Introducing Mobile Collaborative Robots into Bioprocessing Environments: Personalised Drug Manufacturing and Environmental Monitoring
Abstract
1. Introduction
| Therapy Type | Bioreactor Size | Types of Reactors Used | Reference |
|---|---|---|---|
| CAR-T therapies | 100–200 mL | T Flasks, Static culture bags, rocking motion bioreactor | [39] |
| Hematopoietic stem cell therapy | 3–550 mL | Perfusion chamber, stirred tank, fluidised bed, fixed bed, airlift, hollow fiber | [40,41,42] |
| Hematopoietic progenitor cell therapy | 173–600 mL | Stirred suspension, fixed bed reactor | [43,44,45] |
| Mesenchymal cell therapy | 15 mL–5 L | fixed bed disposable reactor | [46,47] |
| Commercial Bioreactors | Bioreactor Size | Reference |
|---|---|---|
| Ambr 15 cell culture system | 24–48 parallel, 10–15 mL micro bioreactors | [39] |
| PBS-MINI MagDrive Bioreactor | 100–500 mL | [43] |
| PALL iCELLi Nano | 1000 mL | [48] |
2. Use Cases
- (a)
- Retrieval and transport of samples stored in rigid containers such as centrifugal tubes.
- Centrifugation is a prevalent method in biomanufacturing for separating various components from the sample and for separating the cells harvested for autologous therapies [19,80]. Centrifugation is also used for harvesting the final product. Other cell separation methods include sedimentation, immunomagnetic cell separation, immunodensity cell isolation, Fluorescence-Activated Cell Sorting (FACS), microfluidic cell separation, etc. use centrifugal tubes or vials similar to centrifugal tubes for processing the sample [81,82,83]. Vials similar in size to centrifugal tubes are also used during the scale out stage of biomanufacturing [84,85] and for laboratory scale production of cell therapies [86]. In a typical biomanufacturing facility, several vials of this kind are moved daily from one place to another. Transport and manipulation of centrifugal tubes can be automated with the help of mobile robots [5]. Hence, a mobile collaborative robot was utilised to transport centrifugal tubes in this use case.
- (b)
- Retrieval and transport of individual samples stored in flexible containers such as blood bags.
- Infusion/blood bags are the most preferred device for storing blood and its components and are suitable for a storage period of up to 42 days [87]. Such bags are also used for packaging pharmaceuticals [88,89,90]. The autologous therapy sector uses these bags to harvest the donor blood for cryopreservation, and to transport the product [91,92]. Vials with blood bag style closure and access systems are available to store and transport autologous cells [93]. Cellbags are used as bioreactor vessels [94,95,96,97]. In short, blood bags/infusion bags are very common methods used in biomanufacturing for moving fluids. These bags are deformable, making them more challenging for robots to grip, which is why this area has not been explored yet for robotic automation. However, this is an application that robots could potentially carry out. This use case demonstrates the potential use of a mobile collaborative robot for manipulating blood bags.
- (c)
- Environmental monitoring through the placement and retrieval of Petri dishes at various locations throughout the facility.
- Environmental samples are collected to monitor the quality of the biomanufacturing environment. Environmental sampling using settle plates is one of the prevalent environmental monitoring methods [98,99]. Typically in a commercial-scale biomanufacturing facility, microbiologists routinely place hundreds of open Petri dishes containing growth media to collect the samples of the viable particles present in the environment [100]. These Petri dishes are later scanned for detecting the label (QR code), collected, and incubated, and are subjected to qualitative and quantitative analysis. The data relating to the sampling is stored manually on a Laboratory Information Management System (LIMS). Hence, labelling, transport, correct placement, and collection of settle plates are of utmost importance. Considering humans are the primary source of contamination, another manual intervention with the picking up of the Petri dishes to scan them is far from an optimal method of recording information for environmental monitoring.
- In this use case, a mobile collaborative robot is utilised to collect the sampled Petri dishes and read a QR code attached to the Petri dish. The information is then updated in the LIMS for future reference. The information from the QR code can further be used to fetch data from a cloud database or to initiate a digital twin [77].
3. Experimental Methods
3.1. Configuring the Mobile Robotic Platform for the Experiments
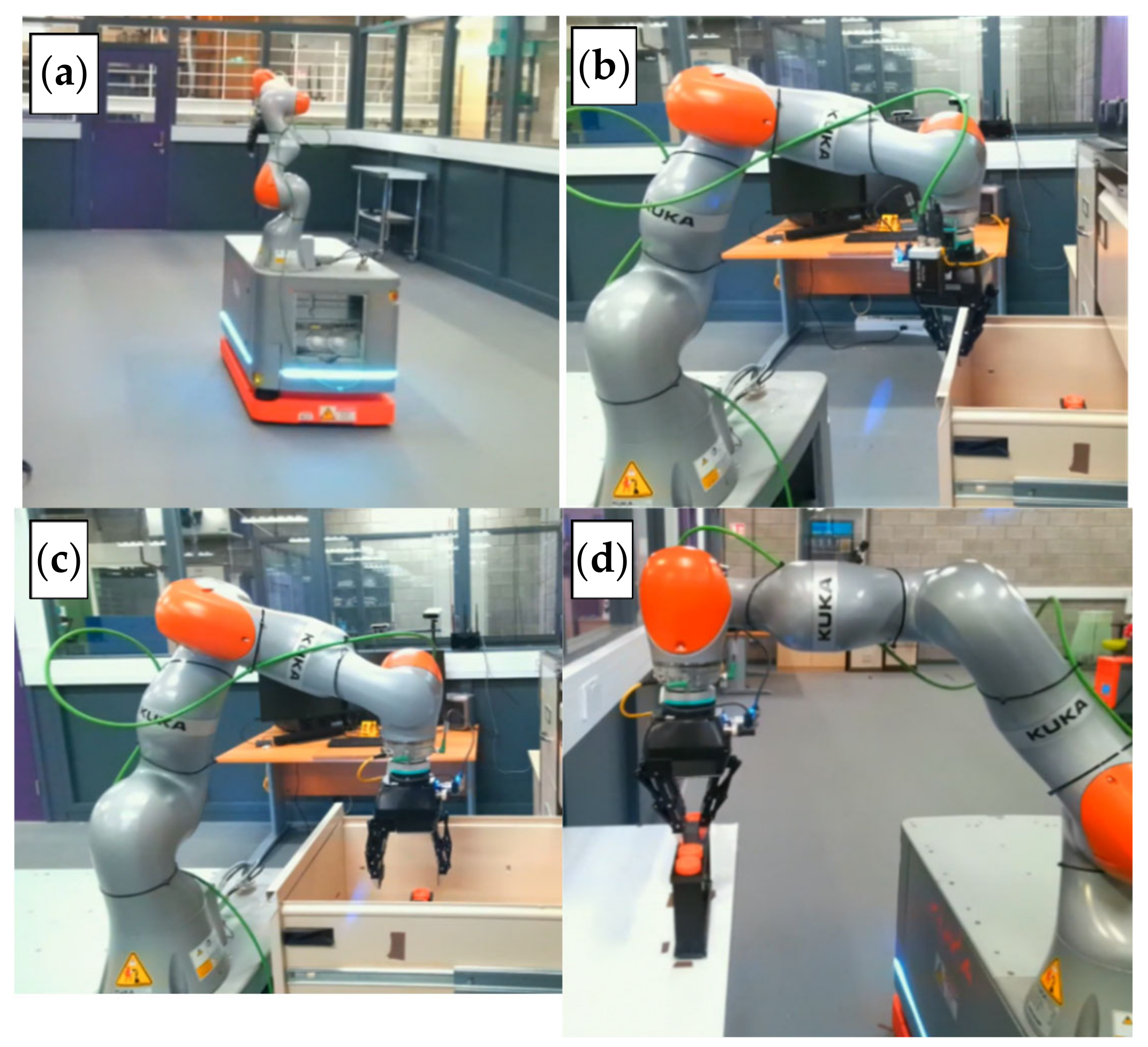
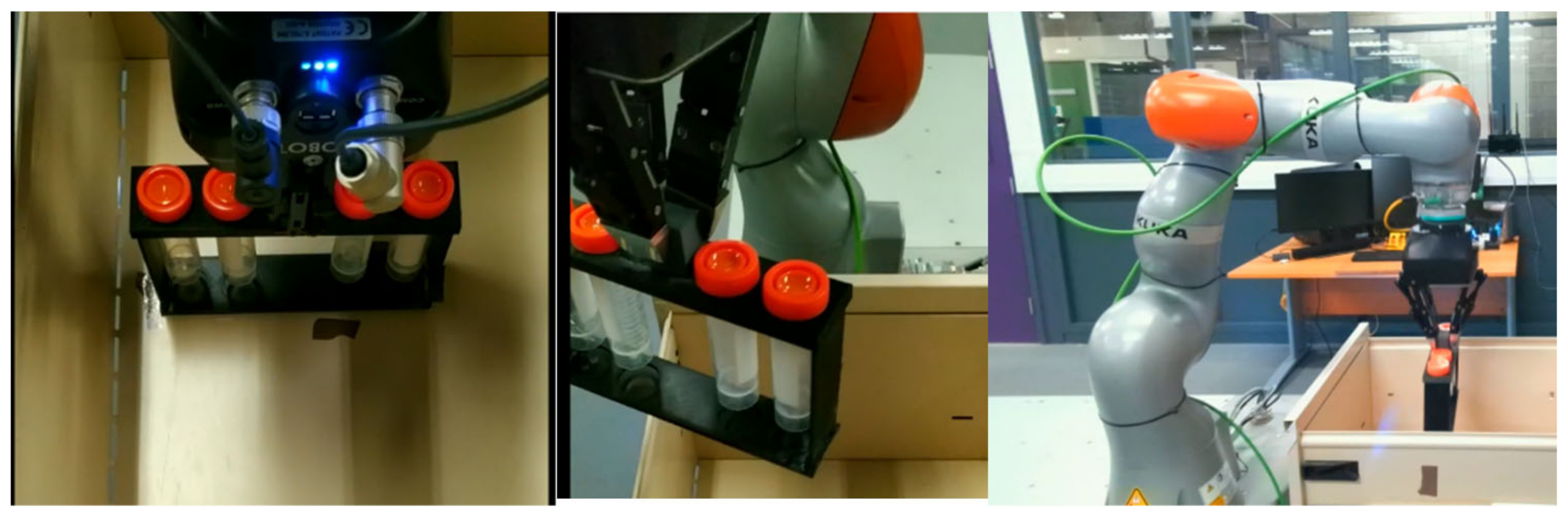
3.2. Experiment 1: Manipulation and Transportation of Centrifugal Tubes
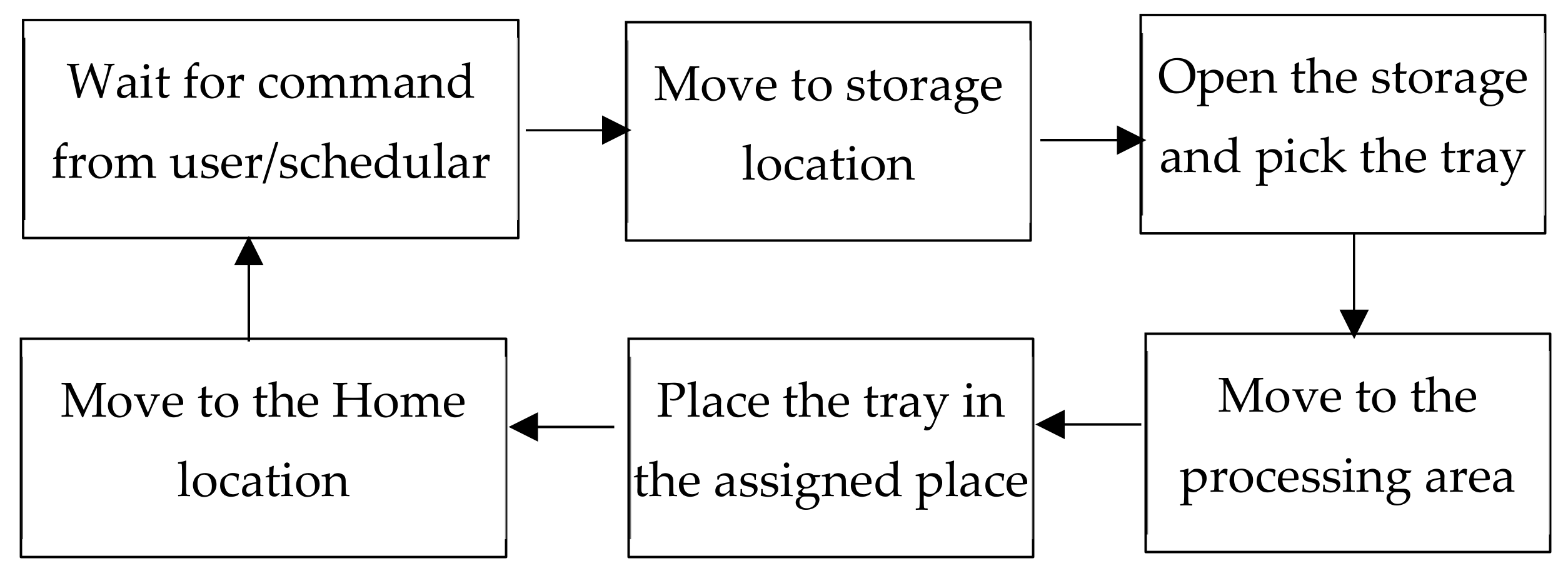
- The mobile robot waits at its home location for the task to be assigned.
- Once a task is assigned, the robot will move to the storage location. The robot uses the preprogrammed navigation algorithm and the map of the environment to plan the path, velocity etc. Here, the storage location is used to emulate the cryostorage area.
- The mobile robot reaches the storage location, the arm is moved towards the storage unit, and the handle of the storage is grasped with the help of the gripper. Then the arm is moved back towards the robot to open the storage. Once the storage is opened, the robot will pick and raise the tray containing the centrifugal tubes. The robot then moves the arm back to a suitable configuration, so that the mobile robot can move without the arm clashing with any other objects nearby.
- The mobile robot navigates to the material transfer area.
- Upon reaching the material transfer area, the arm is moved towards the location where the tray must be placed. After reaching this location, the gripper releases the tray and the arm is retracted.
- The robot navigates back to the home position, waiting for the next task.
3.3. Experiment 2: Manipulation of Deformable Fluid Bags
- The mobile robot waits at its home location.
- Once the task is assigned, the robot moves to the bag collection area.
- On reaching the bag collection area, the robot extends its arm toward the holder, which is kept towards the right side of the robot.
- The robot collects the bag and places it on top of the robotic platform in the assigned location.
- The robot moves back to the bag delivery location.
3.4. Experiment 3: Manipulation of Petri Dishes for Scanning the QR Code
- The robot waits at the home location for the task to be assigned.
- The robot moves to the sampling location on receiving the scanning task. Information about the sampling location, such as location ID, stand height etc. is communicated to the robot.
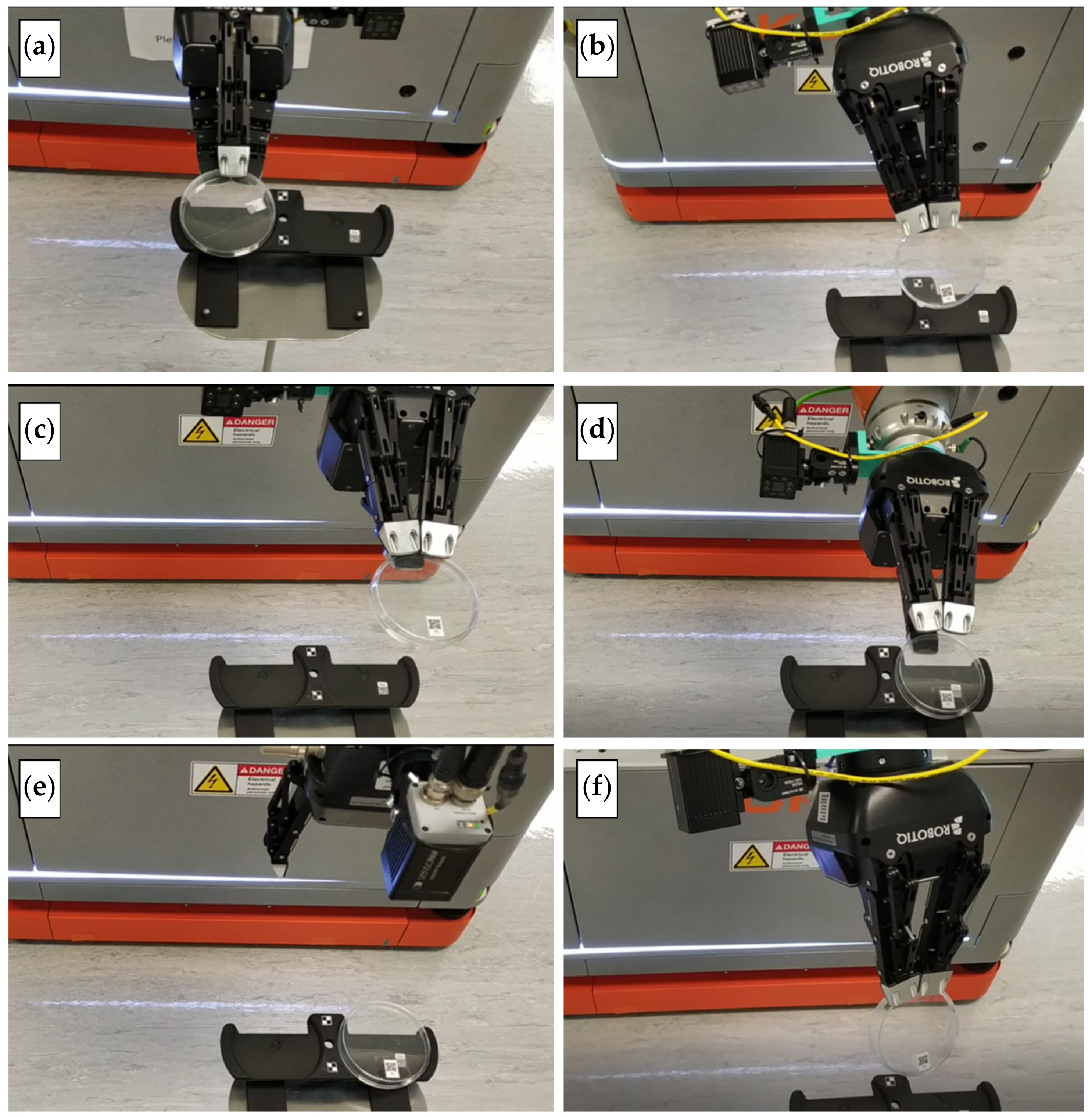
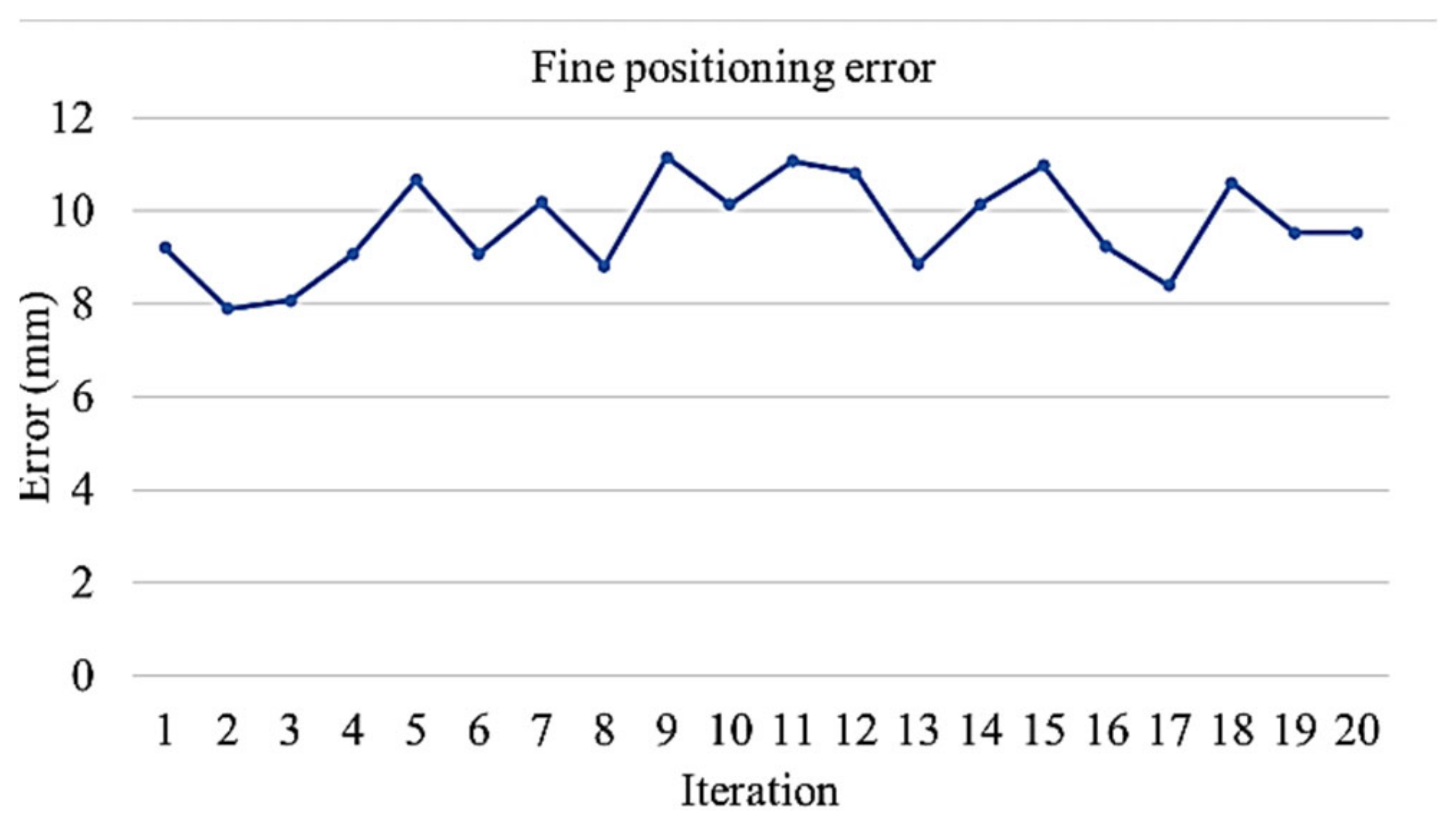
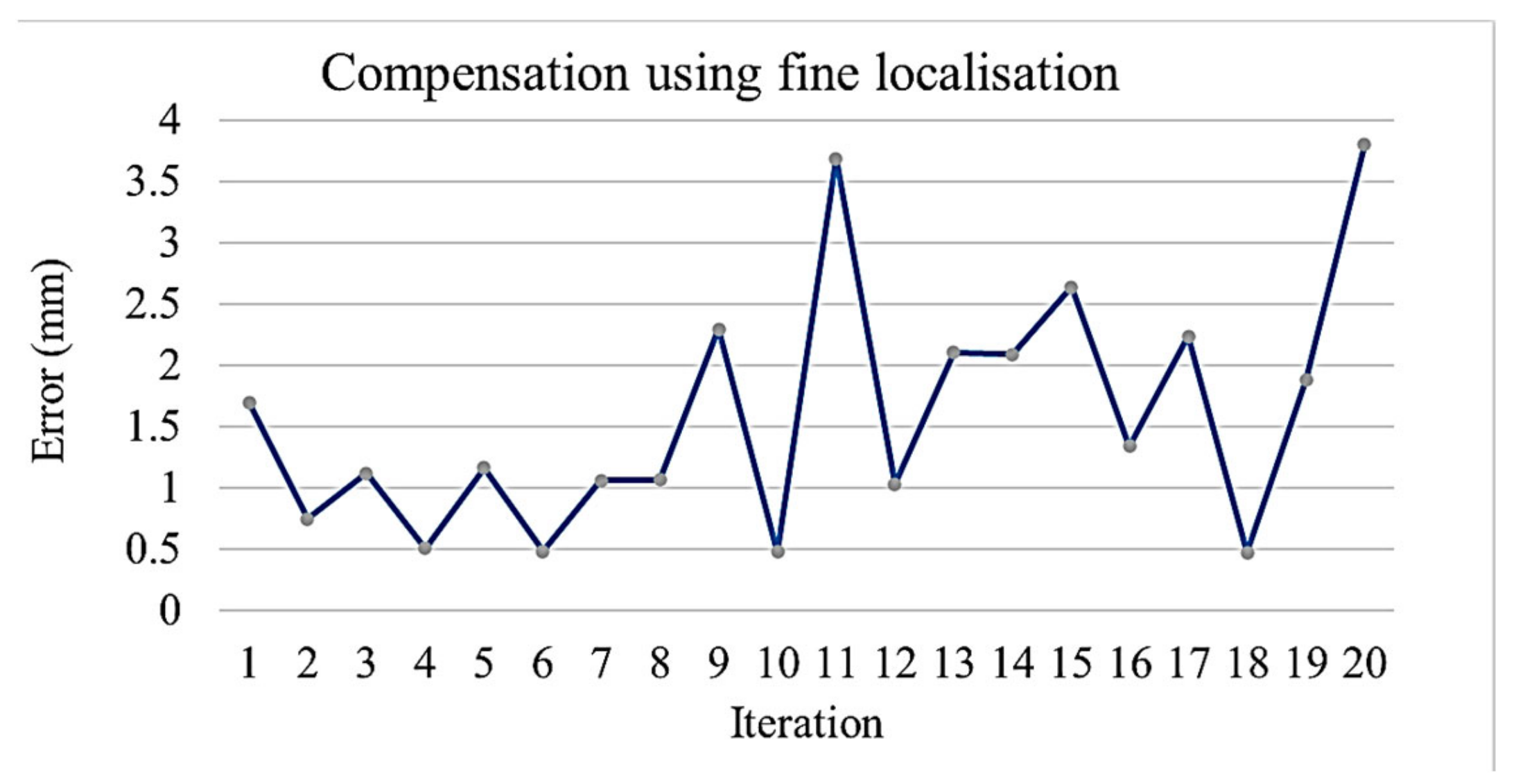
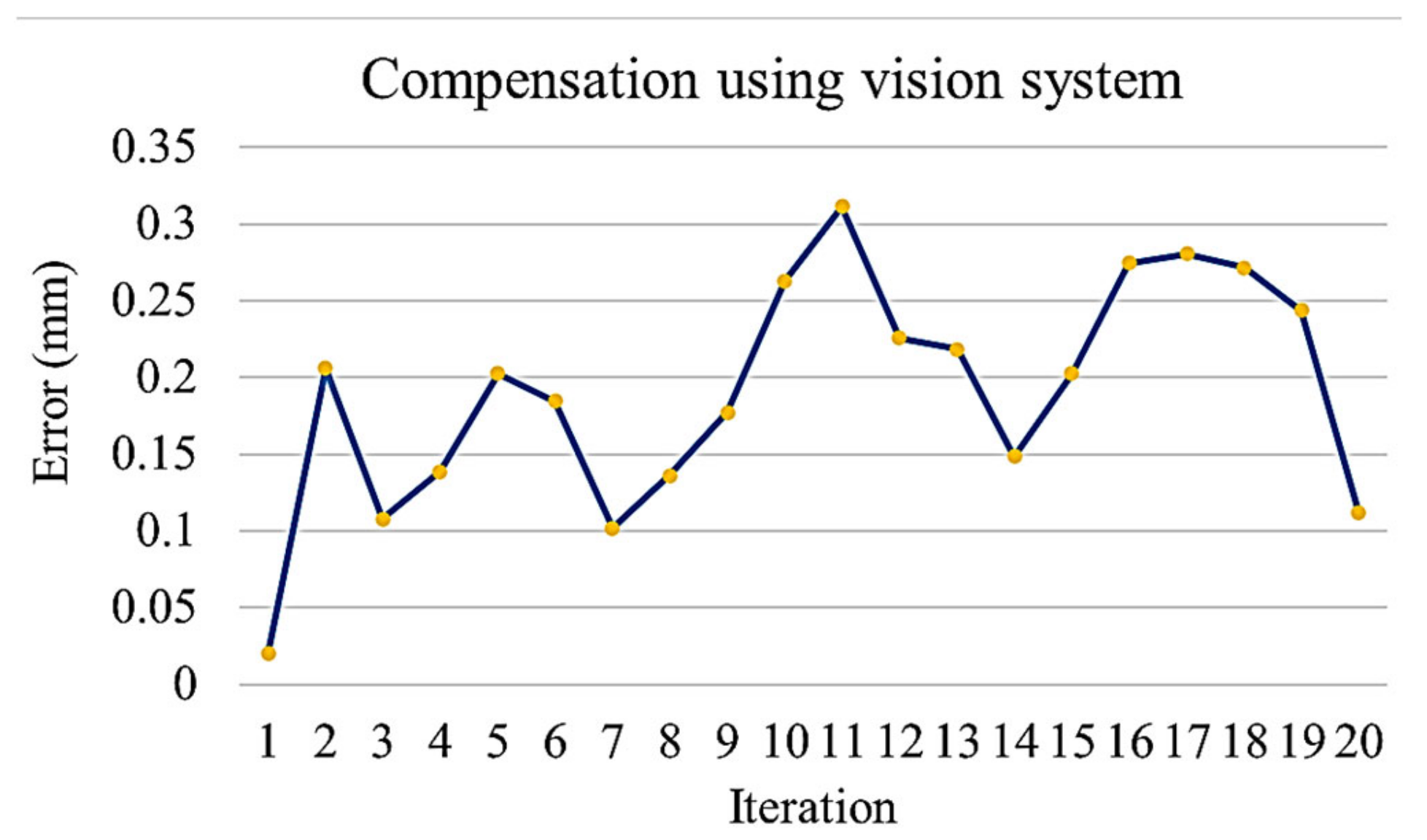
- On reaching the sampling location, the robot extends the arm toward the sampling stand on top of which the dish and the lid is kept. An image of the stand is taken using the vision system to calculate the positioning error of the mobile platform. The positioning error of the platform in turn causes an error while picking the Petri dish. The robotic arm compensates for the platform error by providing the required additional motion corresponding to the error calculated using the vision system. The Petri dish is presented in an open condition with lid placed nearby on an assigned area.
- The robot moves the arm to the pickup position with the gripper in open status. The robot closes the gripper to pick the lid up and place it on the dish.
- The robot picks the Petri dish from the sampling stand, flips it, and places it back on the stand for scanning. The Petri dish is flipped so that the QR code placed at the bottom is visible. The robotic arm is then moved toward the scanning pose. The QR code is scanned using the camera attached to the robotic arm.
- The robot picks up the Petri dish again and places it onto the carrying device kept on top of the robot. The information collected on scanning the dish is communicated to the LIMS system. The robot then waits for the next instructions.

4. Results and Discussion
4.1. Experiment 1: Manipulation and Transportation of Centrifugal Tubes
4.2. Experiment 2: Manipulation of Deformable Fluid Bags
4.3. Experiment 3: Manipulation of Petri Dishes for Scanning the QR Code
4.4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuster, S. COBOTS in Cleanrooms—The New Dimension of Automated Semiconductor Production; KUKA Deutschland GmbH: Augsburg, Germany, 2021. [Google Scholar]
- Sandle, T. People in Cleanrooms: Understanding and Monitoring the Personnel Factor. Available online: https://www.ivtnetwork.com/article/people-cleanrooms-understanding-and-monitoring-personnel-factor (accessed on 16 November 2021).
- Sandle, T. The people factor: Investigating the gown. Eur. Pharm. Rev. 2017, 22, 23–26. [Google Scholar]
- Hyde, W.A. Origin of bacteria in the clean room and their growth requirements. PDA J. Pharm. Sci. Technol. 1998, 52, 154–158. [Google Scholar]
- Beri, R.G.; Wolton, D.; Coulon, C.-H. Opportunities for Modern Robotics in Biologics Manufacturing. Bioprocess Int. 2019, 17, 8–15. [Google Scholar]
- Miller, S.; Zakharyevich, K.; Kavanaugh, D.; Lenich, R.; Salim, J.; Iles-Smith, P.; Schelble, C.; Sheehy, P.; Merkle, S.; Beri, R. Biomanufacturing Technology Roadmap: 4. Automated Facilities; BioPhorum Operations Group, Ltd.: London, UK, 2017; p. 34. [Google Scholar]
- Stanton, D. The 4 Degrees of Automation in Cell Therapy Manufacture. Available online: https://bioprocessintl.com/bioprocess-insider/therapeutic-class/the-4-degrees-of-automation-in-cell-therapy-manufacture/?utm_medium=email&_hsmi=206184161&_hsenc=p2ANqtz-_26kfRxjy7VRDuc6uWRAcpaCQVkUqNZSsHBjPTBTftQxSo5WhBqNvBgBqo587pLd7WvCek1B0L6Wcy3W9Tmhxm (accessed on 13 March 2022).
- Burger, B.; Maffettone, P.M.; Gusev, V.V.; Aitchison, C.M.; Bai, Y.; Wang, X.; Li, X.; Alston, B.M.; Li, B.; Clowes, R.; et al. A mobile robotic chemist. Nature 2020, 583, 237–241. [Google Scholar] [CrossRef]
- Hardner, M.; Schneider, D. Development of a multi camera calibration for an analysis and manipulation system of biological samples in petri dishes. ISPRS—Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, XLII-2/W18, 67–72. [Google Scholar] [CrossRef]
- Lange, O.; Erhard, M.; Teutsch, C.; Sander, J. MIROB: Automatic rapid identification of micro-organisms in high through-put. Ind. Robot Int. J. 2008, 35, 311–315. [Google Scholar] [CrossRef]
- Sabatini, L.; Paolucci, D.; Marinelli, F.; Pianetti, A.; Sbaffo, M.; Bufarini, C.; Sisti, M. Microbiological validation of a robot for the sterile compounding of injectable non-hazardous medications in a hospital environment. Eur. J. Hosp. Pharm. 2019, 27, 63–86. [Google Scholar] [CrossRef]
- Takalo-Kippola, H.; Lappalainen, P. Novel laboratory robot for the manufacturing of microbiological culture media. In Proceedings of the 16th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Baltimore, MD, USA, 3–6 November 1994; Volume 16, pp. 1055–1056. [Google Scholar] [CrossRef]
- U.S Food and Drug Administration. Personalized Medicine: A Biological Approach to Patient Treatment. Available online: https://www.fda.gov/drugs/news-events-human-drugs/personalized-medicine-biological-approach-patient-treatment (accessed on 3 June 2022).
- Kumar, K.; Kon, O.M. Personalised medicine for tuberculosis and non-tuberculous mycobacterial pulmonary disease. Microorganisms 2021, 9, 2220. [Google Scholar] [CrossRef]
- Pritchard, D.E.; Moeckel, F.; Villa, M.S.; Housman, L.T.; McCarty, C.A.; McLeod, H.L. Strategies for integrating personalized medicine into healthcare practice. Pers. Med. 2017, 14, 141–152. [Google Scholar] [CrossRef]
- Horgan, D. Access for All: A Personalised Approach. Public Health Genom. 2016, 19, 129–131. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef]
- Dos Santos, F.F.; Andrade, P.Z.; Da Silva, C.L.; Cabral, J.M.S. Bioreactor design for clinical-grade expansion of stem cells. Biotechnol. J. 2013, 8, 644–654. [Google Scholar] [CrossRef]
- Eaker, S.; Armant, M.; Brandwein, H.; Burger, S.; Campbell, A.; Carpenito, C.; Clarke, D.; Fong, T.; Karnieli, O.; Niss, K.; et al. Concise review: Guidance in developing commercializable autologous/patient-specific cell therapy manufacturing. Stem Cells Transl. Med. 2013, 2, 871–883. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Li, J.; Wang, B.; Bishop, R.; White, C.; Das, A.; Levine, A.D.; Ho, L.; Levine, B.L.; et al. A multiscale simulation framework for the manufacturing facility and supply chain of autologous cell therapies. Cytotherapy 2019, 21, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Lexchin, J. Affordable biologics for all. JAMA Netw. Open 2020, 3, e204753. [Google Scholar] [CrossRef]
- Marks, P.; Witten, C. Toward a new framework for the development of individualized therapies. Gene Ther. 2020, 28, 615–617. [Google Scholar] [CrossRef]
- Löfving, M.; Almström, P.; Jarebrant, C.; Wadman, B.; Widfeldt, M. Evaluation of flexible automation for small batch production. Procedia Manuf. 2018, 25, 177–184. [Google Scholar] [CrossRef]
- Hamburg, M.A.; Collins, F.S. The path to personalized medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef]
- Miossec, P.; Verweij, C.L.; Klareskog, L.; Pitzalis, C.; Barton, A.; Lekkerkerker, F.; Reiter, S.; Laslop, A.; Breedveld, F.; Abadie, E.; et al. Biomarkers and personalised medicine in rheumatoid arthritis: A proposal for interactions between academia, industry and regulatory bodies. Ann. Rheum. Dis. 2011, 70, 1713–1718. [Google Scholar] [CrossRef]
- Meybodi, H.R.A.; Hasanzad, M.; Larijani, B. Path to personalized medicine for type 2 diabetes mellitus: Reality and hope. Acta Med. Iran. 2017, 55, 166–174. [Google Scholar]
- Mishra, V.; Chanda, P.; Tambuwala, M.M.; Suttee, A. Personalized medicine: An overview. Int. J. Pharm. Qual. Assur. 2019, 10, 290–294. [Google Scholar] [CrossRef]
- Malik, N. Allogeneic versus Autologous Stem-Cell Therapy. BioPharm Int. 2012, 25, 18–22. [Google Scholar]
- Precision Medicines: Adapting Manufacturing for Small Batch. Available online: https://www.theaccessgroup.com/en-gb/manufacturing/resources/pharma-insights/precision-medicines-adapting-manufacturing-for-the-super-small-batch/ (accessed on 3 June 2022).
- Vormittag, P.; Gunn, R.; Ghorashian, S.; Veraitch, F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018, 53, 164–181. [Google Scholar] [CrossRef]
- Bartel, R.L. Stem Cells and Cell Therapy: Autologous Cell Manufacturing. In Translational Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2015; pp. 107–112. [Google Scholar] [CrossRef]
- Abraham, E.; Ahmadian, B.B.; Holderness, K.; Levinson, Y.; McAfee, E. Platforms for manufacturing allogeneic, autologous and iPSC cell therapy products: An industry perspective. Adv. Biochem. Eng. Biotechnol. 2018, 165, 323–350. [Google Scholar]
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial scale manufacturing of allogeneic cell therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Schubert, H. The Challenges of Personalized Drugs—And Some Possible Solutions. Tech Talk. Available online: https://www.nne.com/techtalk/how-personalized-drugs-are-changing-pharma-manufacturing/ (accessed on 3 March 2022).
- Veronesi, E.; Murgia, A.; Caselli, A.; Grisendi, G.; Piccinno, M.S.; Rasini, V.; Giordano, R.; Montemurro, T.; Bourin, P.; Sensebé, L.; et al. Transportation conditions for prompt use of ex vivo expanded and freshly harvested clinical-grade bone marrow mesenchymal stromal/stem cells for bone regeneration. Tissue Eng.—Part C Methods 2014, 20, 239–251. [Google Scholar] [CrossRef]
- Hanna, J.; Hubel, A. Preservation of stem cells. Organogenesis 2009, 5, 134–137. [Google Scholar] [CrossRef]
- Celikkan, F.T.; Mungan, C.; Sucu, M.; Ulus, A.T.; Cinar, O.; Ili, E.G.; Can, A.L.P. Optimizing the transport and storage conditions of current Good Manufacturing Practice –grade human umbilical cord mesenchymal stromal cells for transplantation (HUC-HEART Trial). Cytotherapy 2019, 21, 64–75. [Google Scholar] [CrossRef]
- Aghayan, H.R.; Arjmand, B.; Ahmadbeigi, N.; Gheisaii, Y.; Vasei, M. Draft of Iranian national guideline for cell therapy manufacturing. Arch. Iran. Med. 2017, 20, 547–550. [Google Scholar]
- Rotondi, M.; Grace, N.; Betts, J.; Bargh, N.; Costariol, E.; Zoro, B.; Hewitt, C.J.; Nienow, A.W.; Rafiq, Q.A. Design and development of a new ambr250Ò bioreactor vessel for improved cell and gene therapy applications. Biotechnol. Lett. 2021, 43, 1103–1116. [Google Scholar] [CrossRef]
- Cabrita, G.J.M.; Ferreira, B.S.; Lobato Da Silva, C.; Gonçalves, R.; Almeida-Porada, G.; Cabral, J.M.S. Hematopoietic stem cells: From the bone to the bioreactor. Trends Biotechnol. 2003, 21, 233–240. [Google Scholar] [CrossRef]
- Ling, Y.; Ae, N.; Chase, H.A. Novel bioreactors for the culture and expansion of aggregative neural stem cells. Bioprocess Biosyst. Eng. 2008, 31, 393–400. [Google Scholar] [CrossRef]
- Sardonini, C.A.; Wu, Y.-J. Expansion and Differentiation of Human Hematopoietic Cells from Static Cultures through Small-Scale Bioreactors. Biotechnol. Prog. 1993, 9, 131–137. [Google Scholar] [CrossRef]
- Palsson, B.O.; Paek, S.-H.; Schwartz, R.M.; Palsson, M.; Lee, G.-M.; Silver, S.; Emerson, S.G. Expansion of human bone marrow progenitor cells in a high cell density continuous perfusion system. BioTechnology 1993, 11, 368–372. [Google Scholar] [CrossRef]
- Meissner, P.; Schröder, B.; Herfurth, C.; Biselli, M. Development of a fixed bed bioreactor for the expansion of human hematopoietic progenitor cells. Cytotechnology 1999, 30, 227–234. [Google Scholar] [CrossRef]
- Yao, C.L.; Liu, C.H.; Chu, I.M.; Hsieh, T.B.; Hwang, S.M. Factorial designs combined with the steepest ascent method to optimize serum-free media for ex vivo expansion of human hematopoietic progenitor cells. Enzyme Microb. Technol. 2003, 33, 343–352. [Google Scholar] [CrossRef]
- Weber, C.; Pohl, S.; Poertner, R.; Wallrapp, C.; Geigle, P.; Czermak, P. Development of a production process for stem cell based cell therapeutic implants using disposable bioreactor systems. IFMBE Proc. 2008, 22, 2277–2280. [Google Scholar]
- Nienow, A.W.; Coopman, K.; Heathman, T.R.J.; Rafiq, Q.A.; Hewitt, C.J. Bioreactor Engineering Fundamentals for Stem Cell Manufacturing; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780444632654. [Google Scholar]
- Nogueira, D.E.S.; Rodrigues, C.A.V.; Carvalho, M.S.; Miranda, C.C.; Hashimura, Y.; Jung, S.; Lee, B.; Cabral, J.M.S. Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel™ bioreactors. J. Biol. Eng. 2019, 13, 74. [Google Scholar] [CrossRef]
- ISO 14644-1:1999(en); Cleanrooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness. International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-1:ed-1:v1:en (accessed on 16 November 2021).
- Niazi, S.K. Handbook of Pharmaceutical Manufacturing Formulations, 3rd ed.; Compressed Solid Products; CRC Press: Boca Raton, FL, USA, 2019; Volume 1, ISBN 9781420081176. [Google Scholar]
- Tim Sandle Best Practices In Environmental Monitoring [White Paper]. Reading Scientific Services Ltd. 2020. Available online: https://www.researchgate.net/publication/342883006_Best_practices_in_environmental_monitoring (accessed on 7 July 2022).
- Kuehn, S. Ascent of the Robots—Advancing Aseptic Proccessing through the Use of Robot Technology. Available online: https://www.pharmamanufacturing.com/articles/2015/ascent-of-the-robots/ (accessed on 9 March 2022).
- Kokubo, M.; Akers, J.E. Aseptic Manufacturing of Regenerative Medicine Products Using Isolator Technology; Springer: Tokyo, Japan, 2016; ISBN 9784431556664. [Google Scholar]
- Bruscolini, F.; Paolucci, D.; Rosini, V.; Sabatini, L.; Andreozzi, E.; Pianetti, A. Evaluation of ultraviolet irradiation efficacy in an automated system for the aseptic compounding using challenge test. Int. J. Qual. Health Care 2015, 27, 412–417. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, H.; Zhao, C.; Che, A.; Wang, Y. Key technologies and progress of pharmaceutical intelligent manufacturing production line. J. Cent. South Univ. Sci. Technol. 2021, 52, 421–433. [Google Scholar] [CrossRef]
- Krämer, I.; Federici, M.; Kaiser, V.; Thiesen, J. Media-fill simulation tests in manual and robotic aseptic preparation of injection solutions in syringes. J. Oncol. Pharm. Pract. 2016, 22, 195–204. [Google Scholar] [CrossRef]
- Hafner, J. Spool production goes aseptic. Cleanroom Technol. 2008, 15, 13–15. [Google Scholar]
- Geersing, T.H.; Franssen, E.J.F.; Pilesi, F.; Crul, M. Microbiological performance of a robotic system for aseptic compounding of cytostatic drugs. Eur. J. Pharm. Sci. 2019, 130, 181–185. [Google Scholar] [CrossRef]
- Keller, M.; Baum, G.; Schweizer, M.; Bürger, F.; Gommel, U.; Bauernhansl, T. Optimized Robot Systems for Future Aseptic Personalized Mass Production. Procedia CIRP 2018, 72, 303–309. [Google Scholar] [CrossRef]
- Alip, S.L.; Kim, J.; Rha, K.H.; Han, W.K. Future Platforms of Robotic Surgery. Urol. Clin. N. Am. 2022, 49, 23–38. [Google Scholar] [CrossRef]
- Khandalavala, K.; Shimon, T.; Flores, L.; Armijo, P.R.; Oleynikov, D. Emerging surgical robotic technology: A progression toward microbots. Ann. Laparosc. Endosc. Surg. 2020, 5, 3. [Google Scholar] [CrossRef]
- Nota, C.L.M.A.; Smits, F.J.; Woo, Y.; Borel Rinkes, I.H.M.; Molenaar, I.Q.; Hagendoorn, J.; Fong, Y. Robotic Developments in Cancer Surgery. Surg. Oncol. Clin. N. Am. 2019, 28, 89–100. [Google Scholar] [CrossRef]
- Kim, Y.J. The future medical science and colorectal surgeons. Ann. Coloproctol. 2017, 33, 207–209. [Google Scholar] [CrossRef][Green Version]
- Jeon, H.; Yang, K.-M.; Park, S.; Choi, J.; Lim, Y. An Ontology-based Home Care Service Robot for Persons with Dementia. In Proceedings of the RO-MAN 2018—27th IEEE International Symposium on Robot and Human Interactive Communication, Nanjing, China, 27–31 August 2018; pp. 540–545. [Google Scholar]
- Salemi, B.; Reis, J.; Saifhashemi, A.; Nikgohar, F. MILO: Personal robot platform. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, IROS, Edmonton, AB, Canada, 2–6 August 2005; pp. 4089–4094. [Google Scholar]
- Yang, G.; Wang, S. Modeling and Control of a Personal Care Robot Considering Posture Adjustment. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation, ICMA 2019, Tianjin, China, 4–7 August 2019; pp. 1414–1418. [Google Scholar]
- Kataria, S.; Ravindran, V. Digital health: A new dimension in rheumatology patient care. Rheumatol. Int. 2018, 38, 1949–1957. [Google Scholar] [CrossRef]
- Baimas-George, M.; Demartines, N.; Vrochides, D. The role of disruptive technologies and approaches in ERAS®: Erupting change through disruptive means. Langenbecks Arch. Surg. 2022, 407, 437–441. [Google Scholar] [CrossRef]
- Palm, E.; Nordgren, A.; Verweij, M.; Collste, G. Ethically sound technology? Guidelines for interactive ethical assessment of personal health monitoring. Stud. Health Technol. Inform. 2013, 187, 105–114. [Google Scholar] [CrossRef]
- Virk, G.S. Personal care robot safety. In Proceedings of the Emerging Trends in Mobile Robotics, 13th International Conference on Climbing and Walking Robots and the Support Technologies for Mobile Machines, CLAWAR 2010, Singapore, 31 August–3 September 2010; pp. 1332–1339. [Google Scholar]
- Villaronga, E.F.; Virk, G.S. Legal Issues for Mobile Servant Robots; Springer: Cham, Switzerland, 2017; Volume 540, ISBN 9783319490571. [Google Scholar]
- Kochan, A. Cleanroom robots become cleaner and more reliable. Ind. Robot 1998, 25, 27–29. [Google Scholar] [CrossRef]
- Kuehn, T.H.; Wu, Y.; Liu, B.Y.H. Particle contamination below a robot arm in a cleanroom. J. IES 1993, 36, 43–50. [Google Scholar] [CrossRef]
- Mathia, K. Robot design for wafer handling. Cleanroom Technol. 2010, 18, 22–23. [Google Scholar]
- Ma, R.-H. Study on simulation of airflow within the enclosure in the semiconductor factory cleanroom. Aerosol Sci. Technol. 2006, 40, 282–292. [Google Scholar] [CrossRef][Green Version]
- Wolton, D. An Autonomous Sampling System. U.S. Patent 16/333,765, 11 July 2019. [Google Scholar]
- Wolf, Á.; Wolton, D.; Trapl, J.; Janda, J.; Romeder-Finger, S.; Gatternig, T.; Farcet, J.B.; Galambos, P.; Széll, K. Towards robotic laboratory automation Plug & Play: The “LAPP” framework. SLAS Technol. 2022, 27, 18–25. [Google Scholar] [CrossRef]
- Bejczy, B.; Bozyil, R.; Vaiekauskas, E.; Petersen, S.B.K.; Bogh, S.; Hjorth, S.S.; Hansen, E.B. Mixed reality interface for improving mobile manipulator teleoperation in contamination critical applications. Procedia Manuf. 2020, 51, 620–626. [Google Scholar] [CrossRef]
- EI Marvin—Robotic Automation in the Biotechnology Sector—UCD Laboratory for Advanced Manufacturing Simulation and Robotics. Available online: https://www.ucd.ie/lams/research/projects/marvin/ (accessed on 17 July 2022).
- Froelich, K.; Pueschel, R.C.; Birner, M.; Kindermann, J.; Hackenberg, S.; Kleinsasser, N.H.; Hagen, R.; Staudenmaier, R. Optimization of fibrinogen isolation for manufacturing autologous fibrin glue for use as scaffold in tissue engineering. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 143–149. [Google Scholar] [CrossRef]
- Cell Separation and Cell Isolation: Techniques, Methods, Applications. Available online: https://www.stemcell.com/cell-separation (accessed on 7 March 2022).
- Himac Practical Applications of Biological Centrifugation. Available online: https://www.himac-science.com/casebook/bio/bio-casebook.pdf (accessed on 7 March 2022).
- Schmolz, M.; Stein, G.M.; Hubner, W.-D. An innovative, centrifugation-free method to prepare human platelet mediator concentrates showing activities comparable to platelet-rich plasma. Wounds 2011, 23, 171–182. [Google Scholar]
- Levinson, Y.; Beri, R.G.; Holderness, K.; Ben-Nun, I.F.; Shi, Y.; Abraham, E. Bespoke cell therapy manufacturing platforms. Biochem. Eng. J. 2018, 132, 262–269. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.J.; Fang, L.; Zhao, T.B. Co-culture of annulus fbrosus cells and bone marrow mesenchymal stem cells. Genet. Mol. Res. 2015, 14, 3932–3938. [Google Scholar] [CrossRef]
- Applikon miniBio—Small Size Multi-Use Glass Autoclavable Bioreactor. Available online: https://www.getinge.com/int/products/applikon-minibio/ (accessed on 8 June 2022).
- Global Market Insights Inc. Medical Fluid Bags Market. Available online: https://www.gminsights.com/industry-analysis/medical-fluid-bags-market (accessed on 10 March 2022).
- LyondellBasell. Infusion Bags. Available online: https://www.lyondellbasell.com/en/products-technology/polymers/application/infusion-bags/ (accessed on 10 March 2022).
- Hermosilla, J.; Pérez-Robles, R.; Salmerón-García, A.; Casares, S.; Cabeza, J.; Navas, N. Stability study over time of clinical solutions of ziv-aflibercept prepared in infusion bags using a proper combination of physicochemical and functional strategies. J. Pharm. Biomed. Anal. 2021, 203, 114209. [Google Scholar] [CrossRef]
- Illum, L.; Bundgaard, H. Sorption of drugs by plastic infusion bags. Int. J. Pharm. 1982, 10, 339–351. [Google Scholar] [CrossRef]
- Leuenberger, N.; Barras, L.; Nicoli, R.; Robinson, N.; Baume, N.; Lion, N.; Barelli, S.; Tissot, J.D.; Saugy, M. Urinary di-(2-ethylhexyl) phthalate metabolites for detecting transfusion of autologous blood stored in plasticizer-free bags. Transfusion 2016, 56, 571–578. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Costantini, A.; Mancini, G.; Bencivenga, R.; Olivieri, J.; Battaglini, G.; Velletri, L.; Viola, N.; Butini, L.; Capelli, D.; et al. A novel method to evaluate prethawing viability of cryopreserved CD34+ hematopoietic stem cells for autologous transplantation. Transfusion 2020, 60, 1529–1535. [Google Scholar] [CrossRef]
- Woods, E.J.; Thirumala, S. Packaging considerations for biopreservation. Transfus. Med. Hemother. 2011, 38, 149–156. [Google Scholar] [CrossRef]
- Mikola, M.; Seto, J.; Amanullah, A. Evaluation of a novel Wave Bioreactor® cellbag for aerobic yeast cultivation. Bioprocess Biosyst. Eng. 2007, 30, 231–241. [Google Scholar] [CrossRef]
- Kadwell, S.H.; Overton, L.K. Protein expression in insect and mammalian cells using baculoviruses in wave bioreactors. In Baculovirus and Insect Cell Expression Protocols; Humana Press: New York, NY, USA, 2016; Volume 1350. [Google Scholar]
- Da Silva, J.S.; Mizukami, A.; Gil, L.V.G.; de Campos, J.V.; Assis, O.B.; Tadeu Covas, D.; Swiech, K.; Torres Suazo, C.A. Improving wave-induced motion bioreactor performance for human mesenchymal stromal cell expansion. Process Biochem. 2019, 84, 143–152. [Google Scholar] [CrossRef]
- Tsai, A.-C.; Pacak, C.A. Bioprocessing of human mesenchymal stem cells: From planar culture to microcarrier-based bioreactors. Bioengineering 2021, 8, 96. [Google Scholar] [CrossRef]
- Whyte, W. In support of settle plates. PDA J. Pharm. Sci. Technol. 1996, 50, 201–204. [Google Scholar]
- Schenk, J.; Herms, O. Examination of the exposition time of settle plates for monitoring sterile production areas | Untersuchungen zur verwendungsdauer von sedimentationsplatten bei der überwachung steriler fertigungsbereiche. Pharm. Ind. 2005, 67, 603–606. [Google Scholar]
- Andon, B.M. Active air vs. passive air (settle plate) monitoring in routine environmental monitoring programs. PDA J. Pharm. Sci. Technol. 2006, 60, 350–355. [Google Scholar]
- Bowne, M. What Is PROFINET?—PROFINET Explained. Available online: https://us.profinet.com/profinet-explained/ (accessed on 23 July 2022).
- KUKA AG. Automation in Semiconductor Manufacturing. Available online: https://www.kuka.com/en-de/industries/electronics-industry/automation-in-semiconductor-fabrication (accessed on 24 April 2022).
- Ramasubramanian, A.K.; Mathew, R.; Kelly, M.; Hargaden, V.; Papakostas, N. Digital Twin for Human–Robot Collaboration in Manufacturing: Review and Outlook. Appl. Sci. 2022, 12, 4811. [Google Scholar] [CrossRef]
- Sinclair, A. How Geography Affects the Cost of Biomanufacturing. Bioprocess Int. 2010, 8, S16–S19. [Google Scholar]
- NIBRT—National Institute for Bioprocessing Research and Training. Available online: https://www.nibrt.ie/ (accessed on 17 July 2022).




| Keyword Used | Web of Science | Scopus |
|---|---|---|
| Personalised medicine | 77,047 | 69,971 |
| Personalised Medicine and Mobile Robot | 17 | 12 |
| Mobile robot and pharmaceutical | 17 | 26 |
| Aseptic production and Robot | 6 | 12 |
| Cleanroom and Mobile robot | 3 | 4 |
| Biomanufacturing and Mobile Robot | 0 | 0 |
| Manufacturing and Mobile robot | 2631 | 1756 |
| Application Area | Related Work |
|---|---|
| Mobile robots for aseptic production/pharmaceutical facility | [5,76,77] |
| Robotic manipulator for biomanufacturing | [52,53,54,55,56] |
| Robotic surgery | [60,61,62,63] |
| Application of robots in cleanrooms | [72,73,74,75] |
| Application of teleoperated robot in contamination critical environment | [78] |
| Application of robots in personal health care | [64,65,66,67,68,69,70,71] |
| Item | Model | Vendor Details |
|---|---|---|
| Common to all experiments | ||
| Mobile robotic platform | KMR 200 | KUKA Deutschland GmbH, Augsburg, Germany |
| Robotic arm | LBR iiwa 14 R820 | KUKA Roboter GmbH, Augsburg, Germany |
| Robotic gripper | Robotiq 3-Finger Adaptive Robot Gripper | Robotiq inc., St-Nicolas, Canada |
| Vision system | Visor V20C-RO-P3-W-W-M2-L | SensoPart Industriesensorik GmbH, Gottenheim, Germany |
| Programming software | Sunrise.Workbench—1.16.1.9 | KUKA Deutschland GmbH, Augsburg, Germany |
| Gripper configuration | KUKA.WorkVisual V5.0.5_Build0600 | KUKA Roboter GmbH, Augsburg, Germany |
| Designing software | Autodesk Inventor 2021 | Autodesk, California, United States |
| 3D printer | Prusa i3 MK3S | Prusa Research a.s., Prague, Czech Republic |
| PLA filament | RS PRO 1.75 mm Black PLA 3D Printer Filament | Radionics Ltd., Dublin, Ireland |
| Experiment 1 specific equipment | ||
| Centrifugal tube | Corning 50 mL clear polypropylene (PP) self-standing centrifuge tubes- 430921 | Corning, New York, United States |
| Experiment 2 specific equipment | ||
| Bioprocessing container bag | 500 mL Labtainer BPC, 2D system | Thermo Scientific, Massachusetts, United States |
| Experiment 3 specific equipment | ||
| Petri dish | Irradiated TSA media 3P environmental monitoring media | bioMérieux Inc., Durham, North Carolina, United States |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, R.; McGee, R.; Roche, K.; Warreth, S.; Papakostas, N. Introducing Mobile Collaborative Robots into Bioprocessing Environments: Personalised Drug Manufacturing and Environmental Monitoring. Appl. Sci. 2022, 12, 10895. https://doi.org/10.3390/app122110895
Mathew R, McGee R, Roche K, Warreth S, Papakostas N. Introducing Mobile Collaborative Robots into Bioprocessing Environments: Personalised Drug Manufacturing and Environmental Monitoring. Applied Sciences. 2022; 12(21):10895. https://doi.org/10.3390/app122110895
Chicago/Turabian StyleMathew, Robins, Robert McGee, Kevin Roche, Shada Warreth, and Nikolaos Papakostas. 2022. "Introducing Mobile Collaborative Robots into Bioprocessing Environments: Personalised Drug Manufacturing and Environmental Monitoring" Applied Sciences 12, no. 21: 10895. https://doi.org/10.3390/app122110895
APA StyleMathew, R., McGee, R., Roche, K., Warreth, S., & Papakostas, N. (2022). Introducing Mobile Collaborative Robots into Bioprocessing Environments: Personalised Drug Manufacturing and Environmental Monitoring. Applied Sciences, 12(21), 10895. https://doi.org/10.3390/app122110895






