Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection and Cultivation
2.3. Phenotypic Identification of S. aureus
2.3.1. Characteristics of Microscopic Examination and Gram Staining
2.3.2. BBL™ Staphyloslide™ Latex Test and Staph ID 32 API System
2.3.3. Kirby–Bauer Disc Diffusion Technique for Detection of MRSA
2.3.4. Vitek 2 Compact System for Identification and Antimicrobial Resistance of MRSA
2.4. Proteomic Screening for Identification of MSSA and MRSA
2.5. Detection of S. aureus and MRSA Genes Using qPCR
2.6. Antimicrobial Susceptibility of MRSA Using AST GP71 Cards
3. Results
3.1. Preliminary Detection of S. aureus Isolates
3.2. Morphological Characterization of S. aureus Strains
3.3. Routine Detection of MRSA Strains
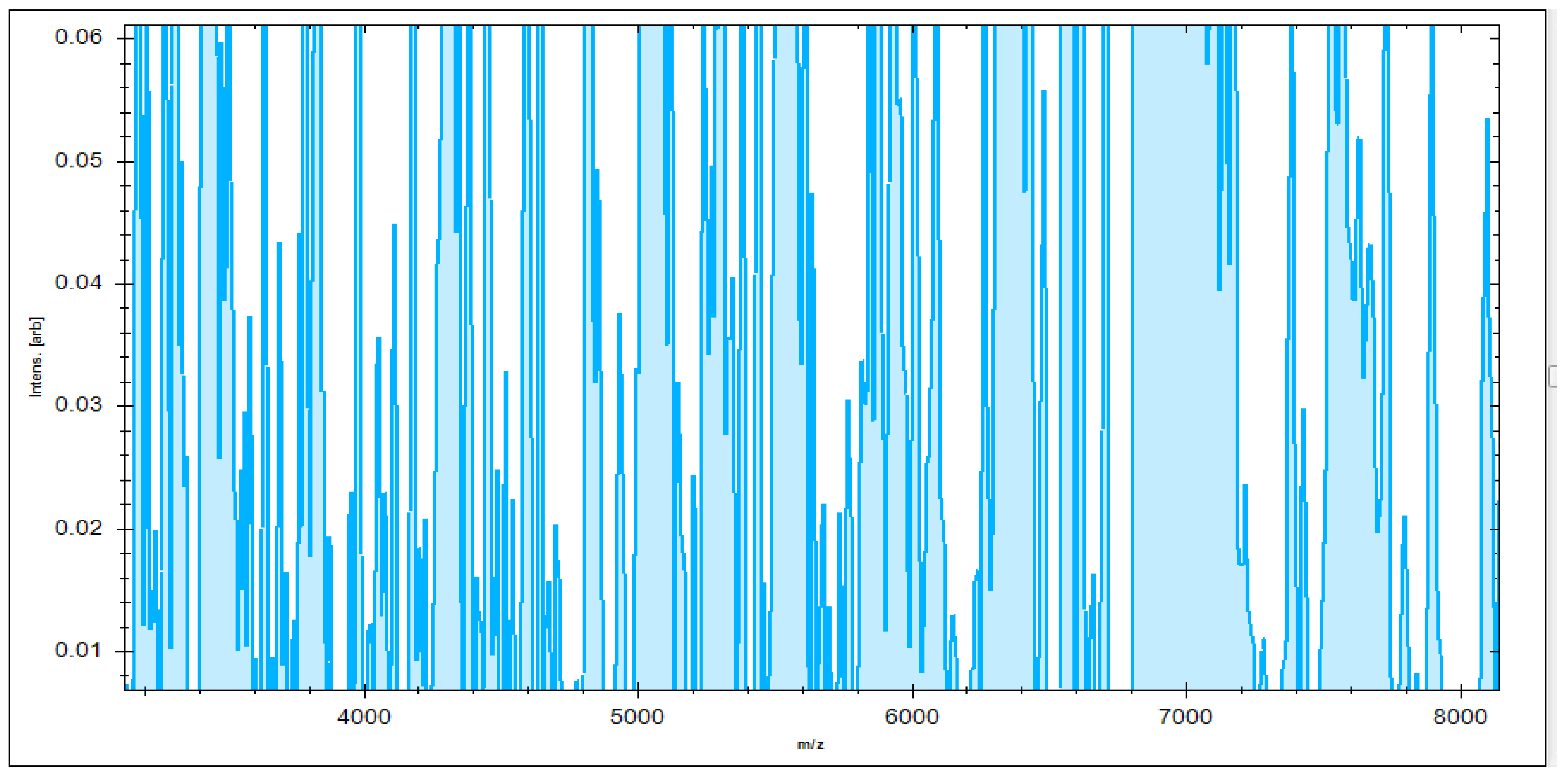
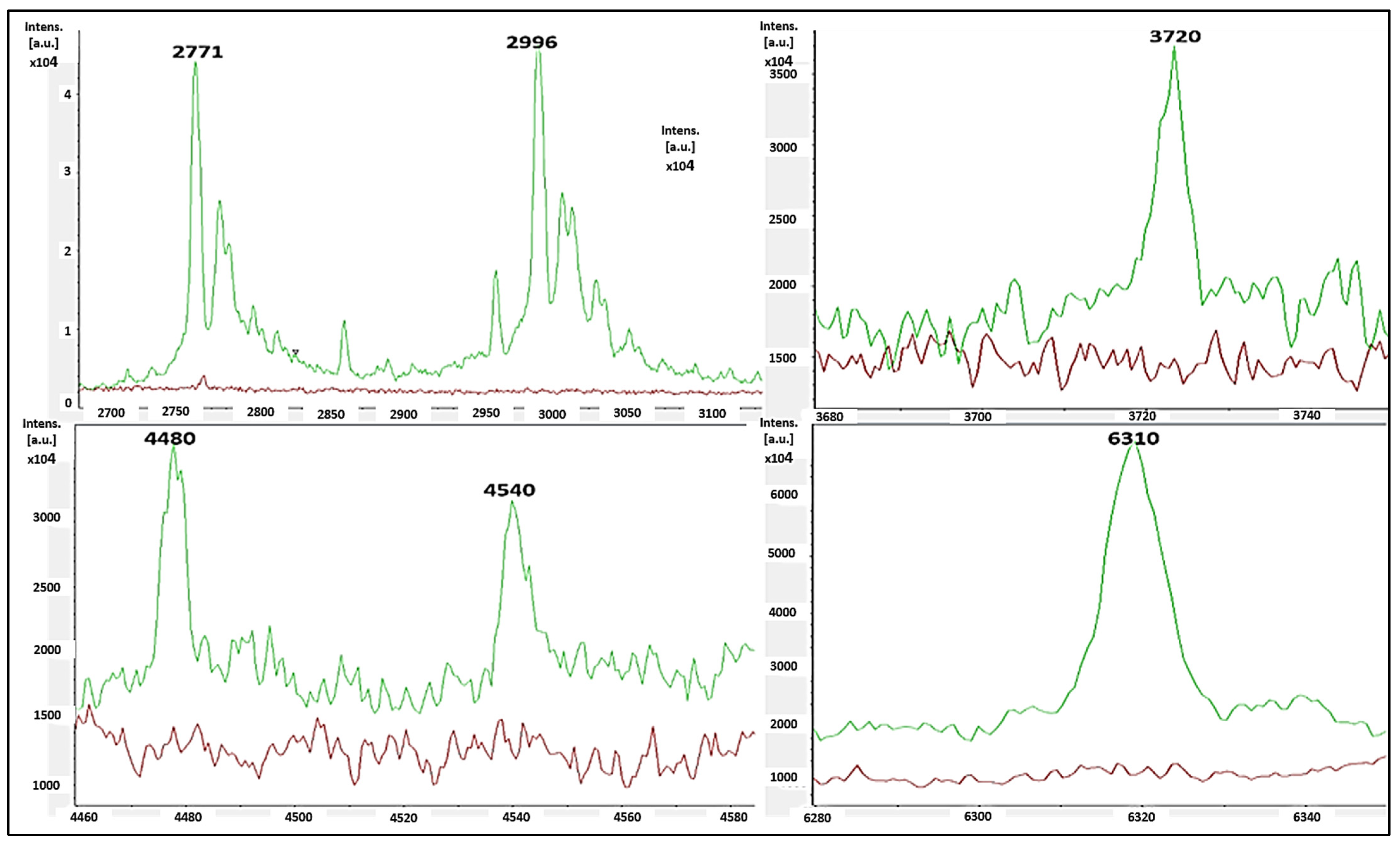
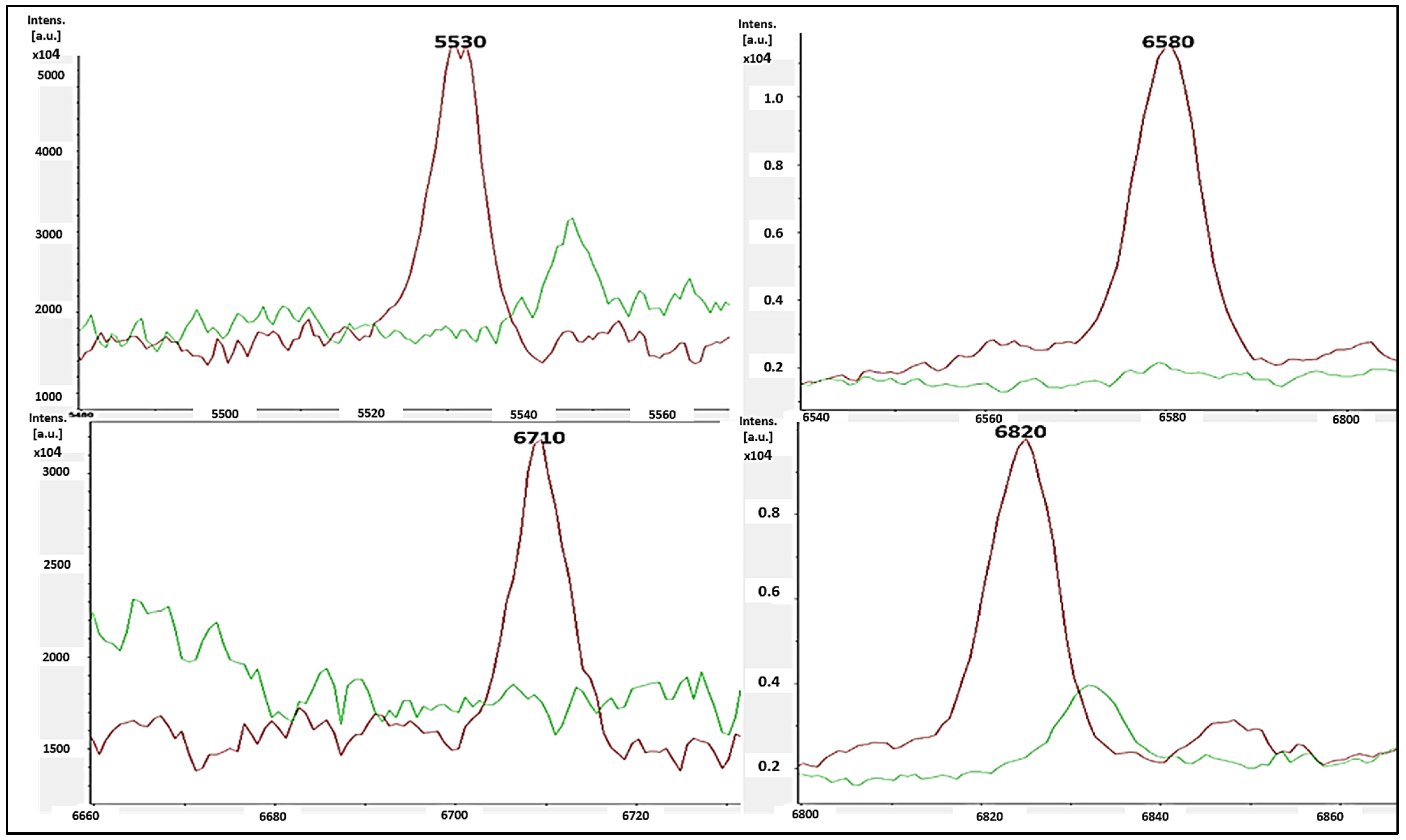
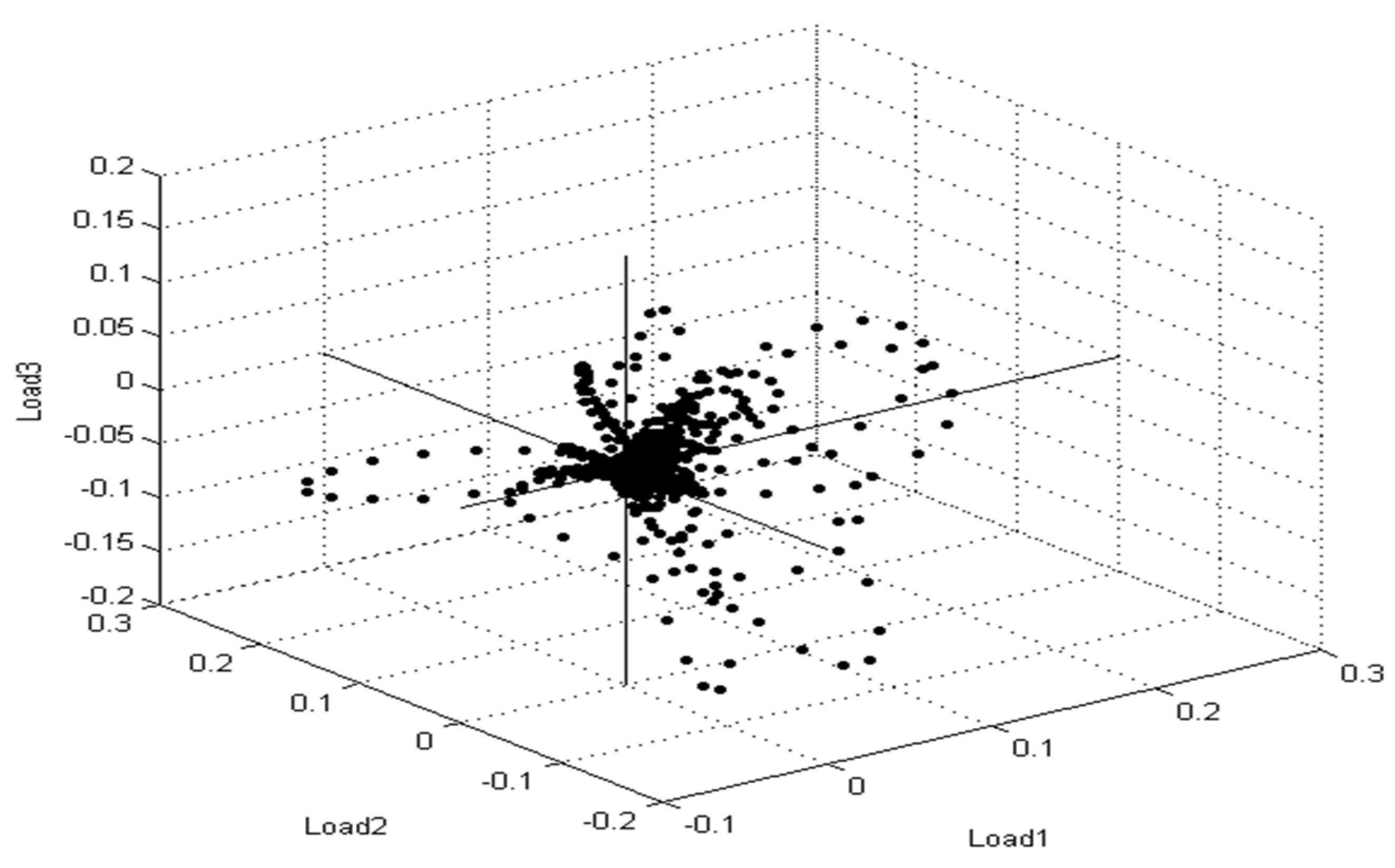
3.4. Identification of S. aureus via Mass Peptide Analysis
3.5. Molecular Identification of MRSA Strains
3.6. Antimicrobial Susceptibility Testing of MRSA Using AST GP71 Cards
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.; Hussein, D.; Salih, J. Antimicrobial susceptibility testing and phenotypic detection of MRSA isolated from diabetic foot infection. Int. J. Gen. Med. 2020, 13, 1349. [Google Scholar] [CrossRef]
- Hinojosa, C.A.; Boyer-Duck, E.; Anaya-Ayala, J.E.; Nunez-Salgado, A.; Laparra-Escareno, H.; Torres-Machorro, A.; Lizola, R. Impact of the bacteriology of diabetic foot ulcers in limb loss. Wound Repair Regen. 2016, 24, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Aragon-Sanchez, J.; Lew, D.; Lipsky, B.A. Diabetic foot infections: What have we learned in the last 30 years? Int. J. Infect. Dis. 2015, 40, 81–91. [Google Scholar] [CrossRef]
- Karmaker, M.; Sanyal, S.K.; Sultana, M.; Hossain, M. Association of bacteria in diabetic and non-diabetic foot infection–An investigation in patients from Bangladesh. J. Infect. Public Health 2016, 9, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ornskov, D.; Kolmos, B.; Horn, P.B.; Nielsen, J.N.; Brandslund, I.; Schouenborg, P. Screening for methicillin-resistant Staphylococcus aureus in clinical swabs using a high-throughput real-time PCR-based method. Clin. Microbiol. Infect. 2008, 14, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mutonga, D.M.; Mureithi, M.W.; Ngugi, N.N.; Otieno, F.C. Bacterial isolation and antibiotic susceptibility from diabetic foot ulcers in Kenya using microbiological tests and comparison with RT-PCR in detection of S. aureus and MRSA. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.; Marques-Costa, A.; Vilela, C.; Neves, J.; Candeias, N.; Cavaco-Silva, P.; Melo-Cristino, J. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res. Clin. Pract. 2012, 95, 153–161. [Google Scholar] [CrossRef]
- Anafo, R.B.; Atiase, Y.; Dayie, N.T.; Kotey, F.C.; Tetteh-Quarcoo, P.B.; Duodu, S.; Osei, M.-M.; Alzahrani, K.J.; Donkor, E.S. Methicillin-resistant Staphylococcus aureus (MRSA) infection of diabetic foot ulcers at a tertiary care hospital in Accra, Ghana. Pathogens 2021, 10, 937. [Google Scholar] [CrossRef]
- Ogba, O.M.; Nsan, E.; Eyam, E.S. Aerobic bacteria associated with diabetic foot ulcers and their susceptibility pattern. Biomed. Dermatol. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Reina-Bueno, M.; Palomo-Toucedo, I.C.; Castro-Méndez, A.; Domínguez-Maldonado, G.; del Carmen Vázquez-Bautista, M. Methicillin-Resistant Staphylococcus aureus Diabetic Foot Crossed Infection: A Case Report. Pathogens 2020, 9, 549. [Google Scholar] [CrossRef]
- Jneid, J.; Lavigne, J.; La Scola, B.; Cassir, N. The diabetic foot microbiota: A review. Hum. Microbiome J. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Złoch, M.; Maślak, E.; Kupczyk, W.; Jackowski, M.; Pomastowski, P.; Buszewski, B. Culturomics Approach to Identify Diabetic Foot Infection Bacteria. Int. J. Mol. Sci. 2021, 22, 9574. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Alenzi, A.; Al-Maary, K.S.; Mubarak, A.S.; Alshammari, H.D.; Al-Sarar, D.; Alsubki, R.A.; Hemeg, H.A. Multidrug-resistant Escherichia coli in Raw Milk: Molecular Characterization and the potential impact of camel’s Urine as an Antibacterial Agent. Saudi J. Biol. Sci. 2021, 28, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Nicoleta, M.; Gheorghe, I.; Popa, M.; LAZĂR, V.; Banu, O.; Bolocan, A.; Grigore, R.; Berteșteanu, Ș.V.; Octav, P. Phenotypic and genotypic investigation of resistance and virulence features of methicillin resistant Staphylococcus aureus strains isolated from hospitalized patients. Rom. Biotechnol. Lett. 2016, 21, 11591. [Google Scholar]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Osman, S.; Edrees, H. Performance of MALDI biotyper compared with Vitek™ 2 compact system for fast identification and discrimination of Staphylococcus species isolated from bovine mastitis. MicrobiologyOpen 2016, 5, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, J.; Chen, R.; Hu, L.; Xia, Q.; Wu, W.; Wang, J.; Hu, F. Multicenter evaluation of three different MALDI-TOF MS systems for identification of clinically relevant filamentous fungi. Med. Mycol. 2021, 59, 81–86. [Google Scholar] [CrossRef]
- Burckhardt, I.; Zimmermann, S. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J. Clin. Microbiol. 2011, 49, 3321–3324. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Qian, J.; Ge, Y.; Ye, K.; Zhou, C.; Zhang, H. Principal component analysis of MALDI-TOF MS of whole-cell foodborne pathogenic bacteria. Anal. Biochem. 2020, 592, 113582. [Google Scholar] [CrossRef]
- Boudreau, M.A.; Fishovitz, J.; Llarrull, L.I.; Xiao, Q.; Mobashery, S. Phosphorylation of BlaR1 in manifestation of antibiotic resistance in methicillin-resistant Staphylococcus aureus and its abrogation by small molecules. ACS Infect. Dis. 2015, 1, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmadishooli, A.; Davoodian, P.; Shoja, S.; Ahmadishooli, B.; Dadvand, H.; Hamadiyan, H.; Shahriarirad, R. Frequency and antimicrobial susceptibility patterns of diabetic foot infection of patients from Bandar Abbas District, Southern Iran. J. Pathog. 2020, 2020, 1057167. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; McDougal, L.K.; Gorwitz, R.J.; Mayer, K.H.; Patel, J.B.; Sennott, J.M.; Fontana, J.L. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J. Clin. Microbiol. 2007, 45, 1350–1352. [Google Scholar] [CrossRef]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Akhi, M.T.; Ghotaslou, R.; Memar, M.Y.; Asgharzadeh, M.; Varshochi, M.; Pirzadeh, T.; Alizadeh, N. Frequency of MRSA in diabetic foot infections. Int. J. Diabetes Dev. Ctries. 2017, 37, 58–62. [Google Scholar] [CrossRef]
- Mota-Meira, M.; Lapointe, G.; Lacroix, C.; Lavoie, M.C. MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob. Agents Chemother. 2000, 44, 24–29. [Google Scholar] [CrossRef]
- Renneberg, J.; Rieneck, K.; Gutschik, E. Evaluation of Staph ID 32 system and Staph-Zym system for identification of coagulase-negative staphylococci. J. Clin. Microbiol. 1995, 33, 1150–1153. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493. [Google Scholar] [CrossRef] [PubMed]
- Spanu, T.; Sanguinetti, M.; Ciccaglione, D.; D’Inzeo, T.; Romano, L.; Leone, F.; Fadda, G. Use of the VITEK 2 system for rapid identification of clinical isolates of staphylococci from bloodstream infections. J. Clin. Microbiol. 2003, 41, 4259–4263. [Google Scholar] [CrossRef] [PubMed]
- Sastry, A.S.; Bhat, S. Essentials of Medical Microbiology; JP Medical Ltd.: Puducherry, India, 2018. [Google Scholar]
- Bhatta, D.R.; Cavaco, L.M.; Nath, G.; Kumar, K.; Gaur, A.; Gokhale, S.; Bhatta, D.R. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: A matter of concern for community infections (a hospital based prospective study). BMC Infect. Dis. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Jana, D.; Dutta, K.; Dua, P.; Ghosh, C. Prevalence of Panton-Valentine leukocidin gene among community acquired Staphylococcus aureus: A real-time PCR study. J. Pathog. 2018, 2018, 4518541. [Google Scholar] [CrossRef] [PubMed]
- Bobenchik, A.M.; Hindler, J.A.; Giltner, C.L.; Saeki, S.; Humphries, R.M. Performance of Vitek 2 for antimicrobial susceptibility testing of Staphylococcus spp. and Enterococcus spp. J. Clin. Microbiol. 2014, 52, 392–397. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.; Bush, K.; Dudley, M.; Eliopoulos, G.; Hardy, D. Clinical and laboratory standards institute. In Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Karadağ, F.Y.; Saltoğlu, N.; Ak, Ö.; Aydın, G.Ç.; Şenbayrak, S.; Erol, S.; Özatağ, D.M.; Kadanalı, A.; Küçükardalı, Y.; Çomoğlu, Ş. Foot self-care in diabetes mellitus: Evaluation of patient awareness. Prim. Care Diabetes 2019, 13, 515–520. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Pecoraro, R.E.; Wheat, L.J. The diabetic foot: Soft tissue and bone infection. Infect. Dis. Clin. N. Am. 1990, 4, 409–432. [Google Scholar] [CrossRef]
- Reacher, M.H.; Shah, A.; Livermore, D.M.; Wale, M.C.; Graham, C.; Johnson, A.P.; Heine, H.; Monnickendam, M.A.; Barker, K.F.; James, D. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: Trend analysis. BMJ 2000, 320, 213–216. [Google Scholar] [CrossRef]
- Al-Moyed, K.A.; Al-Haddad, A.M.; Al-Areqi, B.A.; Al-Danani, D.A. Prevalence of Staphylococcus aureus infection among diabetic foot patients in Sana’a city-Yemen. Alandalus J. Appl. Sci. 2014, 6, 58–77. [Google Scholar] [CrossRef][Green Version]
- Lawes, T.; López-Lozano, J.-M.; Nebot, C.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.; Edwards, G.F.; Gould, I.M. Turning the tide or riding the waves? Impacts of antibiotic stewardship and infection control on MRSA strain dynamics in a Scottish region over 16 years: Non-linear time series analysis. BMJ Open 2015, 5, e006596. [Google Scholar] [CrossRef]
- Tentolouris, N.; Jude, E.; Smirnof, I.; Knowles, E.; Boulton, A. Methicillin-resistant Staphylococcus aureus: An increasing problem in a diabetic foot clinic. Diabet. Med. 1999, 16, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.J.; Lipsky, B.A. Diagnosis and management of infection in the diabetic foot. Med. Clin. 2013, 97, 911–946. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M.; Goldstein, E.J.; Merriam, C.V.; Lipsky, B.A.; Abramson, M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007, 45, 2819–2828. [Google Scholar] [CrossRef]
- Anvarinejad, M.; Pouladfar, G.; Japoni, A.; Bolandparvaz, S.; Satiary, Z.; Abbasi, P.; Mardaneh, J. Isolation and antibiotic susceptibility of the microorganisms isolated from diabetic foot infections in Nemazee Hospital, Southern Iran. J. Pathog. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, M.; Mutluoglu, M.; Turhan, V.; Uzun, G.; Lipsky, B.A.; Sevim, E.; Demiraslan, H.; Eryilmaz, E.; Ozuguz, C.; Memis, A. Causative pathogens and antibiotic resistance in diabetic foot infections: A prospective multi-center study. J. Diabetes Its Complicat. 2016, 30, 910–916. [Google Scholar] [CrossRef]
- Katz, D.E.; Friedman, N.D.; Ostrovski, E.; Ravid, D.; Amrami, N.; Avivi, D.; Mengesha, B.; Zaidenstein, R.; Lazarovitch, T.; Dadon, M. Diabetic foot infection in hospitalized adults. J. Infect. Chemother. 2016, 22, 167–173. [Google Scholar] [CrossRef]
- Cervantes-García, E.; García-González, R.; Reséndiz-Albor, A.; Salazar-Schettino, P.M. Infections of diabetic foot ulcers with methicillin-resistant Staphylococcus aureus. Int. J. Low. Extrem. Wounds 2015, 14, 44–49. [Google Scholar] [CrossRef]
- Eleftheriadou, I.; Tentolouris, N.; Argiana, V.; Jude, E.; Boulton, A.J. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs 2010, 70, 1785–1797. [Google Scholar] [CrossRef]
- Egyir, B.; Guardabassi, L.; Esson, J.; Nielsen, S.S.; Newman, M.J.; Addo, K.K.; Larsen, A.R. Insights into nasal carriage of Staphylococcus aureus in an urban and a rural community in Ghana. PLoS ONE 2014, 9, e96119. [Google Scholar] [CrossRef]
- Donkor, E.S.; Kotey, F.C.; Dayie, N.T.; Duodu, S.; Tetteh-Quarcoo, P.B.; Osei, M.-M.; Tette, E.M. Colonization of HIV-infected children with methicillin-resistant Staphylococcus aureus. Pathogens 2019, 8, 35. [Google Scholar] [CrossRef]
- Dayie, N.T.; Sekoh, D.N.; Kotey, F.C.; Egyir, B.; Tetteh-Quarcoo, P.B.; Adutwum-Ofosu, K.K.; Ahenkorah, J.; Osei, M.-M.; Donkor, E.S. Nasopharyngeal Carriage of Methicillin-Resistant Staphylococcus aureus (MRSA) among Sickle Cell Disease (SCD) Children in the Pneumococcal Conjugate Vaccine Era. Infect. Dis. Rep. 2021, 13, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Grmek-Kosnik, I.; Dermota, U.; Ribic, H.; Storman, A.; Petrovic, Z.; Zohar-Cretnik, T. Evaluation of single vs pooled swab cultures for detecting MRSA colonization. J. Hosp. Infect. 2018, 98, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Kim, I.; Chung, S.H.; Chung, Y.; Han, M.; Kim, J.-S. Rapid discrimination of methicillin-resistant Staphylococcus aureus by MALDI-TOF MS. Pathogens 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, J.; Yang, H.; Yu, J.; Wei, H. Accurate detection of methicillin-resistant Staphylococcus aureus in mixtures by use of single-bacterium duplex droplet digital PCR. J. Clin. Microbiol. 2017, 55, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Van Belkum, A.; Rochas, O. Laboratory-based and point-of-care testing for MSSA/MRSA detection in the age of whole genome sequencing. Front. Microbiol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Pomastowski, P.; Szultka, M.; Kupczyk, W.; Jackowski, M.; Buszewski, B. Evaluation of intact cell matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for capillary electrophoresis detection of controlled bacterial clumping. Anal. Bioanal. Tech. 2015, 13, 1–7. [Google Scholar]
- Manukumar, H.; Umesha, S. MALDI-TOF-MS based identification and molecular characterization of food associated methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 1–16. [Google Scholar]
- Harris, L.G.; El-Bouri, K.; Johnston, S.; Rees, E.; Frommelt, L.; Siemssen, N.; Christner, M.; Davies, A.P.; Rohde, H.; Mack, D. Rapid identification of staphylococci from prosthetic joint infections using MALDI-TOF mass-spectrometry. Int. J. Artif. Organs 2010, 33, 568–574. [Google Scholar] [CrossRef]
- Bergeron, M.; Dauwalder, O.; Gouy, M.; Freydiere, A.-M.; Bes, M.; Meugnier, H.; Benito, Y.; Etienne, J.; Lina, G.; Vandenesch, F. Species identification of staphylococci by amplification and sequencing of the tuf gene compared to the gap gene and by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 343–354. [Google Scholar] [CrossRef]
- Lasch, P.; Fleige, C.; Stämmler, M.; Layer, F.; Nübel, U.; Witte, W.; Werner, G. Insufficient discriminatory power of MALDI-TOF mass spectrometry for typing of Enterococcus faecium and Staphylococcus aureus isolates. J. Microbiol. Methods 2014, 100, 58–69. [Google Scholar] [CrossRef]
- Østergaard, C.; Hansen, S.G.; Møller, J.K. Rapid first-line discrimination of methicillin resistant Staphylococcus aureus strains using MALDI-TOF MS. Int. J. Med. Microbiol. 2015, 305, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Wang, H.; Karichu, J.; Richter, S.S. The presence of a single MALDI-TOF mass spectral peak predicts methicillin resistance in staphylococci. Diagn. Microbiol. Infect. Dis. 2016, 86, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Víquez-Molina, G.; Aragon-Sanchez, J.; Perez-Corrales, C.; Murillo-Vargas, C.; López-Valverde, M.E.; Lipsky, B.A. Virulence factor genes in Staphylococcus aureus isolated from diabetic foot soft tissue and bone infections. Int. J. Low. Extrem. Wounds 2018, 17, 36–41. [Google Scholar] [CrossRef]
- Baddour, M.; AbuElKheir, M.; Fatani, A. Comparison of mecA polymerase chain reaction with phenotypic methods for the detection of methicillin-resistant Staphylococcus aureus. Curr. Microbiol. 2007, 55, 473–479. [Google Scholar] [CrossRef]
- Deplano, A.; Vandendriessche, S.; Nonhoff, C.; Denis, O. Genetic diversity among methicillin-resistant Staphylococcus aureus isolates carrying the mecC gene in Belgium. J. Antimicrob. Chemother. 2014, 69, 1457–1460. [Google Scholar] [CrossRef]
- Mottola, C.; Semedo-Lemsaddek, T.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Molecular typing, virulence traits and antimicrobial resistance of diabetic foot staphylococci. J. Biomed. Sci. 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Kavitha, Y.; Mohan, S.; Moinuddin, S.K. Bacteriological profile of diabetic foot infection with special reference to ESBL and MRSA in a coastal tertiary care teaching hospital. Indian J. Microbiol. Res. 2017, 4, 68–73. [Google Scholar]
- Stappers, M.H.; Hagen, F.; Reimnitz, P.; Mouton, J.W.; Meis, J.F.; Gyssens, I.C. Direct molecular versus culture-based assessment of Gram-positive cocci in biopsies of patients with major abscesses and diabetic foot infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.-P. Staphylococcus aureus toxins and diabetic foot ulcers: Role in pathogenesis and interest in diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.H.M.; Guler, E.; Baddal, B. Prevalence of Panton-Valentine leukocidin in methicillin-resistant Staphylococcus aureus clinical isolates at a university hospital in Northern Cyprus: A pilot study. BMC Res. Notes 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; Pathrose, E.; Bal, A.; Bhalekar, P.; Sekar, P.; Krishnan, P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Telles, J.P.; Cieslinski, J.; Tuon, F.F. Daptomycin to bone and joint infections and prosthesis joint infections: A systematic review. Braz. J. Infect. Dis. 2019, 23, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Hammerschlag, M.R. Treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in children: A reappraisal of vancomycin. Curr. Infect. Dis. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Sekhar, S.M.; Vyas, N.; Unnikrishnan, M.; Rodrigues, G.; Mukhopadhyay, C. Antimicrobial susceptibility pattern in diabetic foot ulcer: A pilot study. Ann. Med. Health Sci. Res. 2014, 4, 742–745. [Google Scholar] [CrossRef]
- Bansal, E.; Garg, A.; Bhatia, S.; Attri, A.; Chander, J. Spectrum of microbial flora in diabetic foot ulcers. Indian J. Pathol. Microbiol. 2008, 51, 204. [Google Scholar]
- Gadepalli, R.; Dhawan, B.; Sreenivas, V.; Kapil, A.; Ammini, A.; Chaudhry, R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care 2006, 29, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Perim, M.C.; da Costa Borges, J.; Celeste, S.R.C.; de Freitas Orsolin, E.; Mendes, R.R.; Mendes, G.O.; Ferreira, R.L.; Carreiro, S.C.; da Silva Pranchevicius, M.C. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev. Da Soc. Bras. De Med. Trop. 2015, 48, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Reveles, K.R.; Duhon, B.M.; Moore, R.J.; Hand, E.O.; Howell, C.K. Epidemiology of methicillin-resistant Staphylococcus aureus diabetic foot infections in a large academic hospital: Implications for antimicrobial stewardship. PLoS ONE 2016, 11, e0161658. [Google Scholar] [CrossRef]
- Kett, D. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACTT-HAP) Investigators. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: An observational, multicentre cohort study. Lancet Infect. Dis. 2011, 11, 181–189. [Google Scholar]
- Hassan, M.A.; Tamer, T.M.; Rageh, A.A.; Abou-Zeid, A.M.; Abd El-Zaher, E.H.; Kenawy, E.-R. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1261–1270. [Google Scholar] [CrossRef]

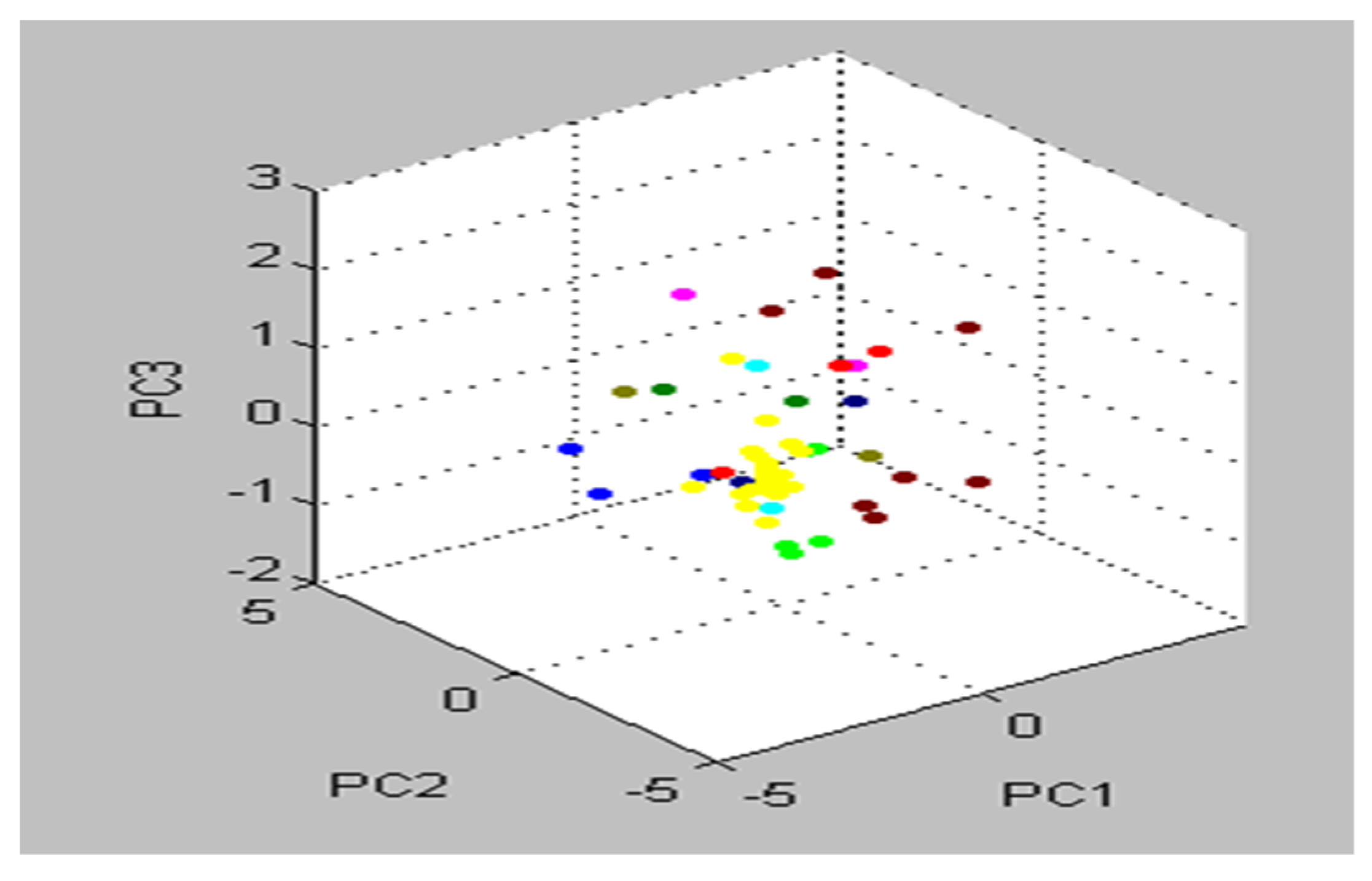
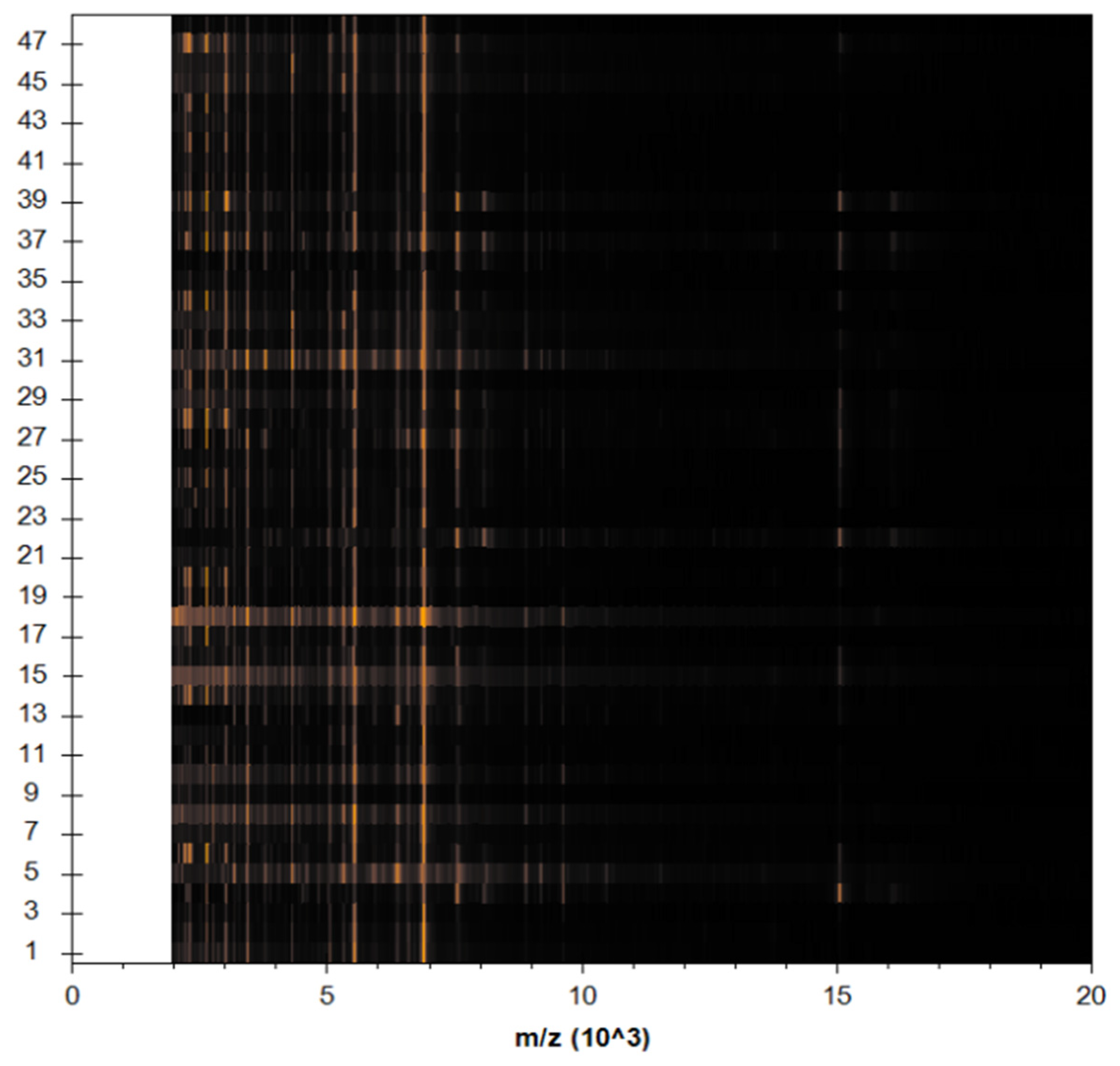
| Gene | Primer Sequence (5′-3′) | Base Pair | Conditions of Real-Time PCR | ||||
|---|---|---|---|---|---|---|---|
| Initial Denaturation | Denaturation | Annealing | Extension | Final Extension | |||
| mecA [27] | GTAGAAATGACTGAACGTCCGATAA CCAATTCCACATTGTTTCGGTCTA | 310 | 94 °C/4 min | 94 °C/30 s | 60 °C/30 s | 72 °C/30 s | 72 °C/5 min |
| PVL [34] | ATCATTAGGTAAAATGTCTGGACATGATCCA GCATCAAGTGTATTGGATAGCAAAAGC | 433 | 94 °C/4 min | 94 °C/40 s | 58 °C/30 s | 72 °C/30 s | 72 °C/5 min |
| No. of Strains | Coagulase Test | Staph ID 32 System | BBL™ Staphyloslide™ | Vitek 2 Compact | ||||
|---|---|---|---|---|---|---|---|---|
| No. of CI Strains | % of CI Strains | No. of CI Strains | % of CI Strains | No. of Cl Strains | % of CI Strains | No. of CL Strains | % of CI Strains | |
| 48 | 45 | 93.75% | 42 | 87.5% | 48 | 100% | 46 | 95.83% |
| Antimicrobial Agent | No. of Isolates | Essential Agreement | Categorical Agreement | Very Major Error | Major Error | Minor Error | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | S | I | R | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Cefoxitin screen | 22 | 2 | 0 | 20 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 22 | 5 | 2 | 15 | 21 | 95.45 | 21 | 95.45 | 0 | 0 | 0 | 0 | 1 | 4.55 |
| Clindamycin | 22 | 10 | 0 | 12 | 16 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 |
| Daptomycin | 22 | 3 | 0 | 19 | 20 | 90.9 | 21 | 95.45 | 0 | 0 | 1 | 4.55 | 0 | 0 |
| Erythromycin | 22 | 4 | 0 | 18 | 20 | 90.9 | 20 | 90.9 | 0 | 0 | 0 | 0 | 2 | 9.1 |
| Gentamicin | 22 | 17 | 1 | 4 | 22 | 100 | 21 | 95.45 | 0 | 0 | 0 | 0 | 1 | 4.55 |
| Linezolid | 22 | 22 | 0 | 0 | 21 | 95.45 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nitrofurantoin | 22 | 22 | 0 | 0 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oxacillin | 22 | 9 | 0 | 13 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzylpenicillin | 22 | 5 | 0 | 17 | 21 | 95.45 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quinupristin–dalfopristin | 22 | 22 | 0 | 0 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rifampin | 22 | 20 | 0 | 2 | 21 | 95.45 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tigecycline | 22 | 22 | 0 | 0 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trimethoprim–sulfamethoxazole | 22 | 18 | 0 | 4 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vancomycin | 22 | 22 | 0 | 0 | 22 | 100 | 22 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abalkhail, A.; Elbehiry, A. Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Appl. Sci. 2022, 12, 10803. https://doi.org/10.3390/app122110803
Abalkhail A, Elbehiry A. Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Applied Sciences. 2022; 12(21):10803. https://doi.org/10.3390/app122110803
Chicago/Turabian StyleAbalkhail, Adil, and Ayman Elbehiry. 2022. "Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance" Applied Sciences 12, no. 21: 10803. https://doi.org/10.3390/app122110803
APA StyleAbalkhail, A., & Elbehiry, A. (2022). Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Applied Sciences, 12(21), 10803. https://doi.org/10.3390/app122110803







