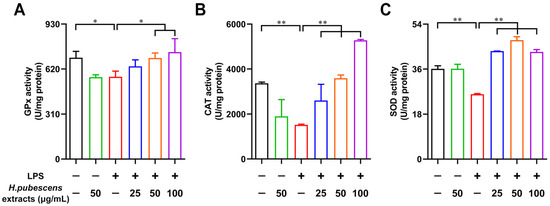
Figure 4.
H. pubescens extracts strengthened the capacities of antioxidant enzymes in LPS-irritated DCs. DCs were collected and washed with PBS after being cultured with the designed concentrations of H. pubescens extracts or LPS (100 ng/mL) for 24 h. The intracellular GPx capacity (A), CAT capacity (B), and SOD capacity (C) of the DC lysate supernatant were tested using commercial kits. All of the data are shown as means ± SD from three experiments. Analysis of variance (ANOVA) was employed. ** p < 0.01, * p < 0.05.
3.5. H. pubescens Extracts Upregulated Both Nrf2 and HO–1 Expression in LPS-Irritated DCs
Nrf2 is an important regulator of oxidative stress, playing a key role in maintaining redox homeostasis [44]. HO–1 is closely related to antioxidant activation [45], which is one of the key and common effectors of Nrf2 [46]. Therefore, we conducted FCM to test the expression of Nrf2 and HO–1 in DCs. As shown in Figure 5, H. pubescens extracts evidently increased the expression level of Nrf2 (A,B) molecules and HO–1 (C,D) molecules in DCs irritated by LPS, implying that H. pubescens extracts are able to suppress oxidative stress via the Nrf2/HO–1 signaling pathway.
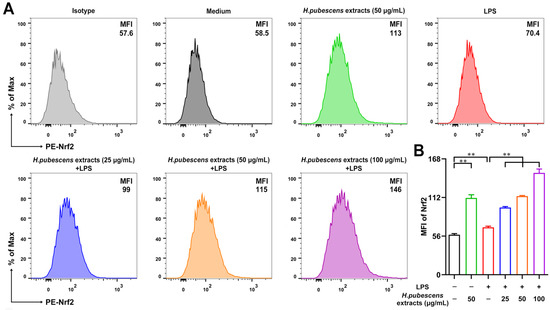
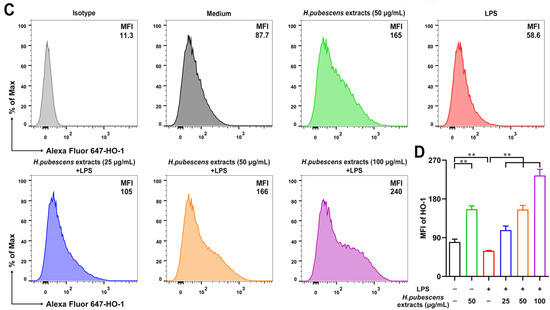
Figure 5.
H. pubescens extracts upregulated both Nrf2 and HO–1 in LPS-irritated DCs. DCs were collected and washed with PBS after being cultured with the designed concentrations of H. pubescens extracts or plus LPS (100 ng/mL) for 24 h. Nrf2 (A,B) and HO–1 (C,D) expression of DCs were tested by FCM. All of the data are shown as means ± SD from three experiments. Analysis of variance (ANOVA) was employed. ** p < 0.01.
4. Discussion
H. pubescens tastes astringent and bitter, and is traditionally used to treat several diseases, including intestinal parasites, animal bites, indigestion, blood-related ailments, and dental or oral ailments [47,48]. H. pubescens, as an important medicinal plant, possesses potential medicinal properties. H. pubescens has been reported to have many activities, including anti-diabetic function, anti-diarrheal effect, anti-inflammatory and analgesic efficacy, and free radical scavenging properties [27]. Here, we systematically evaluated the antioxidative effects of H. pubescens extracts in LPS-irritated DCs in vitro, and found that H. pubescens extracts are able to inhibit LPS-irritated oxidative stress in DCs via the Nrf2/HO–1 axis.
Low-level ROS is required for many important signaling reactions [49]. NO is an important molecule that is considered to regulate cellular functions involving in various pathological and physiological functions, such as endothelial vasodilation, cardiovascular functions, antimicrobial effect, wound healing, immune function, blood pressure regulation, and cytotoxicity, etc. [50,51]. However, oxidative imbalance leads to oxidative stress and the excessive production of ROS and NO [52], leading to apoptosis, resulting in protein and organelle damage, mitochondrial membrane disruption, and cell death [53,54]. The antioxidant system of DCs was unable to effectively clear the over-accumulation of ROS and NO, leading to immune dysfunction in DCs. The data presented here suggest that H. pubescens extracts reduce the level of ROS, NOS, and NO in LPS-irritated DCs.
As an antioxidant, GSH is used as a free radical scavenger and an antidote in cells, and is involved in many processes such as cell proliferation and differentiation. In addition, as the most common metabolite, GSH is usually tested when oxidative stress occurs. When oxidative stress occurs, GSH is converted into GSSG upon its reaction with ROS under the catalysis of GSH-dependent peroxidases [55]. GPx acted as a catalyst to lead hydrogen peroxide (H2O2) to produce reduction in this reaction [56]. Mitochondrial matrix is at a highly reducing environment to conduct appropriate protein folding and maintain protein function. Furthermore, a high GSH/GSSG ratio promotes synthesis of deoxyribonucleotides from ribonucleotides and maintains sulfhydryl groups in proteins for nucleic acid biosynthesis and DNA repairment [40]. Previous studies have confirmed that LPS decreased the GSH/GSSG ratio in DCs [57]. Low GSH/GSSG ratio resulted in a failure to resist the excess expression of ROS and subsequent breakage, leading to oxidative stress in DCs, and then losing lose the immune regulation ability [20,55]. Crucially, the addition of H. pubescens extracts to LPS-irritated DCs dramatically increased the GSH content and raised the GSH/GSSG ratio, and enhanced the level of GPx.
Additionally, CAT and SOD, the other two important antioxidant enzymes, also play a very significant role in preventing cells from being subjected to harm as a result of oxidative stress. Antioxidant enzymes are in charge of maintaining the balance between the functions of free radical formation and removing their excessive amounts. Arguably, superoxide (O2−) and hydrogen peroxide can be seen as the common and most–produced ROS, with the former eliminated by SOD and the latter by CAT, GPx, and peroxiredoxins (PRX) [58,59]. Therefore, the capabilities of CAT and SOD were detected, and the data suggested that H. pubescens extracts significantly upregulated the capabilities of CAT and SOD in DCs irritated by LPS for preventing the oxidative stress.
Nrf2 is a transcription factor that is responsible for the regulation of cellular redox balance and protective antioxidant and phase II detoxification responses in mammals [60]. An increasing number of antioxidant response elements (AREs) have been found and studied, including glutamate-cysteine ligase (GCL), thioredoxin reductase 1 (Txnrd1), NAD(P)H-quinone oxidoreductase 1 (NQO1) and HO–1. The discovery of AREs has led to the conclusion that AREs can combine with Nrf2 to perform functions [61]. This leads to cascading events, which alter oxidative status of the cells and provide powerful protection against oxidative stress in the end [62]. Among Nrf2 signaling pathways, one of the most crucial intracellular protection mechanisms, Nrf2/HO–1 is the most classic approach [63]. Moreover, NO and NOS overproduction can be inhibited by mass expression of HO–1 [64]. Here, our study found that H. pubescens extracts upregulated both Nrf2 and HO–1 in LPS-irritated DCs, making clear that Nrf2/HO–1 signaling pathway may play a vital role in this process.
5. Conclusions
Here, H. pubescens extracts were shown to reduce the intracellular ROS level and weaken the NOS activity and NO level in LPS-irritated DCs. As expected, H. pubescens extracts increased GSH production and decreased GSSG production led by LPS, and the GSH/GSSG ratio was increased during this process. Meanwhile, H. pubescens extracts effectively enhanced the capacities of three antioxidant enzymes. Moreover, H. pubescens extracts significantly improved the expression of the Nrf2 and the HO–1 in LPS-irritated DCs. These findings suggested that H. pubescens extracts is a potential candidate drug target as a novel antioxidant and can be applied to clinical combination therapy in sepsis.
Author Contributions
Conceptualization, T.Q. and C.L.; methodology, Y.Y., B.Z. and T.Q.; software, Y.Y., B.Z. and L.B.; validation, Y.Y. and D.F.; formal analysis, Y.Y., B.Z. and C.L.; investigation, Y.Y., B.Z. and T.Q.; resources, Y.Y. and C.L.; data curation, Y.Y. and B.Z.; writing—original draft preparation, Y.Y., B.Z. and T.Q.; writing—review and editing, B.Z., Y.Y. and T.Q.; visualization, Y.Y. and B.Z.; supervision, T.Q., Y.Y. and C.L.; project administration, Y.Y. and C.L.; funding acquisition, Y.Y., T.Q. and C.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31600113, 31800284), the Key Research and Development Program of Social Development of Jiangsu Province (BE2022774), the Agricultural Science & Technology Independent Innovation Fund of Jiangsu Province (CX(20)3092), the Jiangsu Provincial Natural Science Fund for Excellent Young Scholars (BK20200105), the 2020 Interdisciplinary Project of Yangzhou University Veterinary Special Zone (yzuxk202004), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education (PAPD).
Institutional Review Board Statement
The study was performed in compliance with the guidelines of the Ethical Committee and Jiangsu Laboratory Animal Welfare, and agreed by the Jiangsu Administrative Committee of Laboratory Animals (permission number: SYXK (SU) 2017-0044).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting reported results can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| H. pubescens | Holarrhena pubescens Wall. ex G. Don |
| LPS | Lipopolysaccharide |
| DCs | dendritic cells |
| ROS | reactive oxygen species |
| NOS | nitric oxide synthases |
| NO | nitric oxide |
| GSSG | oxidized glutathione |
| GSH | reduced glutathione |
| GPx | glutathione peroxidase |
| CAT | catalase |
| SOD | superoxide dismutase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| HO–1 | heme oxygenase 1 |
| APCs | antigen-presenting cells |
| MS | multiple sclerosis |
| TBI | traumatic brain injury |
| FBS | Fetal bovine serum |
| Pen Strep | Penicillin and streptomycin |
| IL-4 | Interleukin-4 |
| GM-CSF | recombinant mouse granulocyte macrophage colony stimulating factor |
| RIPA | Radio Immunoprecipitation Assay |
| PMSF | phenylmethanesulfonyl fluoride |
| BMDCs | Murine Bone Marrow-Derived DCs |
| DCFH-DA | 2′,7′ dichlorofluorescein diacetate |
| FCM | flow cytometry |
| NO2− | nitrite |
| NaNO2 | sodium nitrite |
| T-GSH | total glutathione |
| ANOVA | analysis of variance |
| RNS | reactive nitrogen species |
| L-Arg | L-arginine |
| H2O2 | hydrogen peroxide |
| O2− | superoxide |
| PRX | peroxiredoxins |
| ARE | antioxidant response element |
| GCL | glutamate-cysteine ligase |
| Txnrd1 | thioredoxin reductase 1 |
| NQO1 | NAD(P)H-quinone oxidoreductase 1 |
References
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e1147. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Ackermann, M.; Dragon, A.C.; Lachmann, N. The Immune-Modulatory Properties of iPSC-Derived Antigen-Presenting Cells. Transfus. Med. Hemotherapy Off. Organ Der Dtsch. Ges. Fur Transfus. Und Immunhamatol. 2020, 47, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef] [PubMed]
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef]
- Mohammad, M.G.; Hassanpour, M.; Tsai, V.W.; Li, H.; Ruitenberg, M.J.; Booth, D.W.; Serrats, J.; Hart, P.H.; Symonds, G.P.; Sawchenko, P.E.; et al. Dendritic cells and multiple sclerosis: Disease, tolerance and therapy. Int. J. Mol. Sci. 2012, 14, 547–562. [Google Scholar] [CrossRef]
- Wu, D.D.; Li, T.; Ji, X.Y. Dendritic Cells in Sepsis: Pathological Alterations and Therapeutic Implications. J. Immunol. Res. 2017, 2017, 3591248. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Hao, S.; Luan, H. Expression of Peripheral Blood DCs CD86, CD80, and Th1/Th2 in Sepsis Patients and Their Value on Survival Prediction. Comput. Math. Methods Med. 2022, 2022, 4672535. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K.; Punziano, C.; Minopoli, G.; Faraonio, R. MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Redox Control of Cell Senescence. Antioxidants 2022, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef] [PubMed]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef]
- Chuang, C.C.; Shiesh, S.C.; Chi, C.H.; Tu, Y.F.; Hor, L.I.; Shieh, C.C.; Chen, M.F. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit. Care 2006, 10, R36. [Google Scholar] [CrossRef]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, R.Q.; Tong, S.; Dong, N.; Sheng, Z.Y.; Yao, Y.M. Effect of tumor necrosis factor-α induced protein 8 like-2 on immune function of dendritic cells in mice following acute insults. Oncotarget 2016, 7, 30178–30192. [Google Scholar] [CrossRef][Green Version]
- Luan, Y.Y.; Dong, N.; Xie, M.; Xiao, X.Z.; Yao, Y.M. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yin, Y.; Yu, Q.; Yang, Q. Bursopentin (BP5) protects dendritic cells from lipopolysaccharide-induced oxidative stress for immunosuppression. PLoS ONE 2015, 10, e0117477. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, N.; Qin, T.; Zhou, B.; Shi, Y.; Zhao, X.; Ma, B.; Xu, Z.; Li, C. Astaxanthin Provides Antioxidant Protection in LPS-Induced Dendritic Cells for Inflammatory Control. Mar. Drugs 2021, 19, 534. [Google Scholar] [CrossRef]
- Sheng-Ji, P. Ethnobotanical approaches of traditional medicine studies: Some experiences from Asia. Pharm. Biol. 2001, 39 (Suppl. S1), 74–79. [Google Scholar] [CrossRef]
- Sinha, S.; Sharma, A.; Reddy, P.H.; Rathi, B.; Prasad, N.V.S.R.K.; Vashishtha, A. Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica (Wall.): A comprehensive review. J. Pharm. Res. 2013, 6, 488–492. [Google Scholar] [CrossRef]
- Jamadagni, P.S.; Pawar, S.D.; Jamadagni, S.B.; Chougule, S.; Gaidhani, S.N.; Murthy, S.N. Review of Holarrhena antidysenterica (L.) Wall. ex A. DC.: Pharmacognostic, Pharmacological, and Toxicological Perspective. Pharmacogn. Rev. 2017, 11, 141–144. [Google Scholar] [CrossRef]
- Gupta, N.; Choudhary, S.K.; Bhagat, N.; Karthikeyan, M.; Chaturvedi, A. In Silico Prediction, Molecular Docking and Dynamics Studies of Steroidal Alkaloids of Holarrhena pubescens Wall. ex G. Don to Guanylyl Cyclase C: Implications in Designing of Novel Antidiarrheal Therapeutic Strategies. Molecules 2021, 26, 4147. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Dicato, M.; Diederich, M. Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem. Pharmacol. 2012, 84, 1225–1240. [Google Scholar] [CrossRef]
- Koudouvo, K.; Karou, D.S.; Kokou, K.; Essien, K.; Aklikokou, K.; Glitho, I.A.; Simpore, J.; Sanogo, R.; De Souza, C.; Gbeassor, M. An ethnobotanical study of antimalarial plants in Togo Maritime Region. J. Ethnopharmacol. 2011, 134, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, P.; Morganti, M.; Mancini, M.; Signorini, M.A. Traditional healers and laypeople: A qualitative and quantitative approach to local knowledge on medicinal plants in Muda (Mozambique). J. Ethnopharmacol. 2011, 138, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, B.; Bhandari, P.; Gupta, A.P.; Kaul, V.K. Steroidal alkaloids from Holarrhena antidysenterica (L.) WALL. Chem. Pharm. Bull. 2007, 55, 912–914. [Google Scholar] [CrossRef]
- Chakraborty, A.; Brantner, A.H. Antibacterial steroid alkaloids from the stem bark of Holarrhena pubescens. J. Ethnopharmacol. 1999, 68, 339–344. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.H. In vitro biological evaluation of 100 selected methanol extracts from the traditional medicinal plants of Asia. Nutr. Res. Pract. 2014, 8, 151–157. [Google Scholar] [CrossRef][Green Version]
- Yin, Y.; Qin, T.; Wang, X.; Lin, J.; Yu, Q.; Yang, Q. CpG DNA assists the whole inactivated H9N2 influenza virus in crossing the intestinal epithelial barriers via transepithelial uptake of dendritic cell dendrites. Mucosal Immunol. 2015, 8, 799–814. [Google Scholar] [CrossRef]
- Goswamy, D.; Irazoqui, J.E. A unifying hypothesis on the central role of reactive oxygen species in bacterial pathogenesis and host defense in C. elegans. Curr. Opin. Immunol. 2021, 68, 9–20. [Google Scholar] [CrossRef]
- Hameister, R.; Kaur, C.; Dheen, S.T.; Lohmann, C.H.; Singh, G. Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2073–2087. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO–1 Axis in Cancer Cell Growth and Chemoresistance. Oxidative Med. Cell. Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef]
- Soares, M.P.; Marguti, I.; Cunha, A.; Larsen, R. Immunoregulatory effects of HO–1: How does it work? Curr. Opin. Pharmacol. 2009, 9, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Surh, Y.J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. 2014, 67, 353–365. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Badola, H.K. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomed. 2008, 4, 22. [Google Scholar] [CrossRef]
- Panda, S.K. Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Yetik-Anacak, G.; Catravas, J.D. Nitric oxide and the endothelium: History and impact on cardiovascular disease. Vasc. Pharmacol. 2006, 45, 268–276. [Google Scholar] [CrossRef]
- Body, S.C.; Hartigan, P.M.; Shernan, S.K.; Formanek, V.; Hurford, W.E. Nitric oxide: Delivery, measurement, and clinical application. J. Cardiothorac. Vasc. Anesth. 1995, 9, 748–763. [Google Scholar] [CrossRef]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.L.; Wilhelmus, M.M.; de Vries, H.E.; Drukarch, B.; Hoozemans, J.J.; van Horssen, J. Antioxidative defense mechanisms controlled by Nrf2: State-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014, 88, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Grant, R.; Mori, T.A.; Croft, K.D. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS ONE 2014, 9, e85335. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Yamada, H.; Arai, T.; Endo, N.; Yamashita, K.; Fukuda, K.; Sasada, M.; Uchiyama, T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006, 78, 926–933. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anti–Cancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 system in cancers: Stress response and anabolic metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Nasser, M.I.; Masood, M.; Adlat, S.; Huang, Y.; Yang, B.; Luo, C.; Jiang, N. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO–1 signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 126, 110074. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO–1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. CMLS 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R.; Jeon, S.B.; Jeon, W.K.; Chae, H.J.; Chung, H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).