Zeolitization of Diatomite Residues by a Simple Method
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
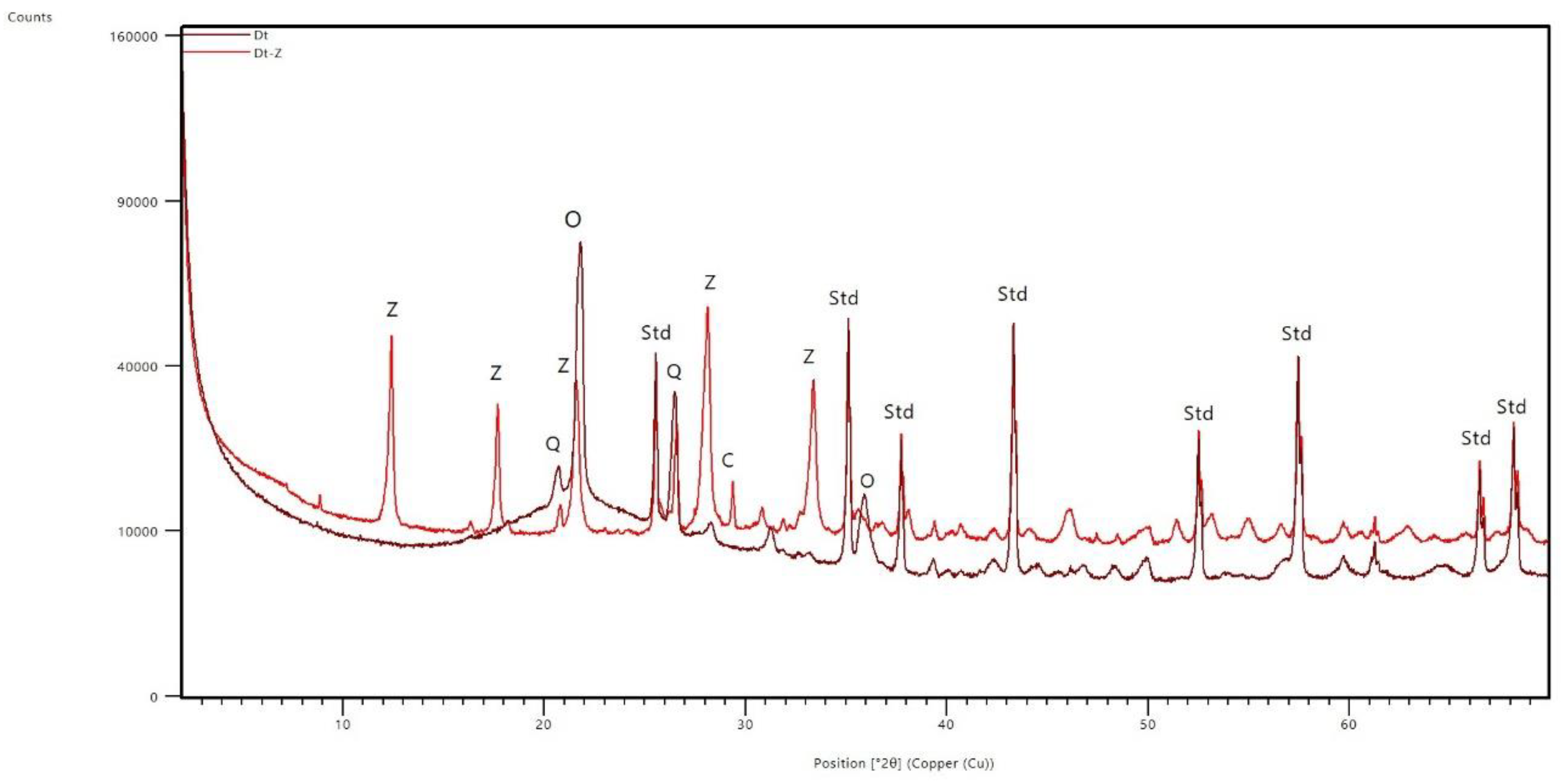
3.1. XRD Analysis: Mineralogical Transformations
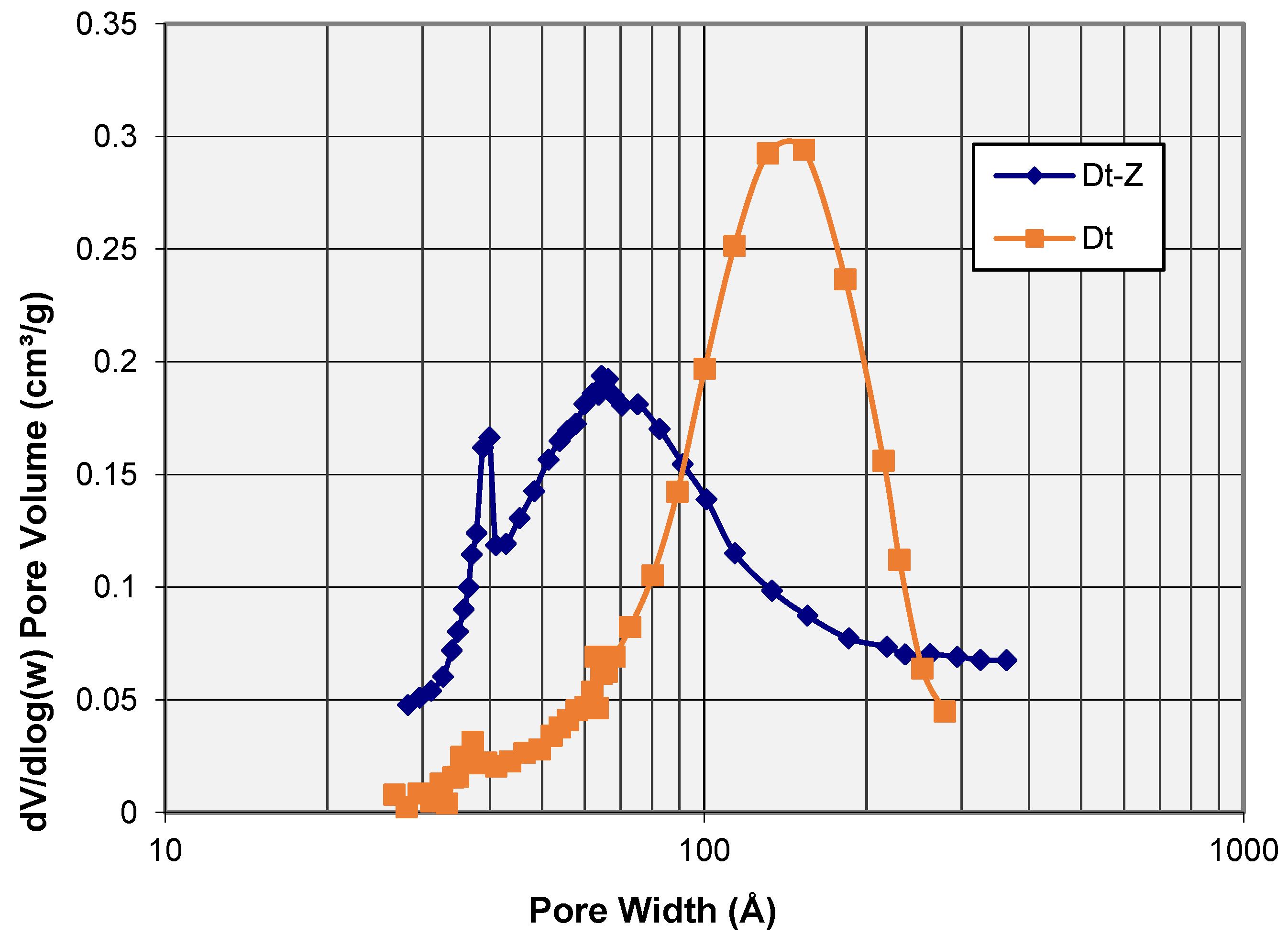
3.2. N2 adsorption–Desorption Isotherms: Porosity and Surface Area
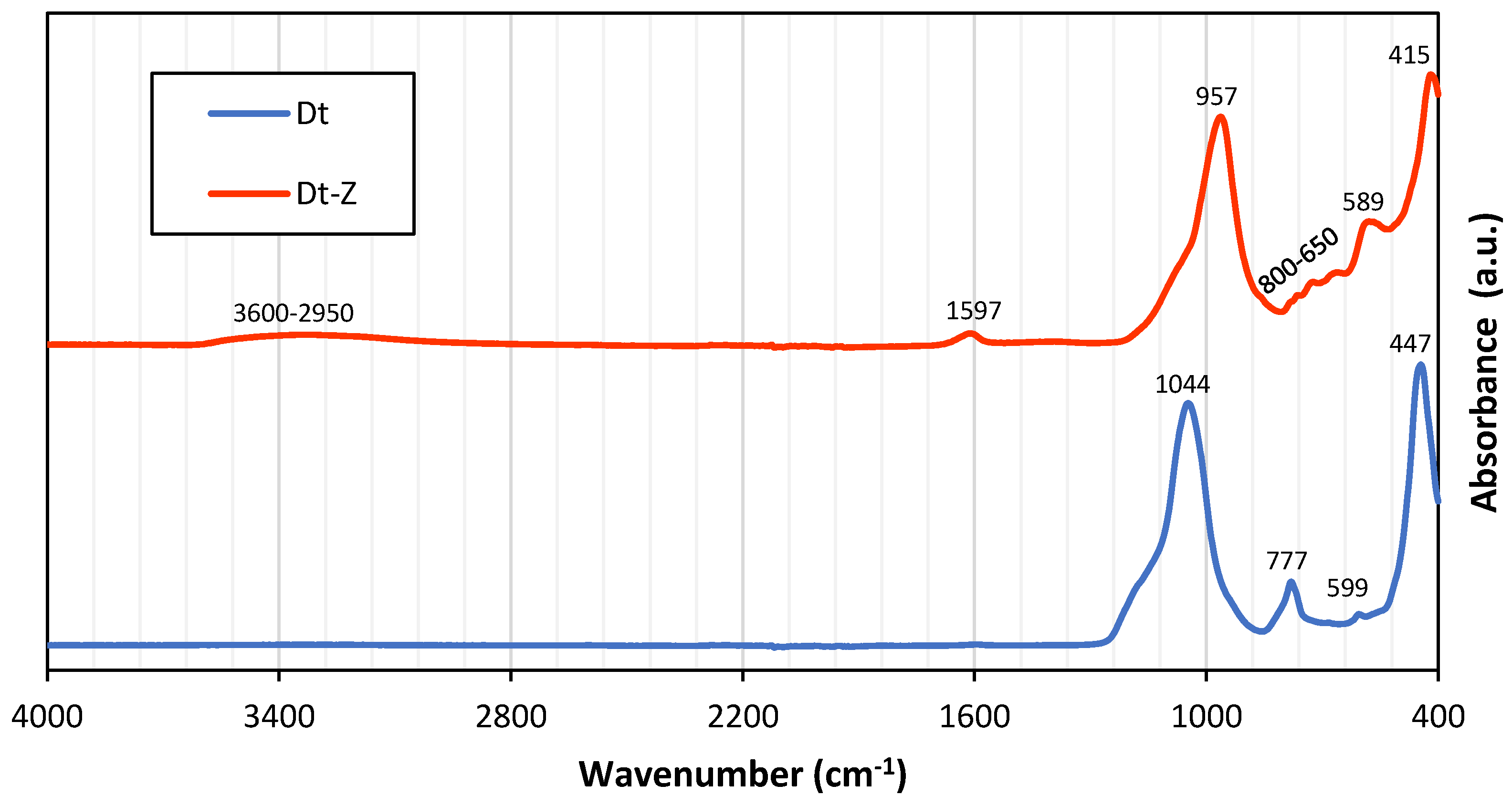
3.3. FTIR Results: Endorsing the Findings on Zeolitization
4. Conclusions
- It has been demonstrated that it is possible to synthesize zeolites (in this case zeolite P) from this type of raw materials without the addition of any aluminum supplying component. Despite this, the volume of sediment obtained was significantly lower than that of the raw material used, so further investigation would be required including some source of aluminum to improve the protocol.
- Quantitative XRD tests have shown that the zeolitization process has been effective, forming a material with 55% zeolite P from the hydrothermal transformation of both the amorphous phase, the opal-CT and, to a lesser extent, the quartz present in the original diatomite-rich residue. Such mineralogical transformations have been corroborated through changes in the bands observed in the FTIR spectra.
- The N2 adsorption–desorption isotherms tests have revealed that although the starting diatomite presented a relatively high specific surface (39.4 m2/g), the zeolitization treatment has involved a positive effect, increasing this property by almost 60% (62.6 m2/g). This specific surface area value is slightly higher than the maximum SBET obtained by Du et al. [9] in zeolites P also produced from diatomites by applying a more laborious method and with the addition of an Al source. Similarly, although the pore volume has hardly changed, the treatment described has favored a reduction in its size by almost 40%, from an average pore size of 118 Å to 71 Å. Therefore, the zeolite formed would be fundamentally mesoporous, like others previously reported in the literature.
- This study shows only a first approach to obtain zeolites from diatomite wastes. Therefore, the findings of this investigation could be useful for future research in order to improve certain properties, such as increasing the purity of the zeolite obtained, increasing its specific surface area and reducing the overall pore size.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biswajit, G.; Dinesh, C.; Subhash, B. Synthesis of zeolite A from calcined diatomaceous clay: Optimization studies. Ind. Eng. Chem. Res. 1994, 33, 2107–2110. [Google Scholar] [CrossRef]
- Chaisena, A.; Rangsriwatananon, K. Effects of thermal and acid treatments on some physico-chemical poroperies of Lampang diatomite. Suranaree J. Sci. Technol. 2004, 11, 289–299. [Google Scholar]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Stafin, G.; Grzebielucka, E.C.; Antunes, S.R.; Ferreira Borges, C.P.; Chaves de Andrade, A.V.; Aparecida Alves, S.; Ferreira de Souza, E.C. Synthesis of zeolites from residual diatomite using a microwave-assisted hydrothermal method. Waste Manag. 2021, 126, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Recio, M.A.; Rodrigues, C.; Jeremias, T.C.; Lapolli, F.R.; Padilla, I.; López-Delgado, A. Highly efficient removal of aluminum, iron, and manganese ions using Linde type-A zeolite obtained from hazardous waste. Chemosphere 2021, 267, 128919. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, Y.; Wang, Y.; Xia, J.; Tang, Y. Synthesis of Meso-/Macroporous Zeolite (Fe,Al)-ZSM-5 Microspheres from Diatomite. Chem. Lett. 2004, 33, 270–271. [Google Scholar] [CrossRef]
- Wajima, T.; Haga, M.; Kuzawa, K.; Ishimoto, H.; Tamada, O.; Ito, K.; Nishiyama, T.; Downs, R.T.; Rakovan, J.F. Zeolite synthesis from paper sludge ash at low temperature (90 °C) with addition of diatomite. J. Hazard. Mater. 2006, 132, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Han, W.; Xiong, G.; Yang, W. A method for diatomite zeolitization through steam-assisted crystallization with in-situ seeding. Mater. Lett. 2008, 62, 2400–2403. [Google Scholar] [CrossRef]
- Du, Y.; Shi, S.; Dai, H. Water-bathing synthesis of high-surface-area zeolite P from diatomite. Particuology 2011, 9, 174–178. [Google Scholar] [CrossRef]
- Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S. One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite. Materials 2018, 11, 906. [Google Scholar] [CrossRef]
- Servatan, M.; Ghadiri, M.; Yazdi, M.K.; Jouyandeh, M.; Mahmodi, G.; Samadi, A.; Zarrintaj, P.; Habibzadeh, S.; Ganjali, M.R.; Saeb, M.R. Synthesis of Cost-Effective Hierarchical MFI-Type Mesoporous Zeolite: Introducing Diatomite as Silica Source. Silicon 2021, 13, 3461–3472. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Frangie, S.; Martinez, V.; Valiente, L.; Webb, O.A. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J. Hazard. Mater. 2012, 241–242, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Bish, D.L.; Post, J.E. Quantitative mineralogical analysis using the Rietveld full pattern fitting method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Rodríguez, L.; Acosta, A. Assessment of crystalline phase changes and glass formation by Rietveld- XRD method on ceramic lightweight aggregates sintered from mineral and polymeric wastes. Ceram. Int. 2018, 44, 11840–11851. [Google Scholar] [CrossRef]
- IZA. International Zeolite Association (IZA). Database of Zeolite Structures. 2022. Available online: http://www.iza-structure.org/databases/ (accessed on 1 August 2022).
- Albert, B.R.; Cheetham, A.K.; Adams, C.J. Investigations on P zeolites: Synthesis and structure of the gismondine analogue, highly crystalline low-silica CaP. Microporous Mesoporous Mater. 1998, 21, 127–132. [Google Scholar] [CrossRef]
- Yang, S.; Lach-hab, M.; Vaisman, I.; Blaisten-Barojas, E.; Li, X.; Karen, V.L. Framework-Type Determination for Zeolite Structures in the Inorganic Crystal Structure Database. J. Phys. Chem. Ref. Data. 2010, 39, 033102. [Google Scholar] [CrossRef]
- Baerlocher, C.; Meier, W.M. The crystal structure of synthetic zeolite Na-P 1, an isotype of gismondine. Z. Für. Krist. -Cryst. Mater. 1972, 135, 339–354. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P. Mesopore determination from nitrogen sorption isotherms: Fundamentals, scope, limitations. Stud. Surf. Sci. Catal. 1979, 3, 663–684. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Shields, J.E.; Lowell, S.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Kluwer Academic Publisher: Boston, MA, USA, 2004; pp. 43–45. [Google Scholar]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Baltork, I.M.; Ghani, K. Alkene epoxidation catalyzed by molybdenum supported on functionalized MCM-41 containing N–S chelating Schiff base ligand. Catal. Commun. 2009, 10, 853–858. [Google Scholar] [CrossRef]
- Madejova, J.; Janek, M.; Komadel, P.; Herbert, H.J.; Moog, H.C. FTIR analyses of water in MX-80 bentonite compacted from high salinary salt solution systems. Appl. Clay Sci. 2002, 20, 255–271. [Google Scholar] [CrossRef]
- Ilia, I.K.; Stamatakis, M.G.; Perraki, T.S. Mineralogy and technical properties of clayey diatomites from north and central Greece. Open Geosci. 2009, 1, 393–403. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Delgado-Plana, P.; Cabezas-Rodríguez, R.; Mejía de Gutiérrez, R.; Eliche-Quesada, D.; Pérez-Villarejo, L.; Galán-Arboledas, R.J.; Bueno, S. Alkaline activation of high-crystalline low-Al2O3 Construction and Demolition Wastes to obtain geopolymers. J. Clean. Prod. 2022, 330, 129770. [Google Scholar] [CrossRef]
- Zainal Abidin, A.; Abu Bakar, N.H.H.; Ng, E.P.; Tan, W.L. Rapid Degradation of Methyl Orange by Ag Doped Zeolite X in the Presence of Borohydride. J. Taibah Univ. Sci. 2017, 11, 1070–1079. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Barczyk, K. FT-IR studies of zeolites from different structural groups. Chemik 2011, 65, 667–674. [Google Scholar]
- López-Delgado, A.; Robla, J.I.; Padilla, I.; López-Andrés, S.; Romero, M. Zero-waste process for the transformation of a hazardous aluminum waste into a raw material to obtain zeolites. J. Clean. Prod. 2020, 255, 120178. [Google Scholar] [CrossRef]
- Milkey, R.G. Infrared spectra of some tectosilicates. Am. Mineral. 1960, 45, 990–1007. [Google Scholar]

| Diatomite | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | CaO | TiO2 | Na2O | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As received | 76.8 | 3.8 | 1.5 | 1.0 | 0.2 | 0.8 | 0.4 | 0.7 | 0.3 | 0.03 | 14.5 |
| Calcined (Dt) | 89.8 | 4.4 | 1.8 | 1.1 | 0.2 | 0.9 | 0.4 | 0.8 | 0.4 | 0.03 | 0.0 |
| Amorphous | Opal-CT | Quartz | Calcite | Zeolite P | |
|---|---|---|---|---|---|
| Dt | 81.2 | 11.1 | 7.7 | – | – |
| Dt-Z | 39.2 | – | 4.3 | 1.6 | 54.9 |
| Δ % | −52.8 | −100.0 | −44.2 | 100.0 | 100.0 |
| SBET (m2/g) | DFT Micro (cm3/g) | BJH Cumulative Pore Volume (cm3/g) | BJH Average Pore Width (Å) | |||||
|---|---|---|---|---|---|---|---|---|
| Adsorption | Desorption | Average | Adsorp. | Desorp. | Average | |||
| Dt | 39.38 | 0.002 | 0.11 | 0.13 | 0.12 | 121.8 | 114.0 | 117.9 |
| Dt-Z | 62.57 | 0.005 | 0.10 | 0.13 | 0.11 | 67.8 | 74.3 | 71.0 |
| Δ% | 58.9 | 124.6 | −10.9 | 0.5 | −4.9 | −44.4 | −34.8 | −39.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Maroto, J.M.; Alonso-Azcárate, J.; Martínez-García, C.; Romero, M.; López-Delgado, A.; Cotes-Palomino, T. Zeolitization of Diatomite Residues by a Simple Method. Appl. Sci. 2022, 12, 10977. https://doi.org/10.3390/app122110977
Moreno-Maroto JM, Alonso-Azcárate J, Martínez-García C, Romero M, López-Delgado A, Cotes-Palomino T. Zeolitization of Diatomite Residues by a Simple Method. Applied Sciences. 2022; 12(21):10977. https://doi.org/10.3390/app122110977
Chicago/Turabian StyleMoreno-Maroto, José Manuel, Jacinto Alonso-Azcárate, Carmen Martínez-García, Maximina Romero, Aurora López-Delgado, and Teresa Cotes-Palomino. 2022. "Zeolitization of Diatomite Residues by a Simple Method" Applied Sciences 12, no. 21: 10977. https://doi.org/10.3390/app122110977
APA StyleMoreno-Maroto, J. M., Alonso-Azcárate, J., Martínez-García, C., Romero, M., López-Delgado, A., & Cotes-Palomino, T. (2022). Zeolitization of Diatomite Residues by a Simple Method. Applied Sciences, 12(21), 10977. https://doi.org/10.3390/app122110977












