1. Introduction
The prevalence of heart failure is approximately 1–2% of the adult population in developed countries, and ≥10% of patients are >70 years of age. The lifetime risk of heart failure at the age of 55 is 33% for men and 28% for women [
1]. HFmrEF is a separate entity as of 2016. The prevalence of HFmrEF in patients with heart failure is 10–25%. HFmrEF can be described as an intermediate clinical entity between HFrEF and HFpEF, more similar to HFrEF, including a high prevalence of coronary artery disease, a lower prevalence of CKD and a lower representation of women in this population. With HFpEF, there are more similarities in terms of higher prevalence of hypertension, higher BMI and lower natriuretic peptides. Some studies have suggested that medications designed for patients with HFrEF could be used effectively in patients with HFmrEF. Furthermore, HFmrEF patients were borderline in the representation of atrial fibrillation or diabetes mellitus, which were more frequent in HFpEF. HFmrEF patients had a lower risk of cardiovascular events but a similar risk of non-cardiovascular events, in contrast to HFpEF patients.
HFmrEF is a heterogeneous group based on the instantaneous measurement of EF in its dynamic evolution. Some patients may experience a decrease due to e.g., myocardial infarction and conversely an increase in LV EF after the introduction of medical, device or reperfusion therapy. For example, in SwedeHF during follow-up of patients with HFmrEF, only 38% remained in this group. Of HfmrEF patients, 37% moved to the HFrEF group and 25% improved EF and moved to the HFpEF category during follow-up.
In patients with HFmrEF, phenotyping or aetiology of heart failure, including new imaging techniques, is important to determine the correct treatment. Pharmacological treatment of HFmrEF has only weak recommendations, based on post hoc analyses and subgroup analyses of heart failure studies already performed that included patients with HFmrEF. Ideally, studies in a special HFmrEF population should be generated, but these are unlikely to be sponsored by industry but will be publicly funded.
It gave us grounds to conduct the current analysis aiming to compare the pharmacotherapy, comorbidities and outcome of patients with HFrEF and HFmrEF.
2. Objectives
The aim of this study was to determine whether patients with HFrEF and HFmrEF differ in clinical manifestations, laboratory findings, epidemiology, comorbidities and pharmacotherapy. This comparison is important given the fact that the group of HFmrEF patients is considered as a kind of “gray zone” and until 2016, according to the ESC Guidelines, these patients were classified mainly as a group with a reduced ejection fraction. Another goal was to compare the 2-year prognosis of patients with HFrEF and HFmrEF. Orthotopic heart transplantation (OHT), left heart support implantation (LVAD) or death from any cause was determined as the primary combined endpoint.
3. Methods
3.1. Study Design
Patients from 3 cardio centers in the Czech Republic (Ist Department of Internal Cardioangiology, St. Anne’s University Hospital in Brno, Faculty of Medicine, Masaryk University; Department of Internal Cardiology, Brno University Hospital, Faculty of Medicine, Masaryk University; Department of Cardiology, Na Homolce Hospital, Prague) were included in the prospective multicentric FARmacology and NeuroHumoraL activation (FAR NHL) registry. These were outpatients and patients hospitalized for ICD implantation or for right-sided catheterization. Data collection took place in 2014 and 2015 and all patients signed informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Brno University Hospital, Czech Republic (protocol code 02-221014/EK, date of approval 22 October 2014).
3.2. Inclusion Criteria
Patients with stable chronic heart failure were included in the FAR NHL registry. We consider stable conditions to be when the symptoms and signs of the disease are unchanged and stable pharmacotherapy for at least a month. Input left ventricular ejection fraction (LVEF) below 50% was divided into HFrEF with EF below 40% and HFmrEF with EF 40–49%. According to the 2016 ESC Guidelines, patients with heart failure are divided into 3 groups according to left ventricular (LV) ejection fraction (EF): reduced EF (HFrEF), mid-range EF (HFmrEF) and preserved EF (HFpEF). The criteria for inclusion in the HFrEF group are EF less than 40%, between 40% and 49% for the HFmrEF group, and 50% or more for patients with HFpEF. In 2021, new ESC Guidelines were published, which adjust the heart failure ejection fraction ranges in different groups. One change concerns an ejection fraction of 40%, which was included in the heart failure with mid-range ejection fraction (HFmrEF) group under the 2016 ESC Guidelines, but is now included in the HFrEF group under the latest 2021 ESC Guidelines or 2022 AHA/ACC/HFSA Guidelines [
1,
2,
3]. In addition, the HFmrEF group has been renamed “mildly reduced” ejection fraction, but the abbreviation has been retained (HFmrEF). However, because most recent studies since 2016 describing the distribution of heart failure according to ejection fraction adhere to the older distribution and to make our study more comparable to this majority of studies, the authors decided to keep the group of patients with LVEF 40% in the HFmrEF group. The patients were assessed for clinical status, comorbidities, ion values, urea, creatinine, uric acid, glomerular filtration rate, hemoglobin and NT-proBNP. An analysis of their pharmacotherapy was performed. Data from two-year follow-up of patients were obtained at outpatient check-ups, during hospitalizations or by telephone.
3.3. Echocardiography
Echocardiography studies were realized on the same clinic visit day by a broadband transducer on commercially available equipment (St. Anne’s University Hospital—Vivid E9, Brno, GE, USA; Faculty Hospital Brno—Vivid S9, Brno, GE, USA; Na Homolce Hospital—Vivid 7, Praha, GE, USA). Left ventricular ejection fraction was determined by transthoracic echocardiography. During the examination, the Simpson’s biplanar method from the apical four-chamber and two-chamber views, respectively, was used to set the ejection fraction.
3.4. Definitions
The prevalence of all comorbidities, such as coronary heart disease, previous myocardial infarction, diabetes mellitus, COPD, hypertension and lower limb ischemic disease, were obtained from the medical history and the set of diagnoses in the previous documentation. Many diagnoses were then also supported by records of these comorbidities, such as percutaneous coronary intervention for coronary artery disease. As a primary composite endpoint, we chose a combination of the hardest endpoint, death from all causes, but also orthotopic heart transplantation or LVAD implantation, whereby the patient essentially ceases to be a heart failure patient and becomes a different type of patient. Deaths were ascertained not only from documentation and by telephone, but also from the state’s IHIS database (Institute of Health Information and Statistics of the Czech Republic). From the documentation and by telephone we collected other endpoints such as hospitalization or cause of death, but especially the telephone history encountered many inaccuracies.
3.5. Statistical Analysis
Data were processed and statistical analysis was performed by the Institute of Biostatistics and Analysis of the Faculty of Medicine of Masaryk University, Brno, Czech Republic. Categorical variables are characterized by absolute and relative frequencies. Continuous basic characteristics are described by mean ± SD, laboratory results are described by median (25th percentile; 75th percentile). The p-value of the Fisher exact test for categorical variables and p-value of the Mann–Whitney U test for continuous variables are shown with Bonferroni correction applied.
4. Results
A total of 1088 patients were enrolled in the registry, in whom we determined LVEF using echocardiography at study entry. In the HFrEF subgroup there were 862 patients with a mean LVEF of 27 ± 7%, while in the HFmrEF group there were 226 patients with an LVEF of 43 ± 3%, (p < 0.001). In the HFrEF group, 83.2% were men, compared to HFmrEF, where 72.1% were men (p = 0.008). The mean age was 63 ± 12 years in the HFrEF group and 67 ± 11 years in the HFmrEF group (p < 0.001).
BMI was 29 ± 5 in both groups. There were 424 (49.2%) non-smokers and 83 (9.6%) smokers in HFrEF, and 128 (56.6%) non-smokers and 30 (13.3%) smokers in HFmrEF. In the HFrEF group, patients had lower systolic blood pressure (p < 0.001), higher heart rate (p < 0.001), and there were fewer patients with ischemic heart disease or prior myocardial infarction compared to patients with HFmrEF (p < 0.001).
Of the other comorbidities, hypertension occurred in the HFrEF group in 64.7% of patients and in the HFmrEF group in 155 (68.6%) patients, followed by dyslipidemia in HFrEF in 75.6% of patients and in HFmrEF in 82.3% of patients. Diabetes mellitus occurred in HFrEF in 40.5% of patients and in HFmrEF in 32.3% of patients and chronic obstructive pulmonary disease (COPD) in HFrEF in 13.6% of patients and in HFmrEF in 12.8% of patients. There were 91 (10.6%) patients who had chronic lower extremity ischemia in the HFrEF group and 31 (13.7%) patients in the HFmrEF group. There were no statistically significant differences between the groups in the proportion of comorbidities.
There were differences between the groups in the functional classification according to the New York Heart Association (NYHA), which divides patients based on a subjective assessment of dyspnea in relation to physical exertion, where patients in NYHA I are not significantly constrained in everyday life with minimal effort and usually at rest. Patients with HFrEF versus HFmrEF were represented in 9.6% vs. 26.5% in NYHA I, 59.7% versus 62.8% in NYHA II and in NYHA III and IV 30.6% versus 10.6% (p < 0.001).
Patients with HFrEF had a huge difference in median NT-proBNP 685 ng/L vs. 197 ng/L in HFmrEF (p < 0.001). HFrEF had higher levels of uric acid (p < 0.001) and urea (p < 0.010), the change in creatinine was not significant.
There were no significant differences in the use of ACE inhibitors or beta-blockers, as basic drugs in the treatment of heart failure significantly reduce morbidity and mortality. However, the differences in medication used between the HFrEF and HFmrEF groups were in the type of diuretic. Patients with HFrEF were more likely to use furosemide (87.4% vs. 55.3%,
p < 0.001) and mineralocorticoid receptor antagonists (MRA) (74.0% vs. 44.7%,
p < 0.001), whereas hydrochlorothiazide was more common in the HFmrEF group (18.6% vs. 7.4%,
p < 0.001), as can be seen in
Table 1 and
Table 2.
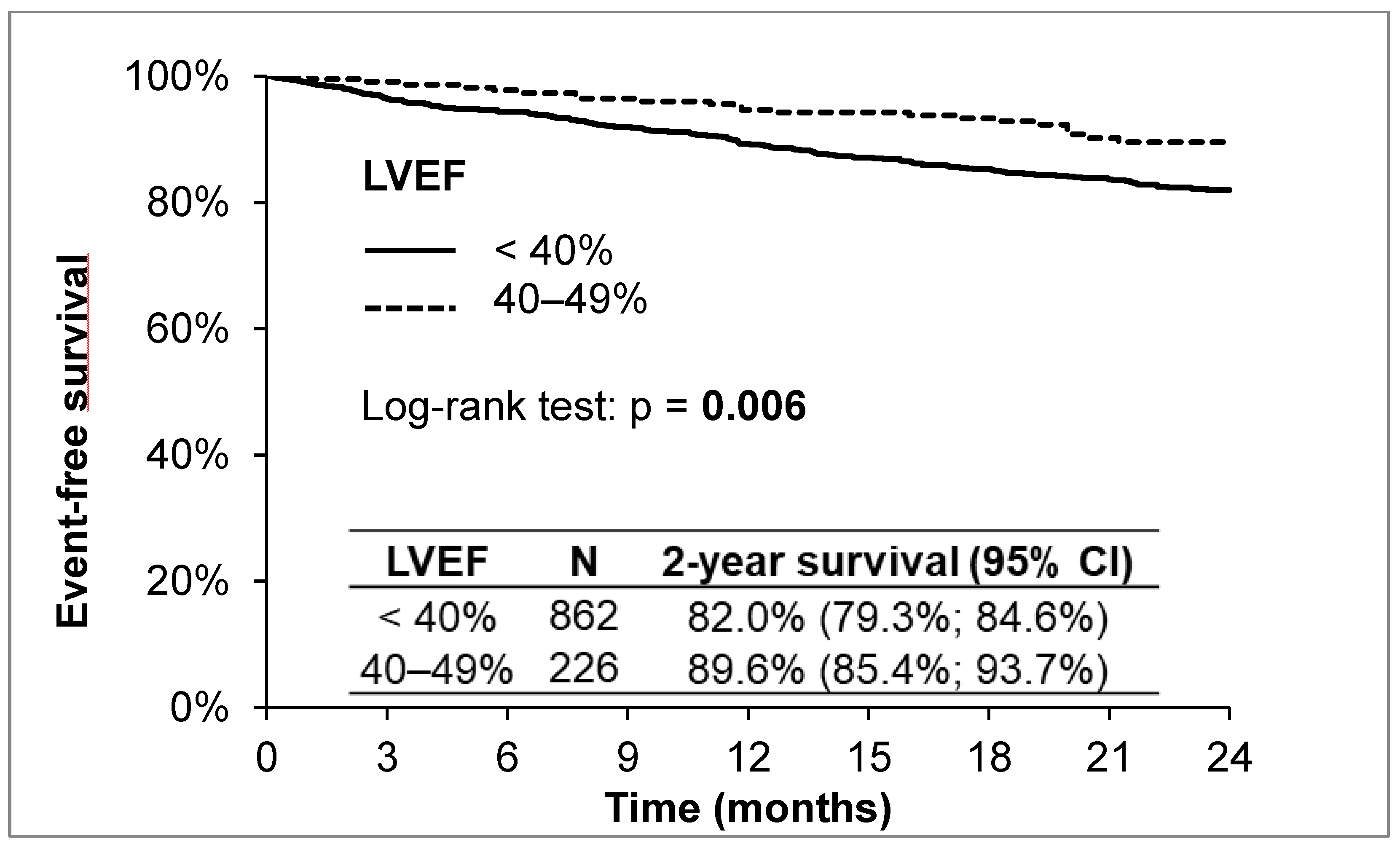
Heart failure is a serious disease with high morbidity and mortality. What can be seen from the above results is that the prognosis of patients in the HFrEF group is worse than in the HFmrEF group. Of the 1088 patients during the 2-year follow-up, 132 (12.1%) died, 141 (13.0%) were hospitalized for acute heart failure, and 34 (3.1%) underwent OHT or LVAD implantation. The 2-year survival without reaching the primary endpoint (all-cause death, OHT, LVAD implantation) was 82.0% in the HFrEF group and 89.6% in the HFmrEF group (according to the Log-rank test,
p = 0.006;
Figure 1).
5. Discussion
In our registry, we found that patients with HFrEF are more symptomatic, have higher NT-proBNP level and lower blood pressure than patients with HFmrEF. They have a worse prognosis than patients in the HFmrEF group. In the HFrEF group, loop diuretics and mineral corticosteroid receptor blockers are more commonly used.
5.1. Comorbidities in HFmrEF
Comorbidities are independent predictors of patients with HFmrEF and should be considered in a clinical trial of HFmrEF. In the study “Comorbidities, fragility and quality of life in patients with HFmrEF”, comorbidities significantly affected patient outcomes (both morbidity and mortality). This effect was unexpectedly higher in patients with HFmrEF than in patients with HFrEF and HFpEF. In particular, hypertension, coronary heart disease, atrial fibrillation and renal failure have a direct link to heart failure. Anemia often develops with heart failure and is associated with a worse prognosis. The relationship between DM and heart failure is two-way and the incidence of DM in heart failure is significant. Patients with DM have an increased risk of heart failure and DM is also associated with a poor prognosis. Renal insufficiency is very common due to the interdependence of the kidneys and the heart. Patients with heart failure and renal insufficiency also have a poor prognosis. Even a slight deterioration in renal parameters is associated with higher mortality. However, there is limited evidence that specific treatment for comorbidities is associated with a lower incidence of serious cardiovascular events regardless of LVEF. This may be due to the fact that comorbidities are more often addressed in patients with HFrEF. Based on the results of the study, patients with HFmrEF may be an ideal group for the targeted treatment of comorbidities, due to the effect of comorbidities on the prognosis and, so far, less severe heart failure [
4].
The study “Non-cardiac comorbidities in heart failure with HFrEF, HFmrEF and HFpEF” looked at other comorbidities, including thyroid dysfunction, obesity, COPD, stroke and peripheral arterial disease. The association of each comorbidity with quality of life, mortality from all causes and hospitalization was evaluated. The highest prevalence of comorbidities was observed in patients with HFpEF. Comorbidities were associated with lower quality of life, but this was more pronounced in patients with HFrEF [
5].
There was no difference in the proportion of comorbidities in the HFmrEF groups compared to HFrEF in our group. Of the monitored comorbidities, i.e., hypertension, dyslipidemia, COPD, DM and ICHDKK, hypertension and dyslipidemia were the most represented in both subgroups.
5.2. Pharmacotherapy in HFmrEF
To date, no prospective study has been performed on patients with HFmrEF. All analyses and recommendations are based on post hoc analyses from studies for HFrEF and HFpEF. These analyses suggest that patients in the HFmrEF subgroup benefit from the medication used similarly for patients in the HFrEF subgroup. These include the use of β-blockers, angiotensin I converting enzyme (ACEI) inhibitors, angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists and sacubitril/valsartan [
6,
7,
8].
5.3. Using ACEI or ARB
Data from the Swedish registry suggest that ACEIs and ARBs are beneficial in the HFmrEF patient group. Of 42,061 patients, 21% had HFmrEF, ACEI/ARBs were associated with a lower risk of death, regardless of the presence or absence of coronary heart disease [
6].
The effect of sartans on clinical outcomes in patients with heart failure across the LVEF spectrum was studied in the CHARM program (post hoc analysis). In a subgroup of 1322 patients with LVEF 40–49% (CHD etiology 66.9%, NYHA III 41.6%, NYHA IV 0.7%, ACEI 27.2%, BB 57.7%, spironolactone 11.4%, diuretics (74.4%)), candesartan reduced the risk of CVD death and hospitalization due to CVD. However, there was no statistical interaction between the LVEF phenotype and candesartan treatment [
9].
In our group of patients in the HFmrEF group, 200 (88.5%) patients used ACEI/ARB and 761 (88.3%) patients in the HFrEF group. There was no statistical difference between the groups. Our results do not focus on monitoring the benefit of ACEI/ARB in patients with HFmrEF, but a number of post hoc analyses on the benefit of ACEI/ARB in patients with HFmrEF have been published [
7,
10].
Our file does not include data on the use of sacubitril/valsartan, which was not commonly used at the time of the start of the study. In the PARADIGM-HF study, sacubitril/valsartan was compared with enalapril. Cardiovascular death as well as hospitalization due to heart failure were identified as the primary endpoint. The study demonstrated a beneficial effect in the treatment of heart failure, regardless of EF [
11] use of beta-blockers in the HfmrEF group.
5.4. Use of Beta-Blockers
According to data from a meta-analysis of 11 clinical studies (stratification according to baseline EF and heart rate), beta-blockers (BB) were found in the group of patients with HFmrEF, and sinus rhythm (575 patients, CHD etiology 91%, NYHA class III-IV, ACE-I/ARB 91%, mineralocorticoid receptor antagonists—MRA 6%, diuretics 65%) reduced the risk of death from all causes, including the risk of cardiovascular death, which is comparable to that in the HFrEF group. BB in the group of patients with HFmrEF and atrial fibrillation did not affect the prognosis (ie the risk of death), which is the same as for HFrEF, but had an effect on the increase in EF [
12,
13].
In our cohort, BB were used by 201 (88.9%) patients in the HFmrEF group and 819 (95.0%) in the HFrEF group, which was on the borderline of significance (p = 0.047) and did not indicate a significant difference in treatment.
5.5. Use of Mineralocorticoid Receptor Antagonists in the HFmrEF Group
According to the RALES [
14], EMPHASIS-HF [
15] and EPHESUS [
16] studies, patients in the HFrEF subgroup received more spironolactone than patients in the HFmrEF subgroup. The TOPCAT post hoc study also reported results for patients with LVEF 44–49% (n = 520). The results showed the effect of spironolactone in reducing the risk of CVD death, hospitalization for CVD causes or resuscitation for sudden cardiac death. However, this is only a small group of patients [
11].
In our group, 101 (44.7%) patients used spironolactone/eplerenone in the HFmrEF group and 638 (74.0%) patients in the HFrEF subgroup (p < 0.001).
5.6. Use of Furosemide and Thiazide Diuretics
Of the other diuretics that we focused on in our study, furosemide was used in the HFrEF group in 87.4% of patients and thiazides in 7.4%. In the HFmrEF group, 55.3% of patients received furosemide and 18.6% used thiazides (p < 0.001). There are no placebo-controlled studies with diuretics in heart failure, but they are undoubtedly a very important drug in the treatment of heart failure to induce euvolemia. HFmrEF patients are patients with less severe heart disease, in our group they need loop diuretics less often and only thiazide diuretics are sufficient for inducing euvolemia.
5.7. Iron Use in the HFmrEF Group
Iron substitution was not studied in our cohort, but there are many studies that have focused on iron deficiency in patients with heart failure. Iron deficiency is associated with worse symptoms, quality of life, and clinical outcomes across the entire spectrum of LVEFs, especially in patients with hemoglobin levels in the blood below 14 g/dl. A total of 1197 patients (71% men) were evaluated (HFrEF, n = 897, HFmrEF, n = 229, HFpEF, n = 72). The overall prevalence of iron deficiency was 53% (50% for HFrEF, 61% for HFmrEF, 64% for HFpEF) and of anemia was 36%. Iron deficiency was more associated with a low volume oxygen maximum (VO2max) than with low hemoglobin. Patients with deficit progression had a higher risk of hospitalization for heart failure and a higher risk of mortality from any cause [
17].
Overall, HFmrEF is a group of patients with clinical characteristics, pathophysiology, therapeutic responses and prognosis that are in many ways similar to HFrEF [
14]. More and more authors are inclined to classify patients from the HFmrEF group back into the HFrEF group due to the high prevalence of ischemic heart disease, and the use of similar “NT-proBNP guided” therapies in HFrEF and HFmrEF, with a confirmed beneficial response to the same drugs, in contrast to HFpEF [
18].
5.8. Coronary Artery Disease and Myocardial Infarction
In our registry, the HFrEF group has fewer cases of ischaemic heart disease or previous myocardial infarction than the HFmrEF group. This may be due to the very good care in the acute phase of myocardial infarction in the Czech Republic, where there is a relatively dense network of Cathlabs and a short distance to direct percutaneous coronary intervention (dPCI). This is also the reason why myocardial infarction is practically not treated by thrombolysis here. We know from a Polish study that the earlier a STEMI patient gets to dPCI, the greater the improvement in LVEF and therefore mortality after one year [
19]. As expected, patients with reduced LVEF had a worse prognosis, as noted in the ACTION registry, where the hazard of mortality increased by 26% for every 5% decrease in LVEF [
20]. Of course, the more comorbidities a patient has, such as diabetes, previous myocardial infarction or renal insufficiency, the worse prognosis the patient has [
21], as we can see in our registry.
6. Conclusions
Patients with HFrEF have a higher NYHA class, NT-proBNP level and lower blood pressure than patients with HFmrEF. They have a worse prognosis than patients in the HFmrEF group, so they more often achieved the endpoint, which was LVAD implantation, achieving OHT or death from any cause.
In the HFrEF group, loop diuretics and mineral corticosteroid receptor blockers are more commonly used, but this reflects the need for symptomatic therapy in patients with more severe heart failure. Otherwise, there is no significant difference between the groups in the therapy used.
The two groups did not differ in the presence of comorbidities.
7. Limitations
The FAR NHL Registry is a Czech multicentric prospective registry. There may be variations between the three different hospitals involved in patient inclusion. In particular, at the I IKAK FNUSA, there are much more often patients with more severe heart failure who are followed up before OHT or LVAD implantation. This may affect both outcomes and event prediction.
Of concern is the ejection fraction of just 40%, which according to the 2016 ESC Guidelines was classified as HFmrEF, whereas now according to the latest 2021 ESC Guidelines, as well as the 1 April 2022 AHA/ACC/HFSA Guideline, is already classified as HFrEF. However, since most of the recent studies since 2016 describing the distribution of heart failure according to ejection fraction adhere to the older distribution, in order to make our study more comparable to this majority of studies, the authors decided to keep the group of patients with LVEF 40% in the HFmrEF group.
The review of 64 prognostic models and meta-analyses and 117 prognostic models revealed less accuracy of the combined endpoint of death or hospitalization alone than mortality prediction alone [
22,
23]. However, other data show that mortality increases strongly as the number of hospitalizations increase [
24]. Given that our centers see patients prior to OHT or LVAD implantation for hospitalization and there is not always a clear distinction between planned hospitalization without deterioration for this purpose alone and decompensated heart failure, the authors ultimately chose not to include hospitalization for deteriorated heart failure in the combined endpoint.
So far, this is a two-year follow-up, and a ten-year prognosis data collection is planned.
Author Contributions
Conceptualization, J.Š. and L.Š.; methodology, J.P. and K.L.; software, K.B.; validation, J.J.; formal analysis, A.T.; investigation, A.T., K.L., M.Š., F.M., O.L. and J.K.; data curation, K.B.; writing—original draft preparation, A.T. and L.Š.; writing—review and editing, K.L. and L.Š.; visualization, K.B.; supervision, L.Š. and J.Š.; project administration, K.L. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by Masaryk University as part of the project “New trends in diagnostics and therapy of cardiomyopathies” number MUNI/A/1685/2020 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2021.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Brno University Hospital, Czech Republic (protocol code 02-221014/EK, date of approval 22 October 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy and ethical restrictions. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Boehm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Gastelurrutia, P.; Lupón, J.; Moliner, P.; Yang, X.; Cediel, G.; de Antonio, M.; Domingo, M.; Altimir, S.; González, B.; Rodríguez, M.; et al. Comorbidities, Fragility, and Quality of Life in Heart Failure Patients with Midrange Ejection Fraction. Mayo Clin. Proc. Innov. Qual Outcomes 2018, 2, 176–185. [Google Scholar] [CrossRef]
- Streng, K.W.; Nauta, J.F.; Hillege, H.L.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Lang, C.C.; Metra, M.; Ng, L.L.; et al. Non-Cardiac Comorbidities in Heart Failure with Reduced, Mid-Range and Preserved Ejection Fraction. Int. J. Cardiol. 2018, 271, 132–139. [Google Scholar] [CrossRef]
- Koh, A.S.; Tay, W.T.; Teng, T.H.K.; Vedin, O.; Benson, L.; Dahlstrom, U.; Savarese, G.; Lam, C.S.P.; Lund, L.H. A Comprehensive Population-Based Characterization of Heart Failure with Mid-Range Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 1624–1634. [Google Scholar] [CrossRef]
- Koufou, E.-E.; Arfaras-Melainis, A.; Rawal, S.; Kalogeropoulos, A.P. Treatment of Heart Failure with Mid-Range Ejection Fraction: What Is the Evidence. J. Clin. Med. 2021, 10, 203. [Google Scholar] [CrossRef]
- Lazarova, M.; Lazar, D.; Malek, F.; Vaclavik, J.; Taborsky, M.; Ignaszewski, A. Heart Failure Disease Management Program, Its Contribution to Established Pharmacotherapy and Long-Term Prognosis in Real Clinical Practice—Retrospective Data Analysis. Biomed. Pap. 2019, 163, 318–323. [Google Scholar] [CrossRef]
- Lund, L.H.; Claggett, B.; Liu, J.; Lam, C.S.; Jhund, P.S.; Rosano, G.M.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Pfeffer, M.A.; et al. Heart Failure with Mid-Range Ejection Fraction in CHARM: Characteristics, Outcomes and Effect of Candesartan across the Entire Ejection Fraction Spectrum. Eur. J. Heart Fail. 2018, 20, 1230–1239. [Google Scholar] [CrossRef]
- Aiglova, R.; Taborsky, M.; Lazarova, M.; Pavlu, L.; Danek, J.; Precek, J.; Schee, A.; Gloger, V.; Cernicek, V.; Vicha, M.; et al. Angiotensin-Converting Enzyme Inhibitors, Angiotensin-II-Receptor Antagonists and Angiotensin-Receptor Blocker/Neprilysin Inhibitor Utilization in Heart Failure Patients: Sub-Analysis of a Nation-Wide Population-Based Study in the Czech Republic. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2022, 166, 322–327. [Google Scholar] [CrossRef]
- Solomon, S.D.; Claggett, B.; Lewis, E.F.; Desai, A.; Anand, I.; Sweitzer, N.K.; O’Meara, E.; Shah, S.J.; McKinlay, S.; Fleg, J.L.; et al. Influence of Ejection Fraction on Outcomes and Efficacy of Spironolactone in Patients with Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2016, 37, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Bunting, K.V.; Flather, M.D.; Altman, D.G.; Holmes, J.; Coats, A.J.S.; Manzano, L.; McMurray, J.J.V.; Ruschitzka, F.; van Veldhuisen, D.J.; et al. Beta-Blockers for Heart Failure with Reduced, Mid-Range, and Preserved Ejection Fraction: An Individual Patient-Level Analysis of Double-Blind Randomized Trials. Eur. Heart J. 2018, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Táborsky, M.; Aiglova, R.; Lazarova, M.; Pavlu, L.; Danek, J.; Přeček, J.; Schee, A.; Gloger, V.; Vicha, M.; Skala, T. Beta-Blockers Utilization in Heart Failure Patients: Sub-Analysis of a Nation-Wide Population-Based Study in the Czech Republic. Biomed. Pap. 2021, 165, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. EMPHASIS-HF Study Group Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M.; et al. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of Iron Deficiency on Exercise Capacity and Outcome in Heart Failure with Reduced, Mid-Range and Preserved Ejection Fraction. Acta Cardiol. 2018, 73, 115–123. [Google Scholar] [CrossRef]
- Rickenbacher, P.; Kaufmann, B.A.; Maeder, M.T.; Bernheim, A.; Goetschalckx, K.; Pfister, O.; Pfisterer, M.; Brunner-La Rocca, H.-P. TIME-CHF Investigators Heart Failure with Mid-Range Ejection Fraction: A Distinct Clinical Entity? Insights from the Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF). Eur. J. Heart Fail. 2017, 19, 1586–1596. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Magielski, P.; Woznicki, M.; Gierach, J.; Jablonski, M.; Fabiszak, T.; Kozinski, M.; Sukiennik, A.; Bronisz, A.; Kubica, J. Occurrence and Predictors of Left Ventricular Systolic Dysfunction at Hospital Discharge and in Long-Term Follow-up after Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Kardiol. Pol. 2012, 70, 329–342. [Google Scholar]
- Sutton, N.R.; Li, S.; Thomas, L.; Wang, T.Y.; de Lemos, J.A.; Enriquez, J.R.; Shah, R.U.; Fonarow, G.C. The Association of Left Ventricular Ejection Fraction with Clinical Outcomes after Myocardial Infarction: Findings from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Medicare-Linked Database. Am. Heart J. 2016, 178, 65–73. [Google Scholar] [CrossRef]
- Lewis, E.F.; Velazquez, E.J.; Solomon, S.D.; Hellkamp, A.S.; McMurray, J.J.V.; Mathias, J.; Rouleau, J.-L.; Maggioni, A.P.; Swedberg, K.; Kober, L.; et al. Predictors of the First Heart Failure Hospitalization in Patients Who Are Stable Survivors of Myocardial Infarction Complicated by Pulmonary Congestion and/or Left Ventricular Dysfunction: A VALIANT Study. Eur. Heart J. 2008, 29, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk Prediction in Patients with Heart Failure: A Systematic Review and Analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, W.; Voors, A.A.; Zwinderman, A.H. Factors Influencing the Predictive Power of Models for Predicting Mortality and/or Heart Failure Hospitalization in Patients with Heart Failure. JACC Heart Fail. 2014, 2, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated Hospitalizations Predict Mortality in the Community Population with Heart Failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).