Abstract
To cope with the problem that the setting time and hardening time of high-belite sulphoaluminate cement (HBCSA) is too fast and not easily controlled, three common retarders, citric acid (CA), β-cyclodextrin (β-CD), and borax (B), were selected and compounded with polycarboxylate superplasticizer (PCE). Based on the cement slurry fluidity, fluidity loss, setting time, and mechanical properties of cement mortar, combined with X-ray diffraction (XRD), scanning electron microscopy (SEM), and Zeta potential tests, the interaction and mechanism of different retarders and high-belite sulphoaluminate cement were studied. The results show that β-CD and CA can significantly delay the hydration process of HBCSA cement, effectively improve the fluidity loss, and make the dispersion more stable, but the strength of cement mortar decreases, which is not conducive to the development of strength. However, the addition of B has no obvious retarding effect on the HBCSA cement, and the dispersibility of the cement paste decreases. In microscopic tests, XRD and SEM tests verified that the combination of retarder and PCE had a certain inhibitory effect on the early hydration of HBCSA, and the zeta potential indicated that the addition of retarder would interact with PCE to improve the fluidity loss of HBCSA. In addition, when the retarders CA and B were, respectively, compounded with β-CD, the two would have a synergistic effect. The retardation effect is more obvious, and the time interval between the initial and final setting is shorter, which is more controllable. The initial fluidity is also improved under the auxiliary plasticization of cement paste by β-CD.
1. Introduction
As the world’s most widely used construction engineering material, cement has a considerable annual output worldwide due to its good hydraulic properties and workability, wide and low-cost raw material sources, and programmed production process. However, cement consumes a lot of energy and generates a lot of CO2 in the production stages such as clinker grinding and limestone calcination. Production technology in the cement industry has also been evolving in recent years, with an increasing focus on sustainable, cost-efficient, and energy-efficient production to reduce energy consumption and greenhouse gas emissions [1,2]. The mineral composition contained in sulphoaluminate cement (CSA) is mainly anhydrous calcium sulphoaluminate (C4A3) and β-dicalcium silicate (β-C2S), which is calcined at a lower temperature, thereby reducing the CO2 emissions caused by the combustion of the fuel. However, the high proportion of C4A3 in CSA can easily lead to problems such as excessive cement setting time and unstable strength growth at later ages [3,4,5,6]. Therefore, by adjusting the ratio of β-C2S and C4A3 in SAC and increasing the content of β-C2S, the development of cement strength in the later stage is effectively guaranteed and the amount of alumina is reduced. It provides the conditions for the utilization of low-quality aluminum resources, forming high-belite sulphoaluminate cement (HBCSA) [5,7,8,9,10,11,12,13,14]. Compared with CSA cement, HBCSA cement has a lower calcination temperature (50–100 °C lower than that of CSA cement), consumes less energy in production, and emits 50–80% of the amount of CO2 that traditional CSA cement emits [15]. It is of great significance to the cement industry’s energy conservation, environmental protection, and sustainable development.
Due to the fast hydration rate of CSA cement, in order to overcome the rapid setting of CSA and ensure the continuous hydration of β-C2S, it is usually necessary to use a retarder to delay the hydration rate of cement [16]. For example, the addition of organic retarders sodium gluconate, tartaric acid, and citric acid can delay the hydration of cement by inhibiting the precipitation or growth of ettringite, and sodium gluconate can slow down the hydration reaction more than tartaric acid. This shows that the interaction with cement hydration is stronger at the same test content, and the retardation effect of citric acid and sodium gluconate gradually weakens with the increase in temperature [16,17,18]. However, tartaric acid will reduce the strength of cement mortar at an early age, but it has little effect on the compressive strength in the long term [19]. Citric acid and sodium gluconate can significantly improve the early strength of CSA cement [16]. In addition, after adding inorganic retarder borax or boric acid, it will precipitate with Ca2+ in the cement particles and adsorb on the cement surface, thereby forming a calcium-based borate layer, preventing the dissolution of ye’elimite [16,17].
In addition, the special properties of rapid setting and high early strength of sulphoaluminate cement result in poor working performance, which requires a water-reducing agent to improve [20,21,22,23]. Therefore, the use of a superplasticizer can improve the fluidity of cement paste, and a retarder can prolong the setting time of the paste. Combining the two is beneficial to maintain good fluidity and workability of the cement paste [22], but the compatibility between the two remains to be studied. Some studies have shown that the initial fluidity of the cement paste will be reduced due to the competitive adsorption of superplasticizer β-naphthalenesulfonic acid-based water-reducing agent (BNS), sulfamic acid-based water-reducing agent (AS), and citric acid after compounding [22,23,24]. However, there is no competitive adsorption of sodium gluconate with BNS and AS. After combining citric acid and sodium gluconate with polycarboxylate superplasticizer (PCE), both the fluidity and flow loss were improved. However, an excessive amount of retarder will lead to a decrease in the activity of cement particles and a decrease in compressive strength [22]. The combination of retarder borax and polycarboxylate superplasticizer will also have competitive adsorption, reducing the superplasticizer’s adsorption capacity and reducing cement paste’s dispersibility [25].
To sum up, the current research mostly focuses on the effect of a single retarder combined with a water reducer on the properties of sulphoaluminate cement. There is insufficient research on the effect of combining two retarders with a water reducer on the properties of high-belite sulphoaluminate cement. Therefore, the setting time of high-belite sulphoaluminate cement is too fast, which is not conducive to operation in practical engineering. Three common retarders (citric acid, beta-cyclodextrin, and borax) were selected for this study. The three retarders were mixed separately and combined with two retarders, combined with polycarboxylate superplasticizer (PCE). Macro performance tests (fluidity, setting time, and mechanical properties) were combined. The effects of retarders on the types of cement hydration products (XRD), microscopic morphology (SEM), and Zeta potential at the interface between the surfaces of cement particles were also studied. The adaptability of different retarders to the cement and the interaction between cement and retarder were further characterized.
2. Materials and Methods
2.1. Materials
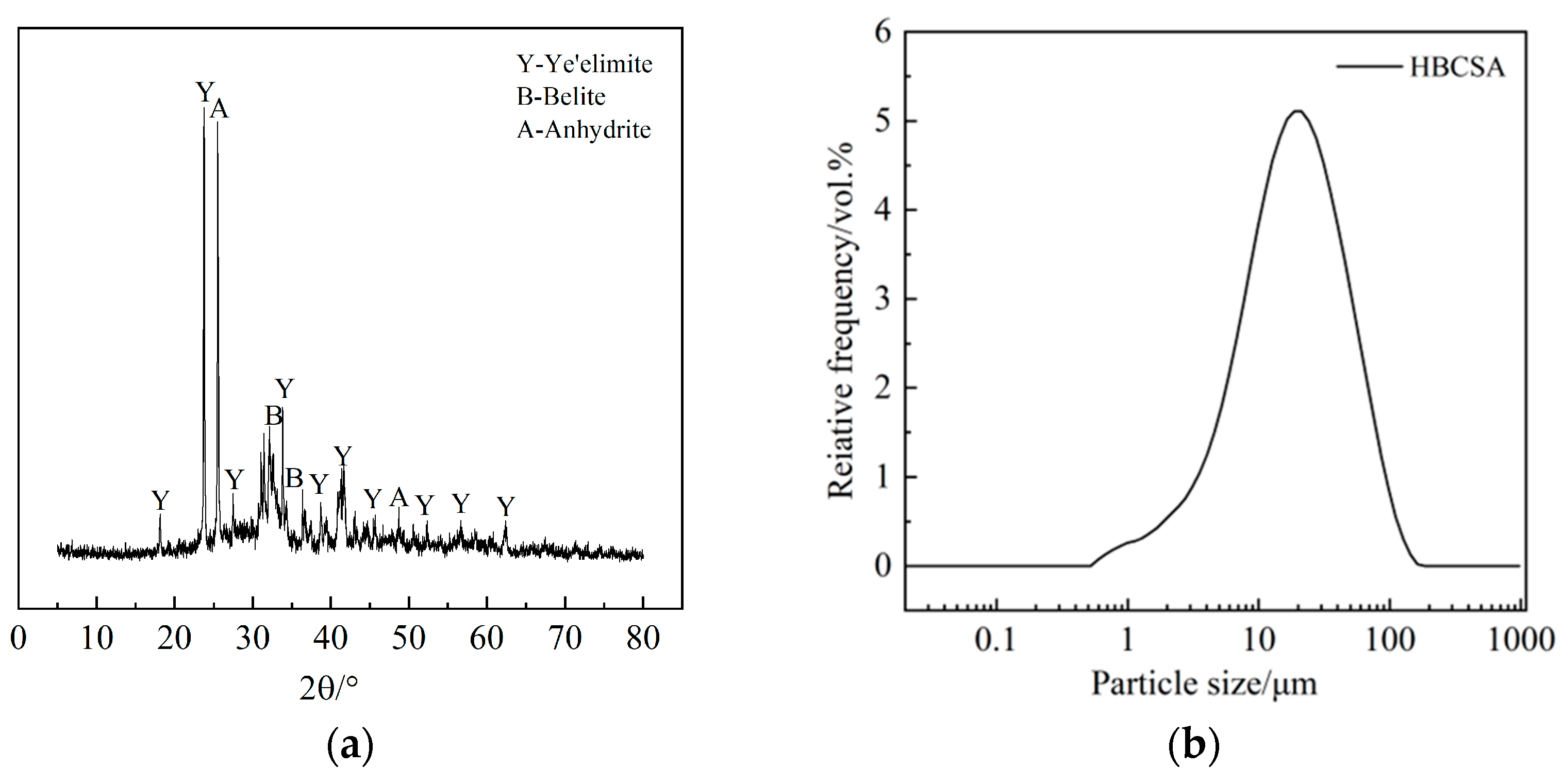
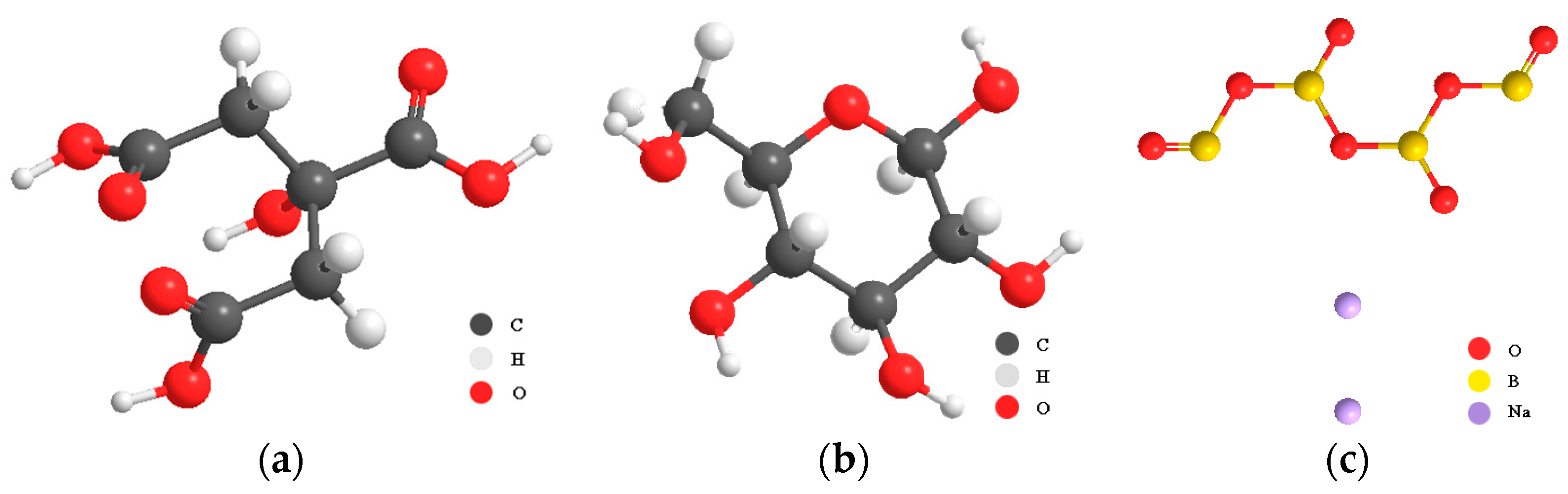
High-belite sulphoaluminate cement with a strength grade of 42.5 produced by Tangshan Polar Bear Building Materials Co., Ltd. (Tangshan, China), was selected. The chemical composition of the cement was obtained by XRF, as shown in Table 1, in which the main chemical composition CaO accounted for 48.03%. The mineral composition of the cement was determined by XRD, as shown in Figure 1a, and was mainly composed of anhydrous calcium sulphoaluminate, dicalcium silicate, and gypsum. The particle size distribution of cement is shown in Figure 1b. The particle size is mainly distributed in the range of 1~102 μm, and the proportion is the highest when the particle size is about 19 μm. The water-reducing agent adopts Polycarboxylate superplasticizer (PCE) with a solid content of 40%. The retarders used are β-cyclodextrin (β-CD), citric acid (CA), and borax (B), all of which are of analytical grade, and their chemical structures are shown in Figure 2. Among them, CA (Figure 2a) is a tricarboxylic acid compound. β-CD consists of seven glucose residues linked by a β-1,4-glycosidic bond (Figure 2b) to form a ring. B (Figure 2c) is an inorganic compound (Na2B4O7).

Table 1.
Chemical composition of HBCSA.
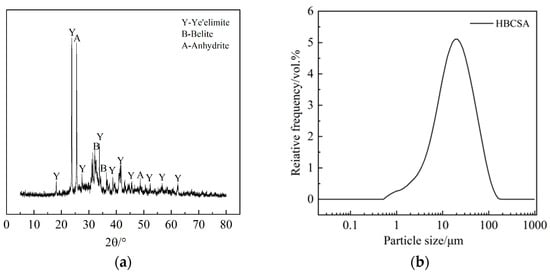
Figure 1.
Mineral composition and particle size distribution of HBCSA: (a) Mineral composition; (b) Particle size distribution.
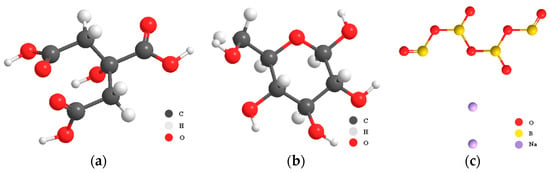
Figure 2.
Chemical structures of three retarders: (a) CA; (b) β-1,4-glucosidic bond; (c) B.
2.2. Testing Method
2.2.1. Preparation of Cement Paste
The cement paste was prepared by adding polycarboxylate superplasticizer (PCE) with contents of 0.8%, 1.0%, 1.2%, 1.4%, and 1.6% to the HBCSA cement paste, respectively. A PCE dosage is determined by a working performance test. Then, three retarders (β-CD, CA, B) are added to the cement according to different contents (0.1%, 0.3%, 0.5%, 0.7%, and 0.9%). Finally, two or more contents of two or more kinds of retarders are determined by testing the working performance and physical and mechanical properties of a single retarder, and the three retarders are compounded in pairs.
2.2.2. Fluidity of Cement Paste
The fluidity of cement paste was tested according to GB/T 8077-2012 “Test method for uniformity of concrete admixtures”. The cement paste was prepared by mixing 300 g cement, 87 g water (w/c = 0.29), and a predetermined amount of admixture, then the prepared slurry was poured into a conical mold (60 mm in height, 36 mm in diameter at the top, and 60 mm in diameter at the bottom). The cone mold was then lifted and the paste was allowed to flow freely on a glass plate for 30 s. The fluidity was determined by measuring the diameter of the cement paste in two perpendicular directions, and the average value was recorded as the fluidity of the cement paste. To determine the fluidity loss, the fluidity of the cement paste was measured repeatedly at 15, 30, 60, and 90 min after stirring.
2.2.3. Setting Time
The setting time was determined according to GB/T 1346-2011 “Water Consumption, Setting Time and Stability of Cement Standard Consistency”. Cement slurry was prepared using 500 g cement, 142.5 g water (w/c = 0.28), and admixtures of different contents, and the setting time was measured by Vicat meter.
2.2.4. Strength
Referring to GB/T 17671-2021 “Inspection Method for Cement Mortar Strength”, the cement mortar ratio was 1:3 and the water-cement ratio was 0.5, and the standard triple test mold of 4 cm × 4 cm × 16 cm was used. After demolding, they were placed in a standard curing box with a temperature of 20 °C and a relative humidity of 95% to the specified age. The compressive and flexural strengths of cement mortar specimens at 1 d, 3 d, 7 d, and 28 d were also measured.
2.2.5. XRD
The clean slurry sample was cured to the test age, the surrounding carbonized surface was removed, and it was sealed and soaked in anhydrous ethanol. In order to prevent the pyrolysis of ettringite in the hydration product, the samples after hydration termination were dried to a constant weight in an oven at 40 °C. The sample was then ground and sieved with a 0.063 mm sieve, and the sieved sample was subjected to a German Bruker D8 Advance X-ray diffractometer. The scanning range was 5°~90°, the scanning speed was 5°/min, and XRD experiments of 1 d and 28 d were carried out.
2.2.6. SEM
Several small pieces measuring 2.5–5 mm were knocked out from the interior of the cement paste specimen that was cured to a specific age. After soaking in absolute ethanol to terminate the hydration and drying, the morphologies of the hydration products at an age of 1 d and 28 d were observed with a Czech TESCAN MIRA LMS scanning electron microscope.
2.2.7. Zeta Potential
Using the Malvern Zetasizer Nano ZS90 instrument in the United Kingdom, the Zeta potentials of cement pastes with different retarders and water reducers were tested within 30 min.
3. Results and Discussion
3.1. Fluidity
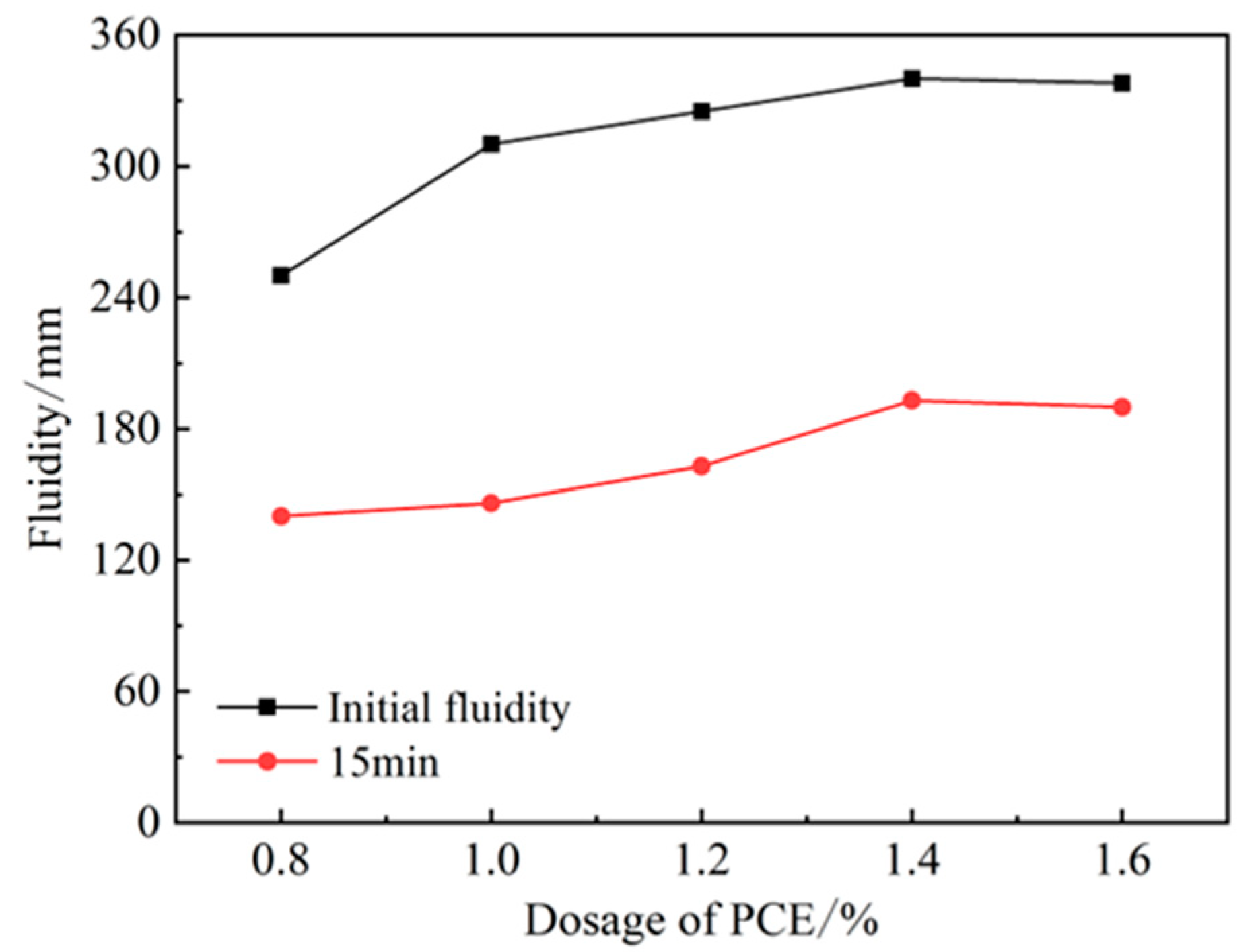
The incorporation of PCE can significantly improve the initial flow of HBCSA cement, as shown in Figure 3. The initial flow rate reached 250 mm when the PCE admixture was at 0.8%. With an increase in admixture, the initial flow rate also increased; when the admixture reached 1.0%, the flow rate increased most significantly to 310 mm. The flow rate increased slowly after the admixture of PCE exceeded 1.0%, the admixture reached adsorption saturation at 1.4%, and the flow rate did not continue to grow. From the viewpoint of the longitudinal flow rate, the flow rate of single-doping PCE has a large longitudinal loss, the flow rate at 15 min decreases by about 50%, and the flow rate disappears after 15 min. Although PCE can improve the initial fluidity of HBCSA, the loss of liquidity is high. It has been reported [26] that this is due to the high activity of HBCSA, which makes the dispersion of PCE lose quickly afterwards, and other studies reported [22] that it may be caused by ettringite produced rapidly resulting from special molecular structure of PCE and its effect of steric hindrance. Therefore, retarders can be used to overcome the rapid loss of PCE dispersion efficiency.
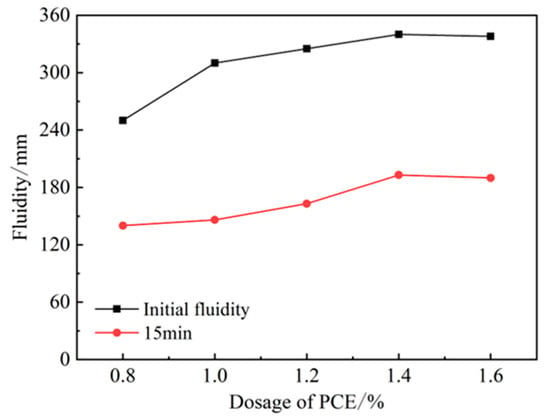
Figure 3.
Effect of PCE on fluidity of HBCSA.
3.1.1. Effect of One-Component Retarder on Fluidity of HBCSA Paste
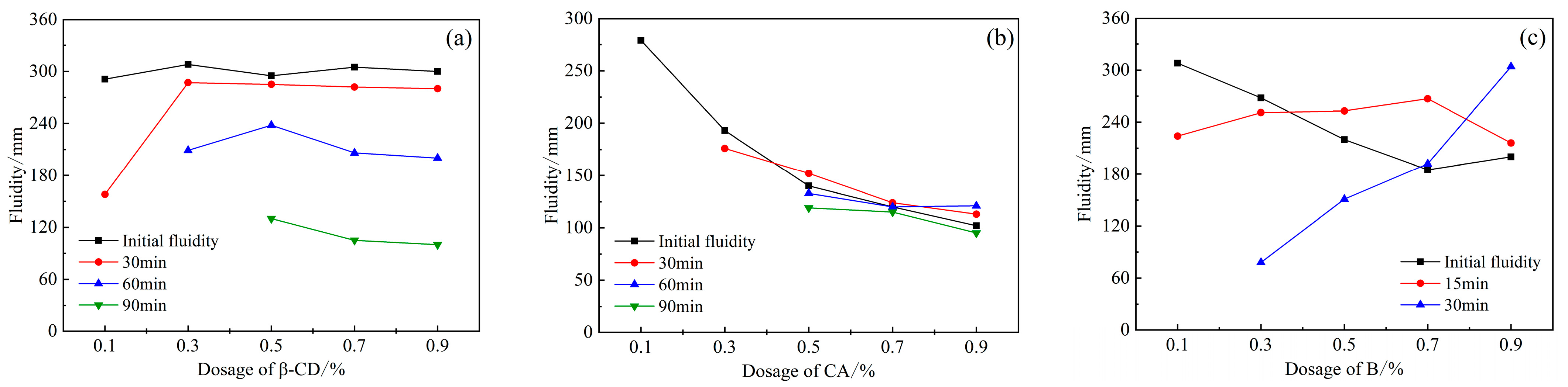
According to the above test results of single-doped PCE, the content of PCE is selected as 1.2%, and different retarders are, respectively, compounded with PCE, as shown in Figure 4. Figure 4a shows the fluidity of the cement slurry after compounding β-CD and PCE. It can be seen that the addition of β-CD and the increase in the content have little effect on the initial fluidity of HBCSA cement. When the content of β-CD is 0.1%, the fluidity decreases greatly over time, and the fluidity drops to 158 mm at 30 min and disappears after 60 min. When the dosage reaches 0.3%, the fluidity loss decreases, and the fluidity disappears after 90 min, and when the dosage is between 0.5% and 0.7%, the fluidity will gradually decrease with the increase in time, and the fluidity can still reach 90 min at about 120 mm. Second, the introduction of CA (Figure 4b) reduced the fluidity of HBCSA cement paste, and the initial fluidity decreased sharply with the increase in its content. When the content of CA is 0.1%, the initial fluidity is 279 mm, and the fluidity disappears after 30 min, and when the content of CA reaches 0.5%, the initial fluidity drops below 150 mm, but the fluidity changes little in each period, and the loss of fluidity decreased significantly with time. This is inconsistent with the conclusion in the literature [22] that the combination of CA and PCE has almost no negative effect on the fluidity of sulphoaluminate cement paste. However, with the introduction of another retarder, B (Figure 4c), compared with retarder CA, the fluidity decreased more slowly with the increase in B dosage. This is consistent with the research results on the influence of borax on the dispersion of PCE in CSA [25]. A large amount of borax dominates the competitive adsorption between B and PCE, thus reducing the adsorption of PCE and the dispersion. However, the fluidity will disappear after 30 min.
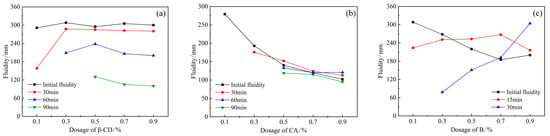
Figure 4.
Effects of PCE compounding β-CD, CA, and B on the fluidity of HB-CSA: (a) β-CD, (b) CA, and (c) B.
Therefore, under the condition of the same dosage, the initial fluidity of the cement paste mixed with β-CD is relatively large, which is similar to the initial fluidity of the single-mixed polycarboxylate superplasticizer (blank group), and with the increase in time, the liquidity decreased regularly, indicating that the liquidity retention ability was excellent. However, the initial fluidity of CA and B was reduced to some extent, and the fluidity over time would exceed the initial fluidity when the dosage was high. Since PCE improves the initial fluidity of cement paste, it is determined by the adsorption amount of PCE on the surface of cement particles [27]. However, the introduction of CA and B will affect the adsorption behavior of PCE, but with the continuous hydration of cement paste, the effect of the retarder is weakened, and the fluidity will increase.
3.1.2. Effect of Composite Retarder on Fluidity of HBCSA Paste
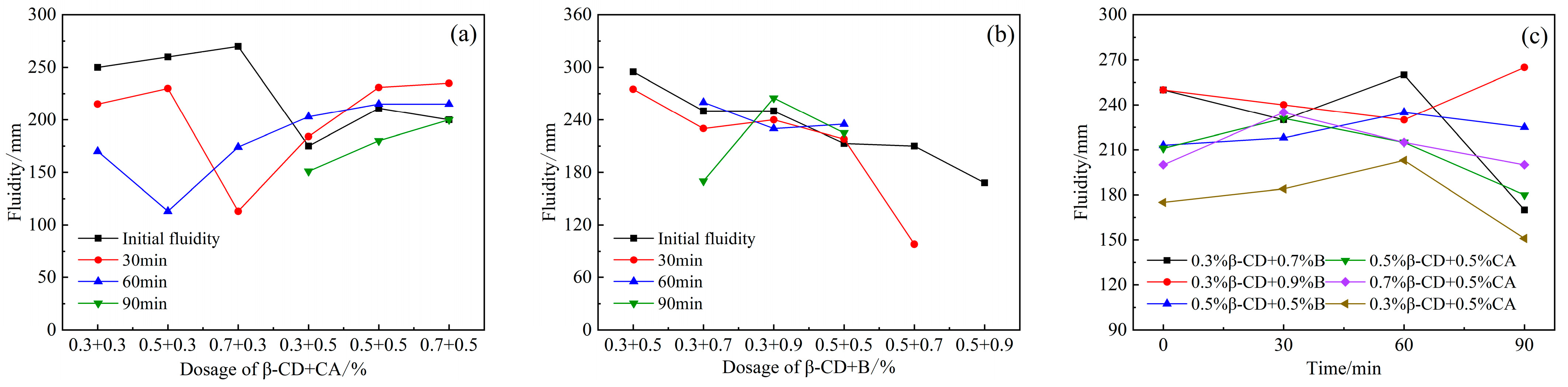
When the retarder β-CD and CA were mixed together (Figure 5a), the initial fluidity decreased to a certain extent. When the fixed content of β-CD was 0.5% and 0.7%, the mobility decreased with the increase in CA content. It can be seen that the fluidity loss over time between 0 and 90 min after the two retarders are remixed is very small. The combined dosage is 0.5% β-CD, 0.3% CA, and its overall fluidity retention ability is good. When the content of CA is 0.3%, and the content of β-CD is 0.5% and 0.7%, the initial fluidity is larger than other content, but the loss of fluidity over time from 0 to 60 min is larger, and there is no fluidity after 90 min. The combined dosage is 0.5% β- CD and 0.3% CA, no matter from the initial fluidity or the change law of the fluidity over time, are better than other dosage. Overall, the fluidity (Figure 5b) also showed a downward trend, and when the β-CD content was 0.3% and the B content was 0.5%, the initial fluidity was the highest; and the greater the B content, the more the fluidity decreased; but the decrease in fluidity over time is not obvious within 60 min. When the content of β-CD and B are both 0.5%, the fluidity over time is not much different from the initial fluidity, but with the increase in the content of B, the loss of fluidity over time increases, even disappearing after 30 min.
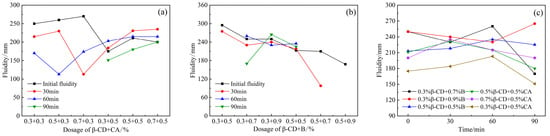
Figure 5.
Effects of PCE compound β-CD+CA and β-CD+B on the fluidity of HB-CSA: (a) β-CD+CA, (b) β-CD+B and (c) comparison of different combination dosage.
According to the results of the above-mentioned compounding of β-CD with CA and B, respectively, the combined dosage with a relatively good fluidity retention ability was selected from the two cases, as shown in Figure 5c. It can be found in the figure that the fluidity of the cement slurry within 60 min is basically higher than the initial fluidity, and even some of the combined dosages have higher fluidity than the initial fluidity at 90 min. This shows that, although the initial fluidity of the two retarders after compounding is lower than that of the blank group (single-doped PCE), the fluidity retention ability of the two compound components is relatively good, and with the inhibition of cement hydration, PCE can be continuously adsorbed on the surface of cement particles and improve its dispersing ability.
3.2. Setting Time
3.2.1. Effect of One-Component Retarder on Setting Time of HBCSA Paste
The setting time of HBCSA cement without retarder is between 17 and 21 min, and PCE mainly produces a dispersing effect in this cement but has little effect on prolonging the setting time of cement. From the setting time of the three single-component retarders (Table 2), it can be seen that the setting time of the cement paste is gradually prolonged when the content of β-CD is 0.1%. When the dosage reaches 0.3%, the retardation effect is more obvious. When the dosage reaches 0.7%, the setting time does not increase significantly, and only increases by 10% compared with the previous dosage. Some studies have shown [28] that with the increase of β-CD content, the setting time also continues to extend. In our research, when the β-CD content reaches 0.5%, the setting time does not increase significantly. So when the dosage of β-CD is 0.5%, the initial and final coagulation effect is the best. When the content of CA is as small as 0.1%, it has little effect on the setting time of HBCSA cement. When the content reaches 0.3%, the setting time increases with the increase in the content, and the maximum can be prolonged by 54 min and 79 min. However, when the content reaches 0.9%, the setting time does not show a regular increase but shows a downward trend. At a low dosage of B, the retardation effect is poor, and the retardation effect of 0.1%, 0.3%, 0.5%, and 0.7% is the same, which also shows that the change in dosage is too small and cannot prolong the setting time of HBCSA cement. When the dosage reaches 0.9%, the setting time of the initial setting and the final setting is only 14 min and 16 min longer than that of the blank group, so the retardation effect of B content on HBCSA cement is not obvious within 1%.

Table 2.
Influence of one-component retarder on setting time of HBCSA.
Therefore, when the dosage is the same and higher than 0.1%, the retardation effect of citric acid and cyclodextrin is more significant. The coagulation time can reach about 1 h. This is reflected in the fact that both β-CD and CA molecules contain a hydrophilic group hydroxyl group. Due to adsorption, β-CD can associate with water molecules through hydrogen bonds, so that a large number of solvated water films are formed on the surface of cement particles, which hinders the direct contact between cement particles and water and effectively inhibits the reaction of (C4A3). Therefore, when the dosage is the same or higher than 0.1%, the retardation effect of citric acid and cyclodextrin is more significant. The coagulation time can reach about 1 h. This is reflected in the fact that both β-CD and CA molecules contain a hydrophilic group hydroxyl group. Due to adsorption, β-CD can associate with water molecules through hydrogen bonds, so a large number of solvated water films are formed on the surface of cement particles, which hinders the direct contact between cement particles and water and effectively inhibits the reaction of (C4A3) and CaSO4, particularly having the effect of inhibiting the dissolution rate of CaSO4, thereby inhibiting the hydration of cement [28]. The B4O72− and Ca2+ in borax will generate calcium borate [25,27], but when the dosage is small, calcium borate is not enough to cover and seal the cement particles, and the cement will still be hydrated, so the retarding effect is not obvious enough.
3.2.2. Effect of Composite Component Retarder on Setting Time of HBCSA Paste
When β-CD and CA are mixed together, the time interval between the initial setting and the final setting is large, which is difficult to control. The time interval between the initial setting and final setting of the mortar after the mixing of the two was significantly shortened, and the setting time was longer than that of the single mixing (Table 3). When the content of β-CD was 0.5% and the content of CA was 0.5%, the coagulation time reached 73 min and 80 min, respectively. It can be seen that after compounding in a certain proportion to the single mixing conditions, the slow setting effect has been improved, the setting time is controlled in about 1 h, and the initial and final setting time interval can also be controlled within the time. However, the retardation effect of the composite components β-CD and B is also more obvious, and the setting time is improved compared with the single-mixed setting time, and the initial setting time is extended to about 60 min or even 90 min. When the content of β-CD is constant, the setting time will also increase with the increase in the content of B. When the content of β-CD reaches 0.9%, the retardation effect is the best, which further indicates that the smaller the B content is, the less obvious the retardation effect is.

Table 3.
Influence of composite component retarder on setting time of HBCSA.
The results from the two composite components show that the retardation effect is more obvious than that of the single retarder, which indicates that the two have a certain synergistic effect. This is also because both β-CD and CA contain hydroxyl groups, which can effectively synergize the adsorption of water molecules in the cement slurry after compounding, so that the cement hydration is slower, thereby effectively prolonging the setting time. When β-CD is added alone, the effect of inhibiting the cement hydration is remarkable. Therefore, after adding B, Ca2+ dissolved in water cannot participate in the reaction and will react with B4O72− ionized by B to generate more calcium borate and form a protective film that covers the cement particles, further preventing cement hydration.
3.3. Strength
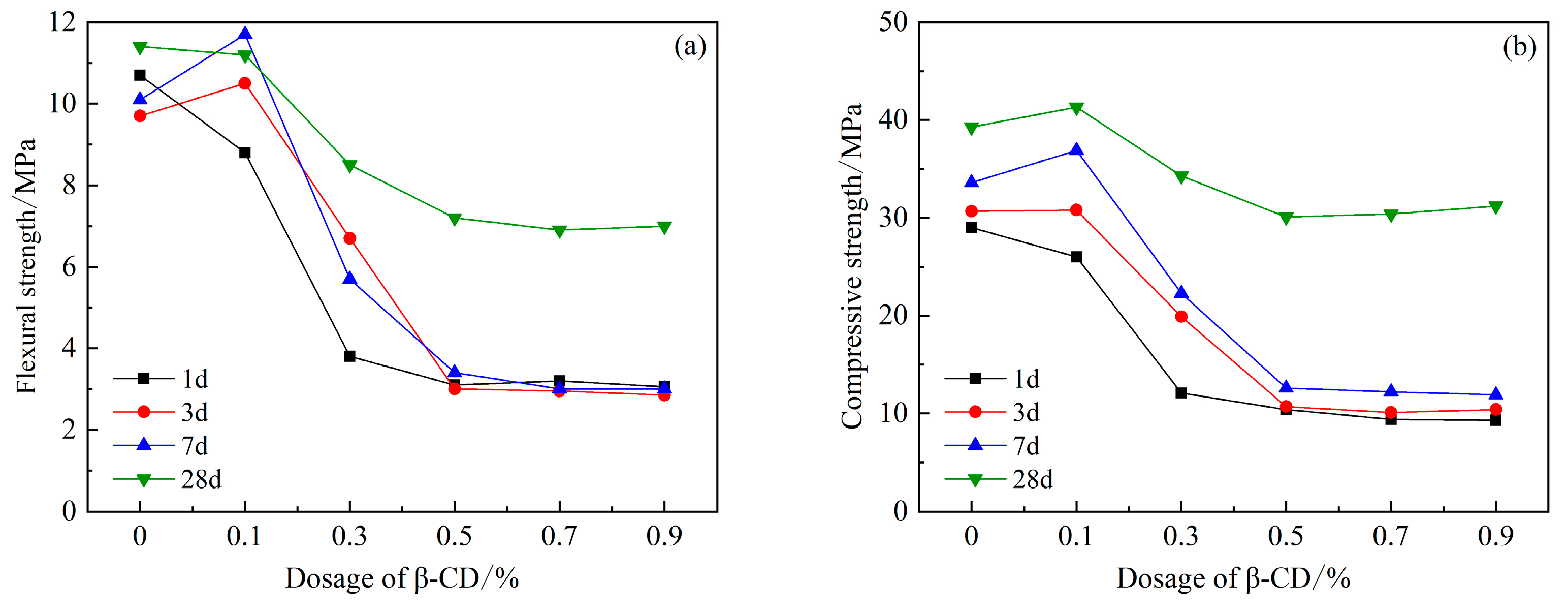
After adding polycarboxylate water reducer and compounding different dosages of β-cyclodextrin into high-belite sulphoaluminate cement, as shown in Figure 6, it can be seen that the compressive and flexural strengths of its 1 d mortar increase slightly when the content is 0.1%, and when the content reaches 0.3%, they both decrease, and the decline is larger, with a decrease of 50%. With the increase in β-CD content, the 1 d, 3 d, and 7 d intensities all showed a decreasing trend compared with the blank group. After the content of β-CD reached 0.5%, the difference between the 7 d intensity and the 1 d and 3 d intensity was small, and there was no obvious improvement. The flexural and compressive strength of 28 d was significantly improved, which indicated that the compounding of β-CD and PCE indeed reduced the early hydration rate of high-belite sulphoaluminate cement, but the later hydration rate increased rapidly. The final hydration degree is not inhibited, which may be due to the auxiliary plasticizing effect of β-CD; and the hydration products in the solution around the cement particles are more uniformly distributed, which is conducive to the sufficient hydration of the cement particles.
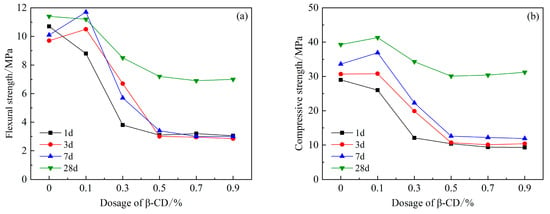
Figure 6.
Effects of PCE compound β-CD on mechanical properties of HBCSA: (a) flexural strength and (b) compressive strength.
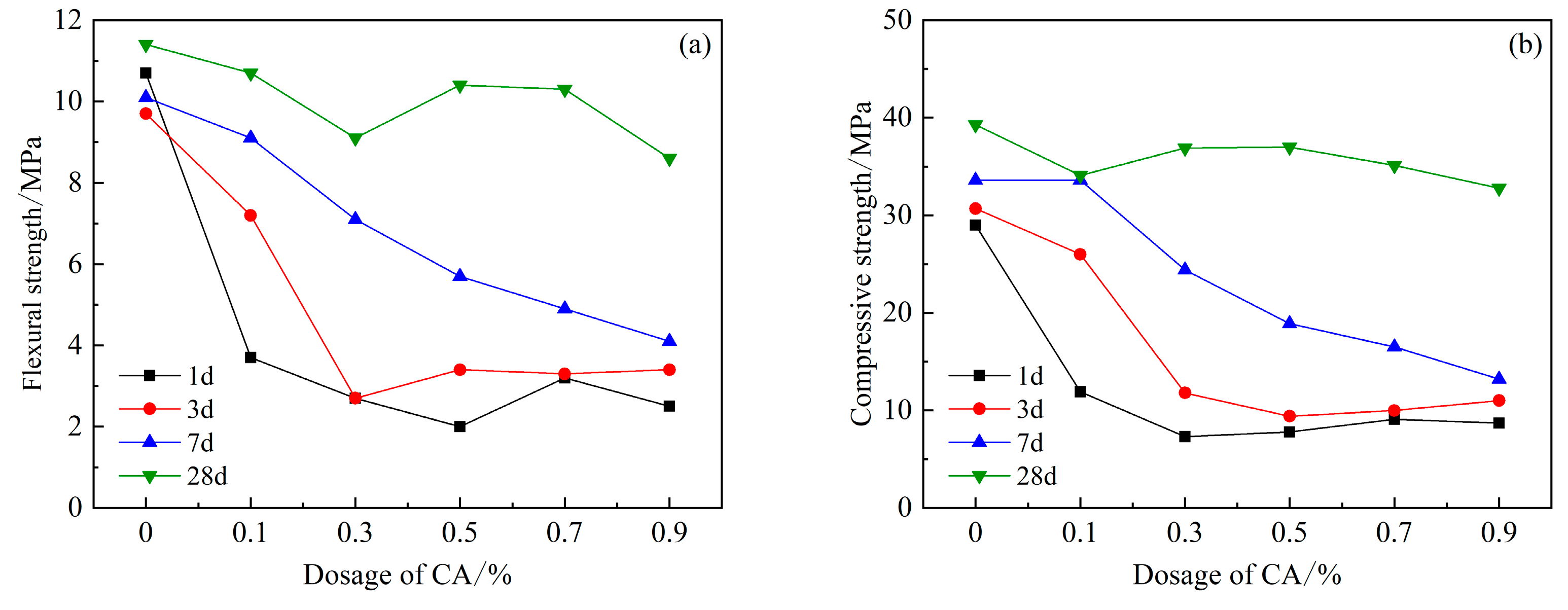
Figure 7 shows the strength of each age after adding the retarder CA. It can be clearly seen that the early strength of 1 d, 3 d, and 7 d is significantly lower than that of the blank group, and the flexural and compressive strengths decrease with the content of CA. Both showed a decreasing trend, and after the dosage reached 0.3%, the flexural and compressive strengths decreased most obviously in the first 3 d. The compressive strength of 28 d with a CA content of 0.3% and 0.5% is basically the same, and the strength is the highest compared with other content, and only decreased by 5.9% compared with the blank group.
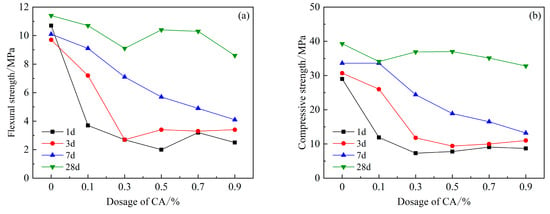
Figure 7.
Effects of PCE compound CA on mechanical properties of HBCSA: (a) flexural strength and (b) compressive strength.
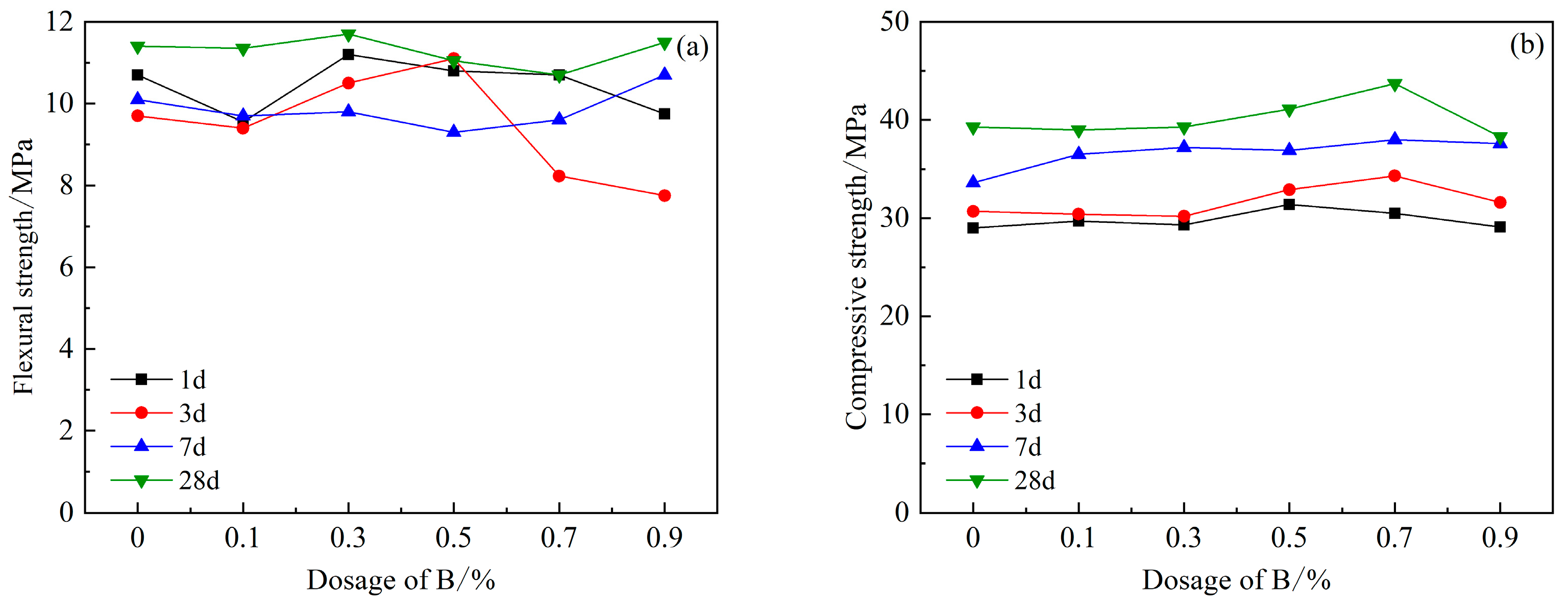
However, the introduction of retarder B, and the increase in B content, did not have an adverse effect on the strength of HBCSA cement (Figure 8), but its 3 d and 28 d flexural strengths showed an increase first and then a decreasing trend. When the content of B is 0.3%, the flexural strength of cement reaches the maximum. The dosage of B is within 0.9%. With the increase in the dosage of B, the compressive strength of cement mortar gradually increases. The compressive strength of cement is the highest when the dosage is 0.7%, and the strength value is 43.7 MPa. When the content of B exceeds 0.7%, the decreasing trend of compressive strength begins to appear at all ages. This shows that an appropriate amount of borax can effectively improve the compressive strength of cement, but too much B content will reduce the compressive strength of cement. When studying the influence of borax on the properties of CSA [27], the 1 d and 3 d compressive strength of CSA gradually increased. However, on the 28th day, the compressive strength of B increased significantly only when the content of B was 0.2%, and the other dosage was basically the same as that of the blank group. In our research, after the incorporation of B and PCE, the 28 d strength variation trend of HBCSA is basically consistent with the above studies. However, when the content of B is 0.5% and 0.7%, the strength increases linearly and is higher than that of the blank group.
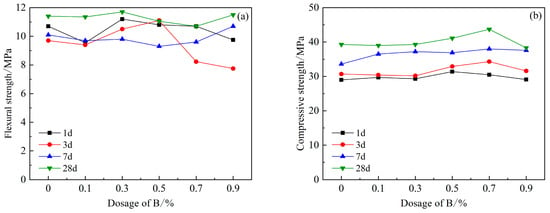
Figure 8.
Effects of PCE compound B on mechanical properties of HBCSA: (a) flexural strength and (b) compressive strength.
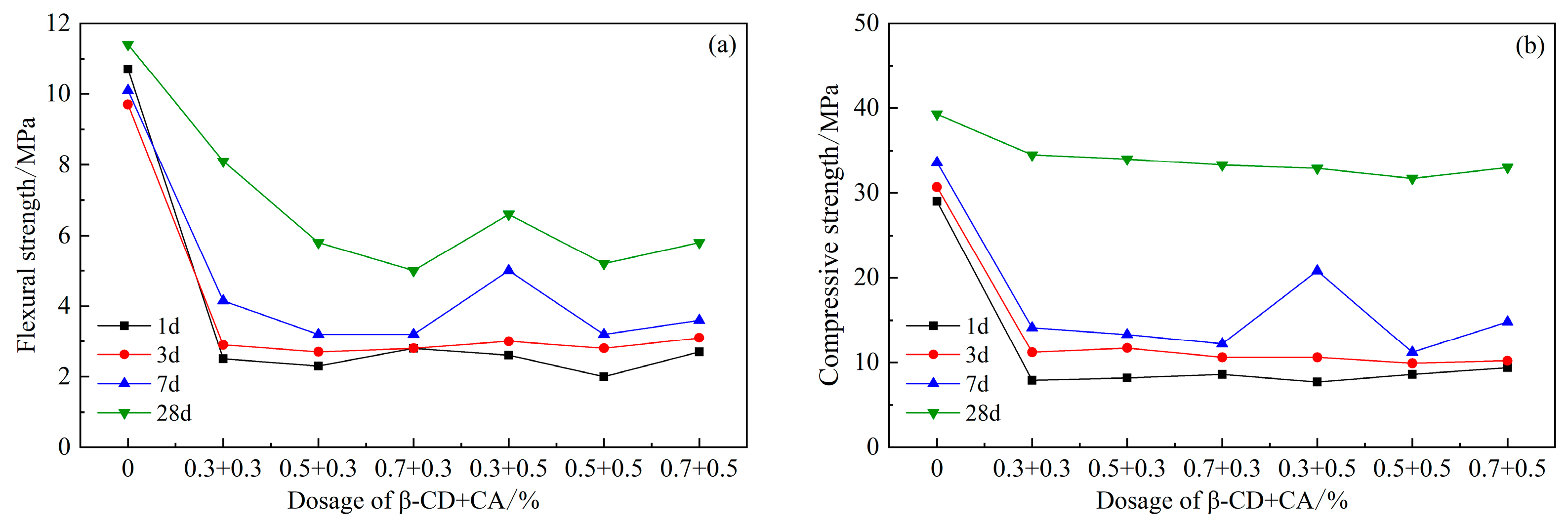
Figure 9 shows the strength of the cement mortar after compounding with β-CD and CA. It can be seen from the figure that the strength decreases after the two are mixed, and the strength does not increase significantly in the first 7 days. When the content of β-CD is 0.3% and the content of CA is 0.5%, the 7 d flexural strength increases to 3.95 MPa and the compressive strength increases to 20.8 MPa; obviously, there is still a big gap compared with the blank group. However, compared with other dosages, the strength of this dosage increased more significantly. According to the 28 d strength, when the content of β-CD and CA are both 0.3%, the flexural and compressive strengths are the highest, which are 8.1 MPa and 34.5 MPa, respectively. According to the situation that the above two retarders are added separately, with a dosage of 0.3% to 0.9%, the strength of the first 3 d is basically the same, and the strength of 7 d has increased. However, the 7 d strength of the composite of β-CD and CA was essentially the same compared to its 3 d strength. This shows that after the two retarders are mixed together, the hydration of cement is inhibited together, the retardation effect is more significant, and the hydration is slower.
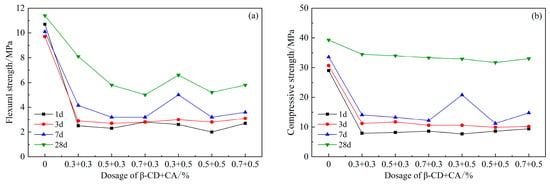
Figure 9.
Effects of PCE compound β-CD and CA on mechanical properties of HBCSA: (a) flexural strength and (b) compressive strength.
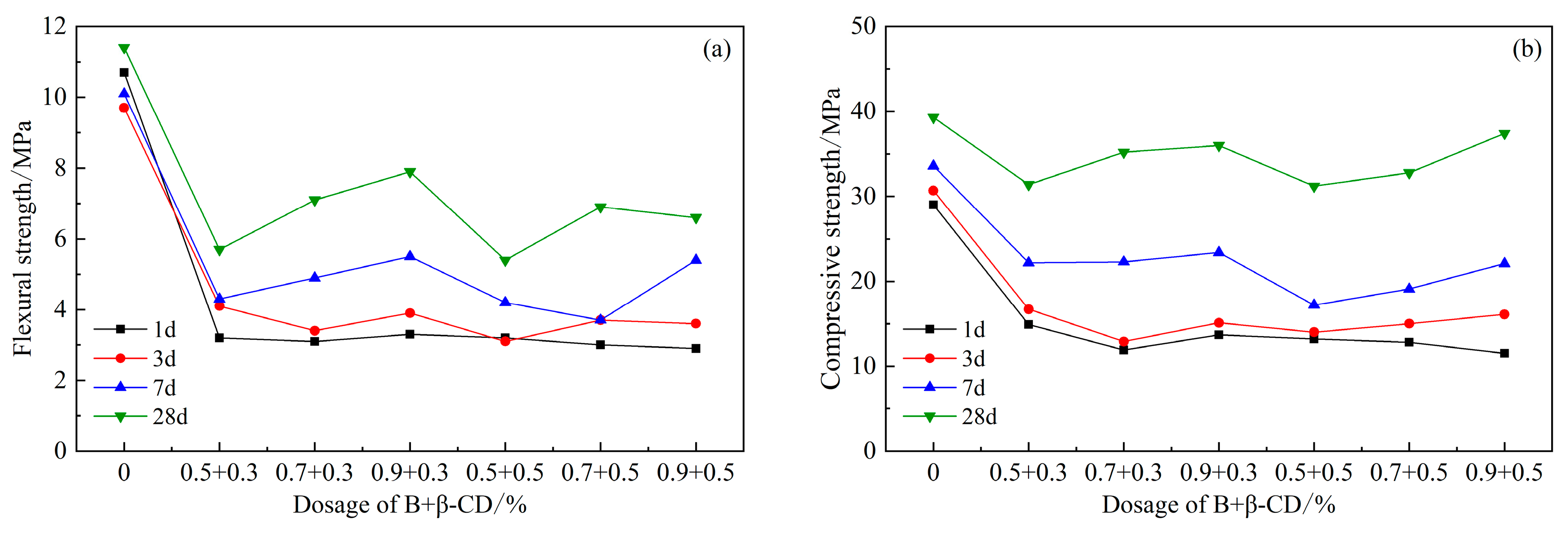
The strength of composite component B and β-CD also showed a decreasing trend compared to the blank group, as shown in Figure 10. The strength of the cementitious sand increased with the amount of β-CD, with the highest strength at 0.9% and a strength of 37.4 MPa at 28 d. The results show that the higher the amount of B, the more favorable the development of the strength of HBCSA cement within the dosing range.
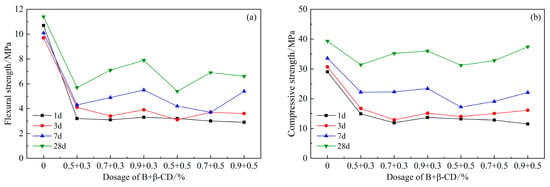
Figure 10.
Effects of PCE compound B and β-CD on mechanical properties of HBCSA: (a) flexural strength and (b) compressive strength.
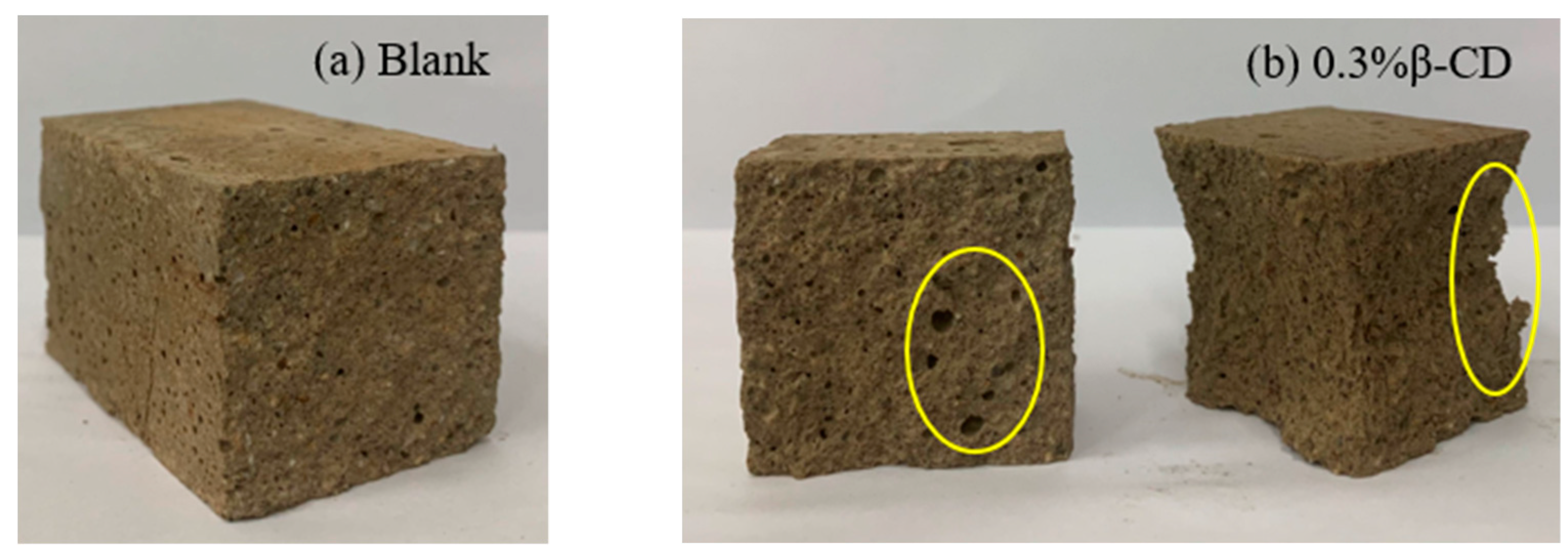
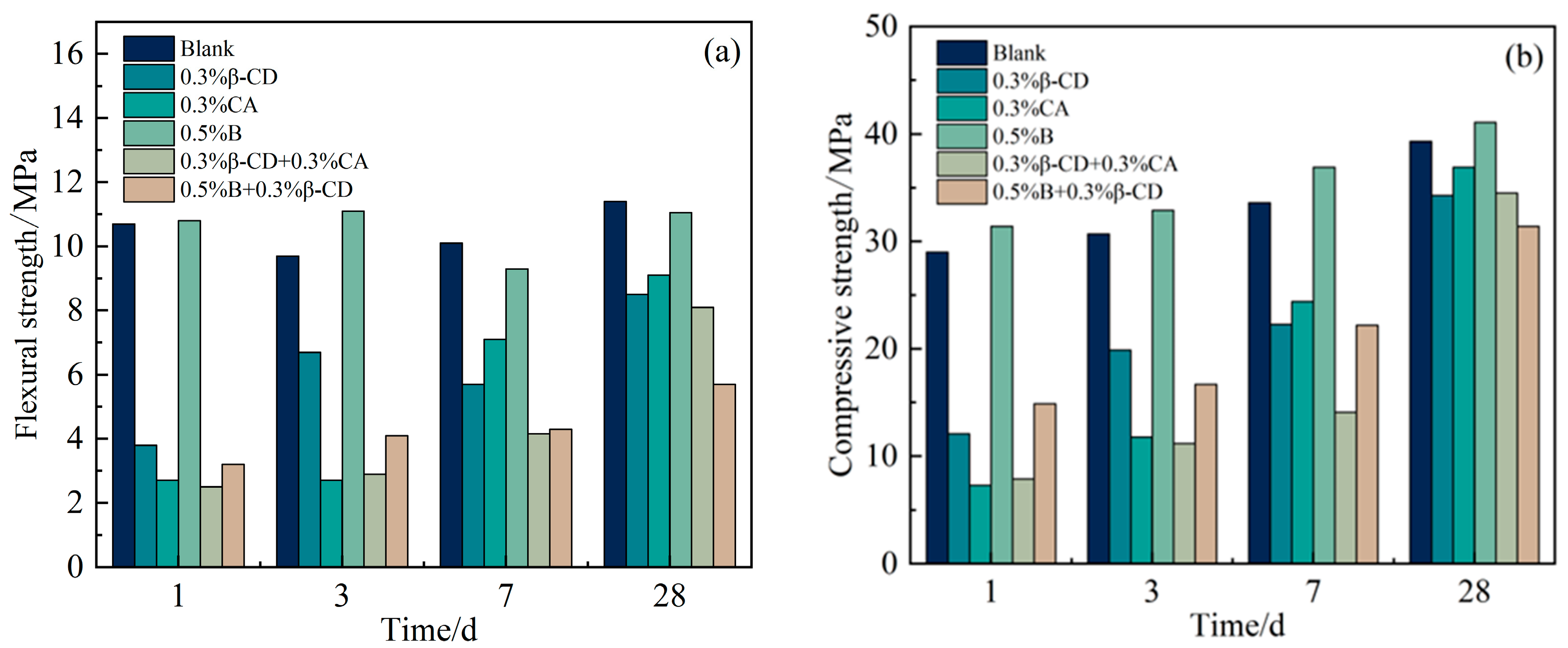
Based on the development pattern of the strength of HBCSA cementitious sand after the addition of different retarders (except for single admixture B) mentioned above, it can be found that the strength of cementitious sand will decrease substantially in the early stage. Taking Figure 11 as an example, Figure 11a shows the damage interface of the pure high-belite sulphoaluminate cement mortar sample; it can be clearly seen that the specimen has a dense structure inside the surface at 3 d, with only some tiny holes. In contrast, with the breaking interface between the flexural and compressive breaking of the cementitious sand specimen at 3 d after the addition of 0.3% β-CD to the cement (Figure 11b), it can be seen that the specimen appears to have more holes and a looser structure, and the damage is more severe after the specimen is compressed. As the strength of cement stone is increased by promoting the dissolution of cement and the precipitation of hydration products to accelerate the hydration of the slurry, however, when the retarder is added, the rate of formation of hydration products is slow and inconsistent, the distribution is not uniform, and the bond between the layers is poor when stacked closely, resulting in a loose structure and slow strength development of the cementitious sand specimen. When these retarders have a good retarding effect, they inhibit the hydration of the cement, and the strength at all ages is lower than that of the blank group, meaning that the flexural strength of the cementitious sand decreases sharply. According to the above, from each retarder in the selection of the content and the blank group strength for comparison (Figure 12a), it is clear that in addition to 0.5% B and the blank group of flexural strength difference not being large, the other retarder components of the flexural strength decline are more obvious, and with the development of age, the strength growth is not obvious, especially after the retarders are mixed in pairs, at the age of 28 d. The difference between the flexural strength of the retarder and the blank group was also large. By observing the compressive strength of each retarder component (Figure 12b), it is clear that the early strength decrease is also more obvious, but with the growth of age, the compressive strength increases regularly. The results showed that the introduction of retarders β-CD and CA had more obvious adverse effects on the flexural strength of HBCSA cement mortar without regularity.
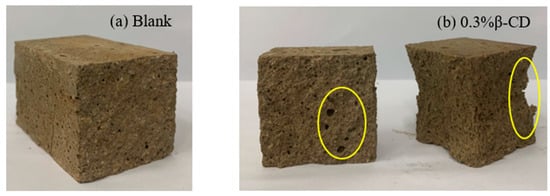
Figure 11.
Damage interface of cement mortar test blocks at 3 d: (a) Blank and (b) 0.3% β-CD (yellow circles represent pores and loose parts).
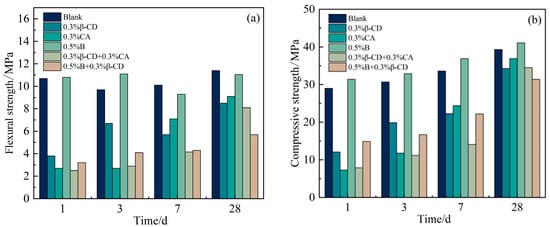
Figure 12.
Strength of cement mortar test blocks with different retarder components at various ages: (a) flexural strength and (b) compressive strength.
3.4. XRD Analysis
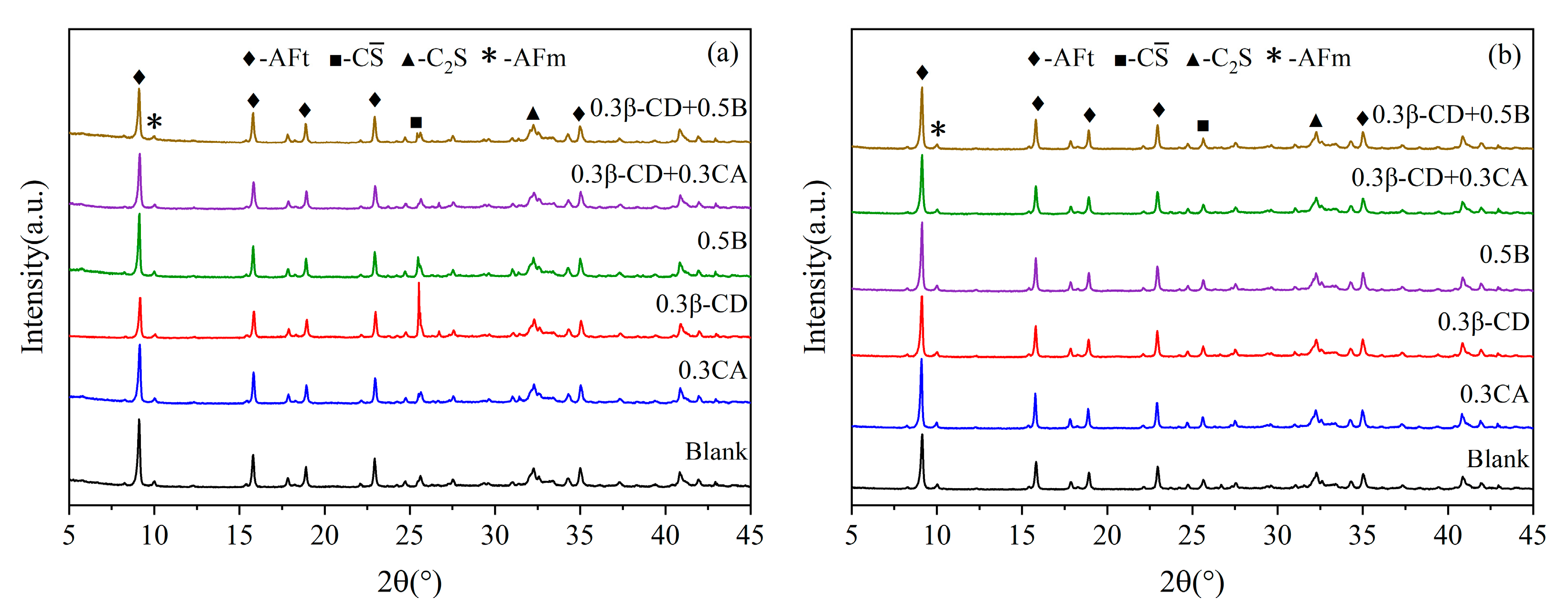
In combination with the results of the macroscopic performance tests described above, five doses were selected from the three retarders that were overall superior (0.3% β-CD, 0.3% CA, 0.5% B, 0.3% β-CD + 0.3% CA, and 0.3% β-CD + 0.5% B); in order to further study the effect of different retarder and water-reducing agent compounding on the hydration products of HBCSA, the XRD analysis was carried out. Figure 13 shows the XRD patterns of different retarders and cement pastes hydrated for 1 d and 28 d, and ettringite AFt is the main hydration product. Figure 13a shows the XRD patterns of different retarders at an age of 1 d, and when the diffraction angle is about 8°, the diffraction intensity of AFt is the highest. The components are all reduced to varying degrees, and there is also unreacted C (diffraction angle of about 26°), dicalcium silicate (diffraction angle of about 32°), and AFm with lower diffraction peaks (diffraction angle of about 10°). The samples mixed with 0.3% CA have a higher diffraction intensity of gypsum, indicating that CA has a strong inhibitory ability on cement hydration. However, the diffraction peaks of ettringite of the samples mixed with 0.3% β-CD and 0.5% B are more obvious than those of CA, and the diffraction peaks of gypsum are also reduced, which also shows that the two retarders have little effect on the hydration rate of cement. When the three retarders are compounded in pairs, the diffraction peaks of the hydrated product ettringite are weaker than those of single-doped β-CD and B, but compared with single-doped CA, when β-CD and CA are compounded, the diffraction peaks of ettringite are somewhat stronger. With the increase in the hydration time, when the hydration age reaches 28 d, gypsum and ye’elimite are further consumed, and ettringite is continuously formed, the position and shape of its characteristic diffraction peaks have not changed. Also, the diffraction peaks of C and C2S have not changed. The hydration degrees of the blank group and the blended retarder were basically the same, and there was no significant difference in the diffraction peak intensity of ettringite. It can also be seen that the intensity and position of the diffraction peaks of AFm and C2S did not change at 1d and 28d, and in different retarders. To sum up, the addition of a retarder significantly weakens the hydration of cement but does not change the types of HBCSA cement hydration products.
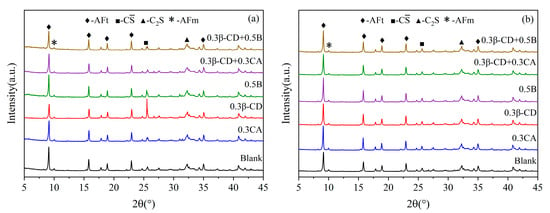
Figure 13.
XRD patterns of hydration products of cement paste hydrated for 1 d and 28 d: (a) 1 d and (b) 28 d.
3.5. SEM Analysis
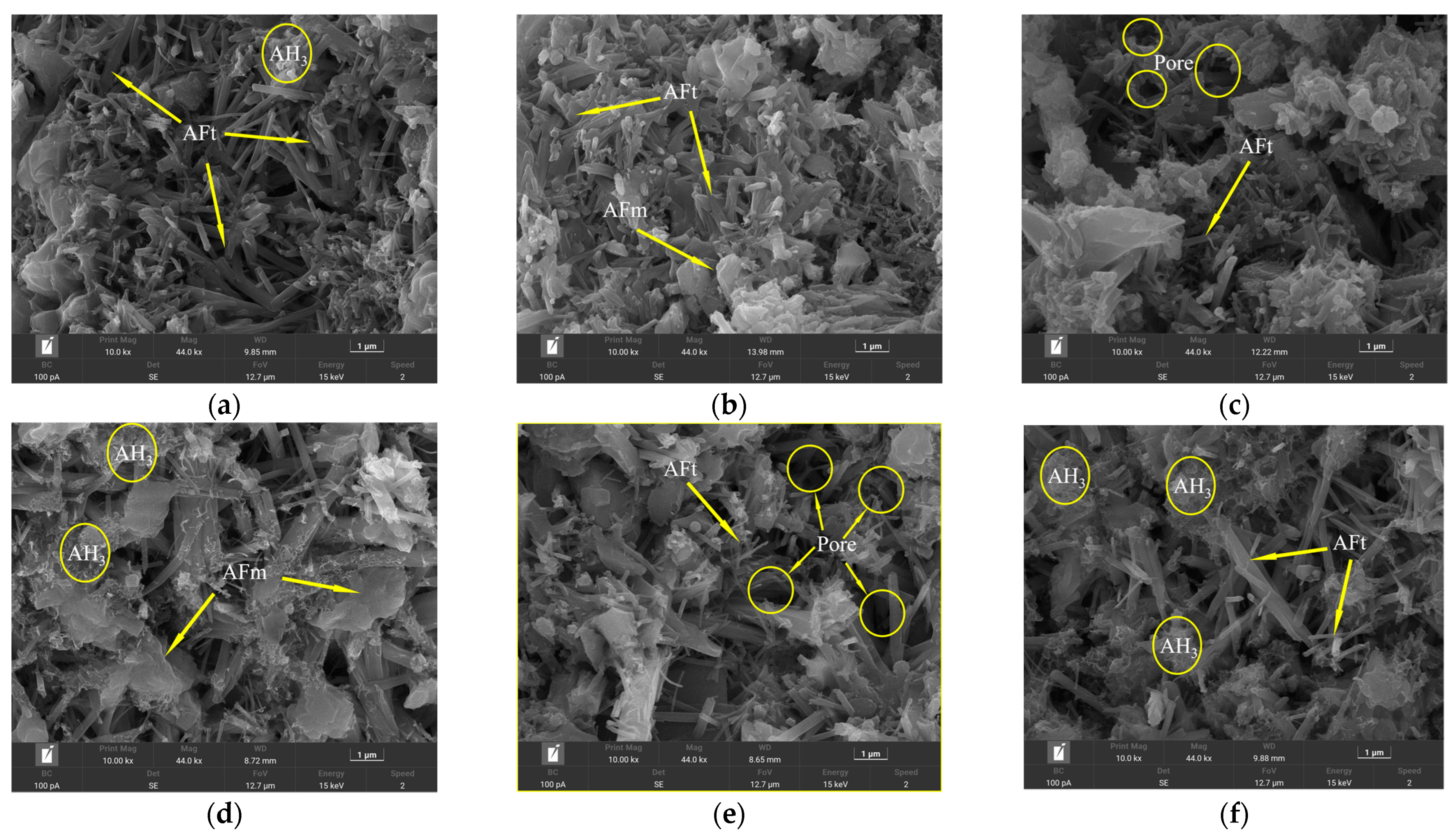
Due to the bonding and interlocking effects between ettringite crystals [29], it has a great contribution to the early strength of cement. Therefore, the dosage consistent with the above XRD test was selected, and the SEM morphology of different retarders at 1 d and 28 d was observed (Figure 14 and Figure 15). It can be seen that most of the AFt generated by the blank group (Figure 14a) has a slender rod-like structure with a staggered distribution, and a small amount of AH3 gel around it adheres to the AFt, which makes the structure relatively dense. After the incorporation of CA (Figure 14b), the development of ettringite 1 d was relatively slow, and the shape of the needle bar was not obvious enough. However, the number of ettringites formed by incorporating β-CD was significantly reduced, and plate-like AFm appeared, with a sparse distribution and more pores. This shows that the formation rate and development of ettringite are delayed after the addition of CA and β-CD, which also verifies the conclusion that the above two will be detrimental to the early strength development of cement. It can be seen from Figure 14d that, after adding B, compared with the blank group, the hydrated product ettringite has a thick and short shape, and the generated AH gel is significantly increased, and filled in the pores, making the structure more stable due to an increase in density. This shows that the incorporation of borax improves the early strength of HBCSA and further indicates that borax cannot effectively inhibit the hydration of HBCSA. It can be seen from Figure 14e,f that after the remixing of β-CD and CA retarders, the ettringite morphology is mostly thin and short, and it is attached to the unhydrated cement particles, so the structure is relatively loose; after the cyclodextrin and borax are mixed together, some ettringites are needle-shaped, and some are short and thin, and AH3 gel is distributed around. After the two retarders are re-mixed, the dosage increases and the two have a synergistic retardation effect, so the amount of ettringite generated is significantly lower than that of single mixing, which will make the early hydration rate of HBCSA cement slower.
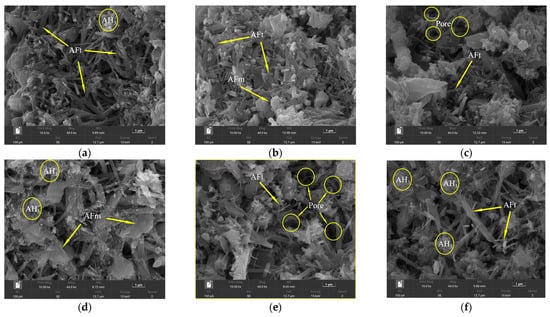
Figure 14.
SEM morphology of cement paste test block at 1 d: (a) blank; (b) CA; (c) β-CD; (d) B; (e) β-CD+ CA; (f) β-CD+ B.
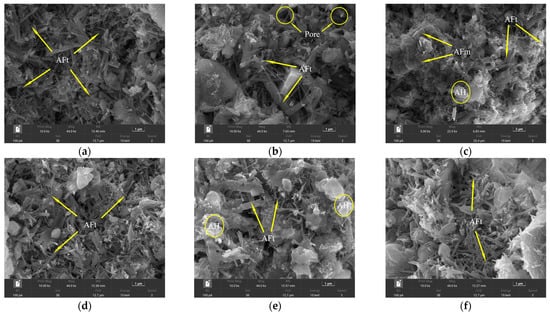
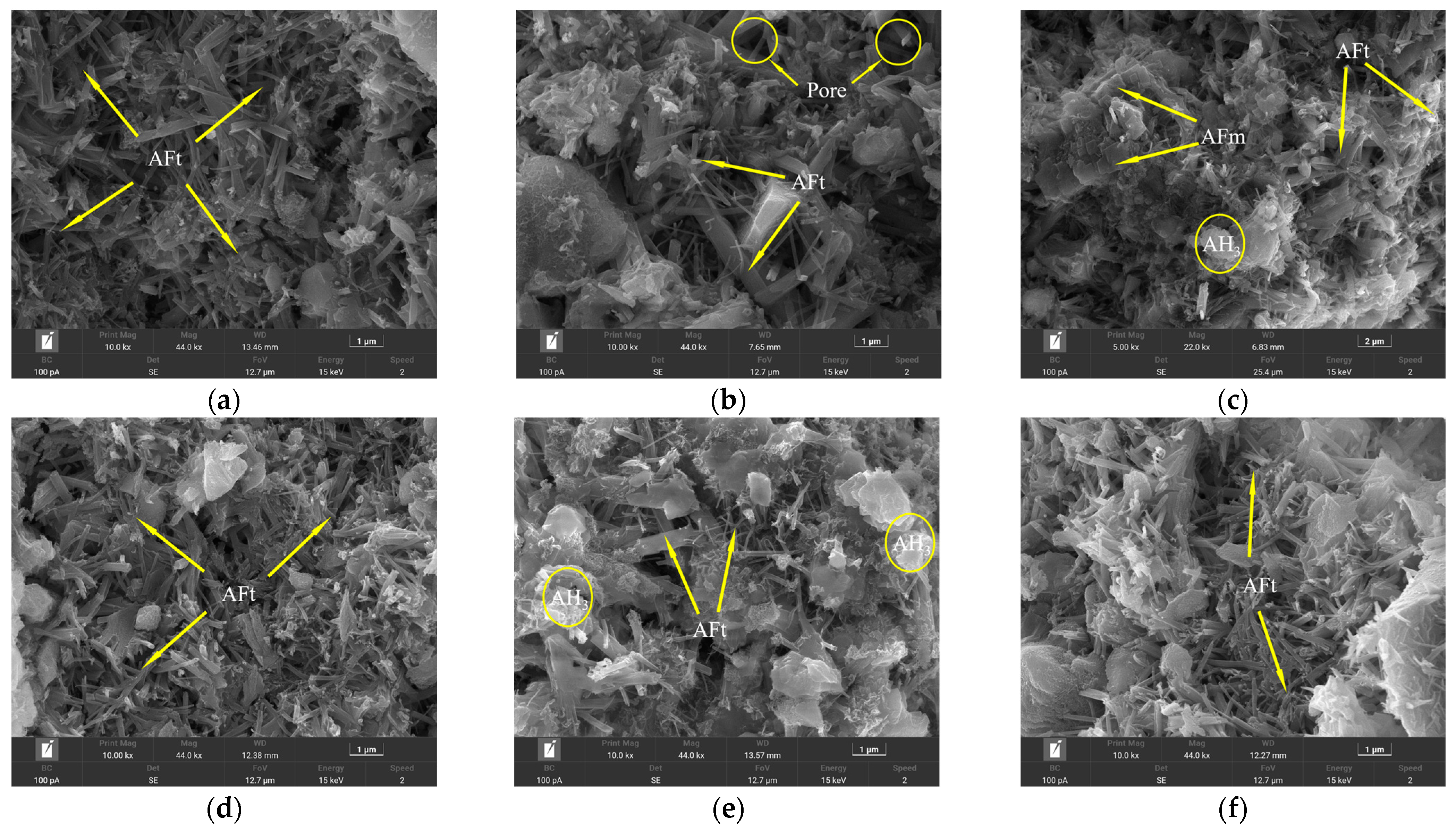
Figure 15.
SEM morphology of cement paste test block at 28 d: (a) blank; (b) CA; (c) β-CD; (d) B; (e) β-CD+CA; (f) β-CD+B.
By observing the SEM morphology of 28 d, it can be seen that the blank group (Figure 15a) has shown a large amount of hydration product AFt at 28 d, and compared with the morphology of 1 d, the grain size is obviously refined and radially grown. The hydration products can be tightly connected. After the incorporation of CA (Figure 15b), thick rod-shaped ettringite was formed, and the growth of small ettringite in the figure was inhibited. Some rods were stacked together, and the gap between the two rods was large, which also caused the structure to be looser. Similarly, with the addition of β-CD (Figure 15c), the resulting ettringite has an uneven morphology and a small amount of AH3 gel. Therefore, the better retardation effect of the retarders CA and β-CD can effectively inhibit the hydration of cement, so that the hydration of cement is insufficient, and the number of ettringites of hydration products is reduced, thereby reducing the strength development of cement mortar. In addition, the addition of B (Figure 15d) does not affect the formation of hydration products and has a certain promotion effect on cement hydration. The morphology of ettringite is relatively consistent, which is similar to that of the blank group, and the structure is compact. However, in these three samples, it can also be clearly seen that petal-like AFm is formed and interspersed in ettringite. Since AFt and AFm are unstable phases, they can be converted into each other under certain conditions. When the solution is insufficient in sulfate and calcium ions, AFt can be naturally converted into AFm [30], which also shows that the addition of a retarder may consume sulfate and calcium ions in the cement paste and promote the generation of AFm.
After mixing the three retarders in pairs, it can be seen that the number of fine and small ettringites in Figure 15e increases, and β-CD improves the dispersibility of cement particles and cooperates with CA to delay the cement hydration, so the ettringite is more dispersed in its position, the interwoven structure is not obvious, and the structure is relatively loose. However, in the re-doping of β-CD and B (Figure 15f), ettringite is closely connected in the middle, but the amount of ettringite is reduced, making the overall structure less uniform.
3.6. Zeta Potential
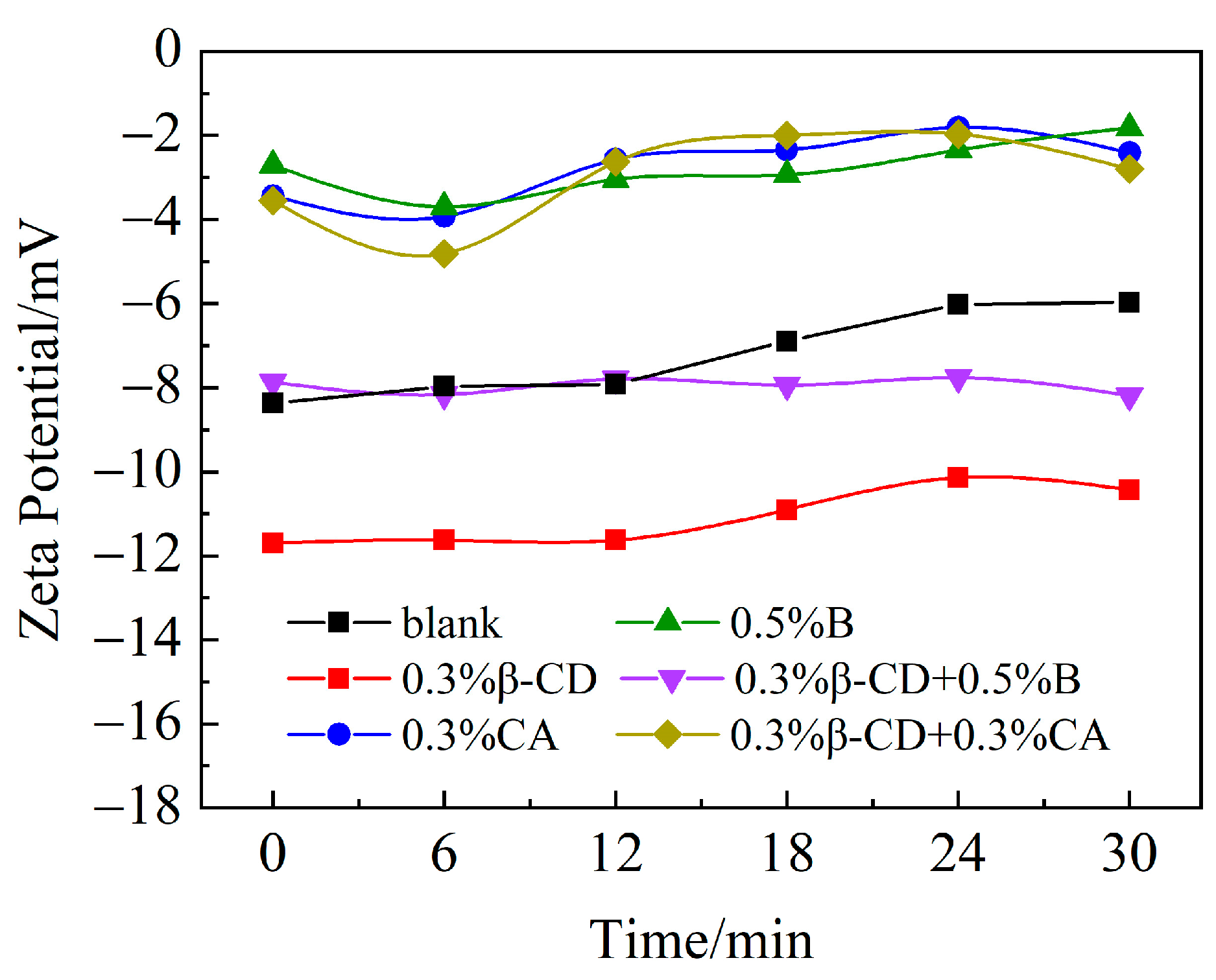
The dispersibility of polycarboxylate superplasticizers is influenced by their structural parameters such as charge density, side-chain length, and functional group composition. PCE with shorter side chains and a lower side-chain density will exhibit a higher anionic charge density, resulting in a higher zeta potential [31]. According to the previous results of the flow tests after different retarders were added to the cement slurry in combination with PCE, the addition of retarders affected the dispersion of PCE in the cement slurry. Figure 16 shows the zeta potential at 30 min after different retarders are added to the cement slurry. It can be seen that the zeta potential (absolute value) of PCE alone is around 8 mv, which is due to the fact that after PCE is added, the main chain of the water-reducing agent containing negative electric groups will be adsorbed on the surface of the cement particles, making the surface of the cement particles negatively charged, while the cement particles themselves are also negatively charged and will repel each other, and gradually, the dispersion of the cement paste will be improved. However, the zeta potential (absolute value) decreases with time, indicating that the PCE alone does not improve the flow loss of the cement paste significantly. After incorporating the different retarders, the Zeta potential of the cement paste with 0.3% CA and 0.5% B decreases and remains unchanged for the cement paste with time, indicating that there is strong competitive adsorption of CA and B, which enables the dispersion of the cement paste to remain stable within 30 min due to the delayed hydration of the cement. After 0.3% of β-CD was incorporated, the absolute value of the zeta potential of cement slurry was about 11 mv, which increased the adsorption on the surface of cement particles and effectively improved the dispersibility of cement particles. After compounding its retarder, it is clear that after compounding β-CD and B, β-CD improves the competitive adsorption with PCE produced when B is mixed alone and provides a negative charge effect. In this way, its absolute potential is basically the same as that of the water-reducing agent alone, and the potential remains stable within 30 min. It may also be due to the reaction of B with Ca2+ first to produce calcium borate [25], which makes the cement hydration process involved in free water increase and the fluidity of the cement paste is enhanced, then the zeta potential increases. After the compounding of β-CD and CA, the potential only increased by 1–2 mv within 12 min compared to that of CA alone, and the Zeta potential after 12 min was basically the same as that of CA alone. The results indicate that the more the retarder and polycarboxylate superplasticizer are adsorbed on the surface of the cement particles, the greater the inter-particle repulsion and the greater the fluidity of the cement paste [32], which is also in general agreement with the test results of fluidity.
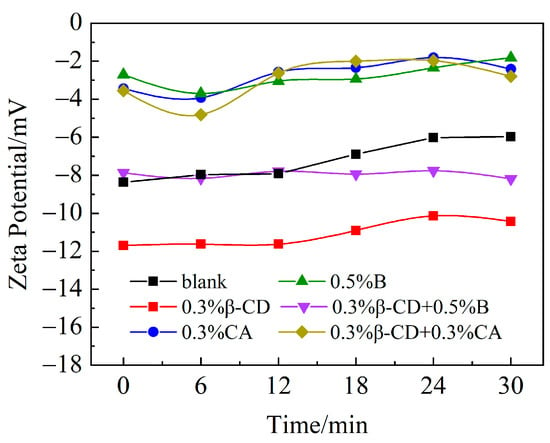
Figure 16.
Zeta potential of different retarders and cement paste hydration within 30 min.
4. Conclusions
- (1)
- Among the three retarders, after compounding β-CD and PCE, the initial fluidity of the HBCSA paste is not much different from that when PCE is used alone, and the dispersion performance is relatively stable. For the incorporation of CA or B, the initial fluidity and Zeta potential of the cement paste decreased due to the competitive adsorption with PCE. When CA and B were, respectively, mixed with β-CD, the fluidity was improved. The addition of a retarder improves the fluidity loss and makes the cement paste plastic within a certain period of time.
- (2)
- The three retarders have different degrees of retardation effect on the HBCSA paste, and after the two retarders are mixed together (β-CD+CA, β-CD+B), the retardation effect is more obvious. When B is added alone, the maximum setting time is only 31min and 37min. Compared with the other two retarders, the setting retardation effect is not obvious enough, but after the synergistic effect with β-CD, the setting time is prolonged.
- (3)
- Retarders β-CD and CA, whether they are single-mixed or mixed together, sustainability reduce the early strength of HBCSA. When B is added alone, it not only improves the early strength of the cement paste but also does not affect the long-term mechanical properties of the cement. When it is mixed with β-CD, it reduces the early strength of cement. However, with the increase of age, the strength of cement motor grows gradually, and it has a more positive effect on the strength of cement.
- (4)
- Through the analysis of XRD and SEM microscopic tests, it is further verified that the combination of retarder and PCE will delay the early hydration of HBCSA, and the development of hydration products will be slow, thus changing the morphology of hydration products, but not changing the type of hydration products.
- (5)
- The Zeta potential of the HBCSA paste within 30 min after adding different retarders was compared. The addition of β-CD will increase the Zeta potential (absolute value). After the addition of β-CD and B, the Zeta potential of the cement paste is basically the same as that of the blank group, and the Zeta potential of other retarder components is decreased, which further characterized that the addition of the retarder would affect the dispersion performance of PCE in the cement paste.
In view of the results, it is feasible to mix the two retarders, β-CD+CA or β-CD+B, to improve the workability of HBCSA. They can effectively inhibit or delayed the initial formation of ettringite. However, considering the intensity development, the combination of β-CD+B is more appropriate. The dosage of β-CD can be 0.3–0.5%, and that of B can be 0.7–0.9%. At the same time, the setting time and fluidity of HBCSA can be regulated by adjusting the amount ofβ-CD in the composite admixture. Further studies will focus on the correlation between the effect of compounding retarders on macroscopic properties and microstructure development on HBCSA, to modify the properties of HBCSA products applied in engineering.
Author Contributions
Conceptualization, H.J. and M.G.; methodology, H.J. and M.G.; validation and formal analysis, M.X.; data curation, M.L. and S.D.; writing—original draft preparation, M.X.; writing—review and editing, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key R&D and Promotion Special Program of Henan Province (Research in Science and Technology) (No. 222102320092) and the Innovative Funds Plan of Henan University of Technology (No. 2021ZKCJ17).
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Tangshan Polar Bear Building Materials Co., Ltd. (Tangshan, China) for the high-belite sulphoaluminate cement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, J.; Huang, J.; Yu, C.; Cui, C. Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis. Appl. Sci. 2021, 11, 8015. [Google Scholar] [CrossRef]
- Chan, M.; Masrom, M.A.N.; Yasin, S.S. Selection of Low-Carbon Building Materials in Construction Projects: Construction Professionals’ Perspectives. Buildings 2022, 12, 486. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. C4A3 hydration in different alkaline media. Cem. Concr. Res. 2013, 46, 41–49. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Xu, L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 80 °C. Cem. Concr. Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Feng, W.; Xiong, Z.; Zhang, G. Effects of temperature on performances and hydration process of sulphoaluminate cement-based dual liquid grouting material and its mechanisms. J. Therm. Anal. Calorim. 2020, 139, 47–56. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Mao, Y.; Shen, X. Synthesis and characterization of high belite sulfoaluminate cement through rich alumina fly ash and desulfurization gypsum. Ceramics - Silikáty 2013, 57, 7–13. [Google Scholar]
- Beltagui, H.; Jen, G.; Whittaker, M.; Imbabi, M.S. The influence of variable gypsum and water content on the strength and hydration of a belite-calcium sulphoaluminate cement. Adv. Appl. Ceram. 2017, 116, 199–206. [Google Scholar] [CrossRef]
- Su, D.; Yue, G.; Li, Q.; Guo, Y.; Gao, S.; Wang, L. Research on the Preparation and Properties of High Belite Sulphoaluminate Cement (HBSAC) Based on Various Industrial Solid Wastes. Materials 2019, 12, 1510. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Lu, Z.; Ng, S.; Niu, Y.; Jiang, J.; Xu, Y.; Lai, Z.; Liu, H. The Role of Brownmillerite in Preparation of High-Belite Sulfoaluminate Cement Clinker. Appl. Sci. 2022, 12, 4980. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.-Z.; Yue, G.; Li, Q.; Ling, T.-C. Synthesis of high belite sulfoaluminate cement with high volume of mixed solid wastes. Cem. Concr. Res. 2022, 158, 106845. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Haha, B.M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Telesca, A.; Matschei, T.; Marroccoli, M. Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes. Appl. Sci. 2020, 10, 8650. [Google Scholar] [CrossRef]
- Tang, R.; Wang, Z.; Lan, M.; Chen, Z. Effects of Retarders on Hydration and Properties of High-Belite Calcium Sulphoaluminate Cement. Bull. Chin. Ceram. Soc. 2020, 39, 3763–3769. (In Chinese) [Google Scholar]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Ben Haha, M. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, Z.; Wang, D. Influence of citric acid and sodium gluconate on hydration of calcium sulfoaluminate cement at various temperatures. Constr. Build. Mater. 2020, 263, 120247. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Coppola, L.; Coffetti, D.; Candamano, S. Tartaric acid effects on hydration development and physico-mechanical properties of blended calcium sulphoaluminate cements. Cem. Concr. Compos. 2021, 124, 104275. [Google Scholar] [CrossRef]
- Tian, H.; Kong, X.; Cui, Y.; Wang, Q.; Wang, D. Effects of polycarboxylate superplasticizers on fluidity and early hydration in sulfoaluminate cement system. Constr. Build. Mater. 2019, 228, 116711. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Li, G.; Tang, S.; Niu, M.; Wu, Y. Effect of Naphthalene-Based Superplasticizer and Polycarboxylic Acid Superplasticizer on the Properties of Sulfoaluminate Cement. Materials 2021, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, G.; Li, Y. Effects of superplasticizers and retarders on the fluidity and strength of sulphoaluminate cement. Constr. Build. Mater. 2016, 126, 44–54. [Google Scholar] [CrossRef]
- Plank, J.; Winter, C. Competitive adsorption between superplasticizer and retarder molecules on mineral binder surface. Cem. Concr. Res. 2008, 38, 599–605. [Google Scholar] [CrossRef]
- Belhadi, R.; Govin, A.; Grosseau, P. Influence of polycarboxylate superplasticizer, citric acid and their combination on the hydration and workability of calcium sulfoaluminate cement. Cem. Concr. Res. 2021, 147, 106513. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Zou, F.; Jian, S.; Ma, B.; Zhi, Z. Effect of borax on rheology of calcium sulphoaluminate cement paste in the presence of polycarboxylate superplasticizer. Constr. Build. Mater. 2017, 139, 277–285. [Google Scholar] [CrossRef]
- Xun, W.; Wu, C.; Li, J.; Yang, C.; Leng, X.; Xin, D.; Li, Y. Effect of Functional Polycarboxylic Acid Superplasticizers on Mechanical and Rheological Properties of Cement Paste and Mortar. Appl. Sci. 2020, 10, 5418. [Google Scholar] [CrossRef]
- Yu, G.; Yi, L.; Zhuo, J. Effect of Borax on the Hydration Behavior of Sulphoaluminate Cement. Bull. Chin. Ceram. Soc. 2016, 35, 3720–3723. (In Chinese) [Google Scholar]
- Tang, R.; Wang, Z.; He, H.; Zhang, L.; Cai, Y.; Wang, J. Effects of Polycarboxylate with β-Cyclodextrin on Properties of High Belite Calcium Sulphoaluminate Cement. Mater. Rev. 2018, 32, 4000–4005. (In Chinese) [Google Scholar]
- Yu, J.; Qian, J.; Tang, J.; Ji, Z.; Fan, Y. Effect of ettringite seed crystals on the properties of calcium sulphoaluminate cement. Constr. Build. Mater. 2019, 207, 249–257. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, H.; Li, Z.; Chen, E.; Shao, H. Hydration stage identification and phase transformation of calcium sulfoaluminate cement at early age. Constr. Build. Mater. 2015, 75, 11–18. [Google Scholar] [CrossRef]
- Liu, M.; Lei, J.; Bi, Y.; Du, X.; Zhao, Q.; Zhang, X. Preparation of polycarboxylate-based superplasticizer and its effects on zeta potential and rheological property of cement paste. J. Wuhan Univ. Technol. Sci. Ed. 2015, 30, 1008–1012. [Google Scholar] [CrossRef]
- Srinivasan, S.; Barbhuiya, S.; Charan, D.; Pandey, S. Characterising cement-superplasticiser interaction using zeta potential measurements. Constr. Build. Mater. 2010, 24, 2517–2521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).