Parametric Design of a Finger Rehabilitation Mechanism with Double Action and Two Degrees of Freedom
Abstract
1. Introduction
- Saeboflex [12], which uses springs anchored at the fingertips to produce extension.
- Mobile Digit Assistive System, MIDAS [13], which has adjustable cams that allow for different assistive forces with springs.
- HANDEXOS [14], a cable-driven mechanism actuated by a DC motor.
- Hand-Spring Operated Movement Enhancer (HandSOME) [15], with adjustable spring anchor points and mechanical stops to limit the Range of Motion (ROM) depending on the patient’s needs.
- The ROM for each finger articulation is increased compared to the prototypes proposed in the current literature by using personalized four-bar mechanisms and cams with virtual centers.
- Quickly personalizing finger rehabilitation devices that suit each patient’s need through a parametric model will provide a unique work area for each individual.
- The mechanism is non-invasive, having very little interference with the finger’s natural movements by placing almost every part of the mechanism above the fingers and avoiding the use of physical hinges located at the articulation level.
- Increments in the variety and flexibility of possible rehabilitation exercises, compared to designs mentioned before, by using two DOFs for each finger and having double action.
- Reduction in manufacturing and design time and costs.
2. Materials and Methods
2.1. General Framework
- 0° to 100° for the metacarpophalangeal (MCP) joint.
- 0° to 105° for the proximal interphalangeal (PIP) joint.
- 0° to 85° for the distal interphalangeal (DIP) joint.
2.2. Parametric Model
- The reduction in the time it takes to produce a 3D model of a rehabilitation mechanism that is adapted to an individual’s anatomical dimensions.
- The maximization of the patient articulations’ ROM by making the mechanism fit their individual anatomy.
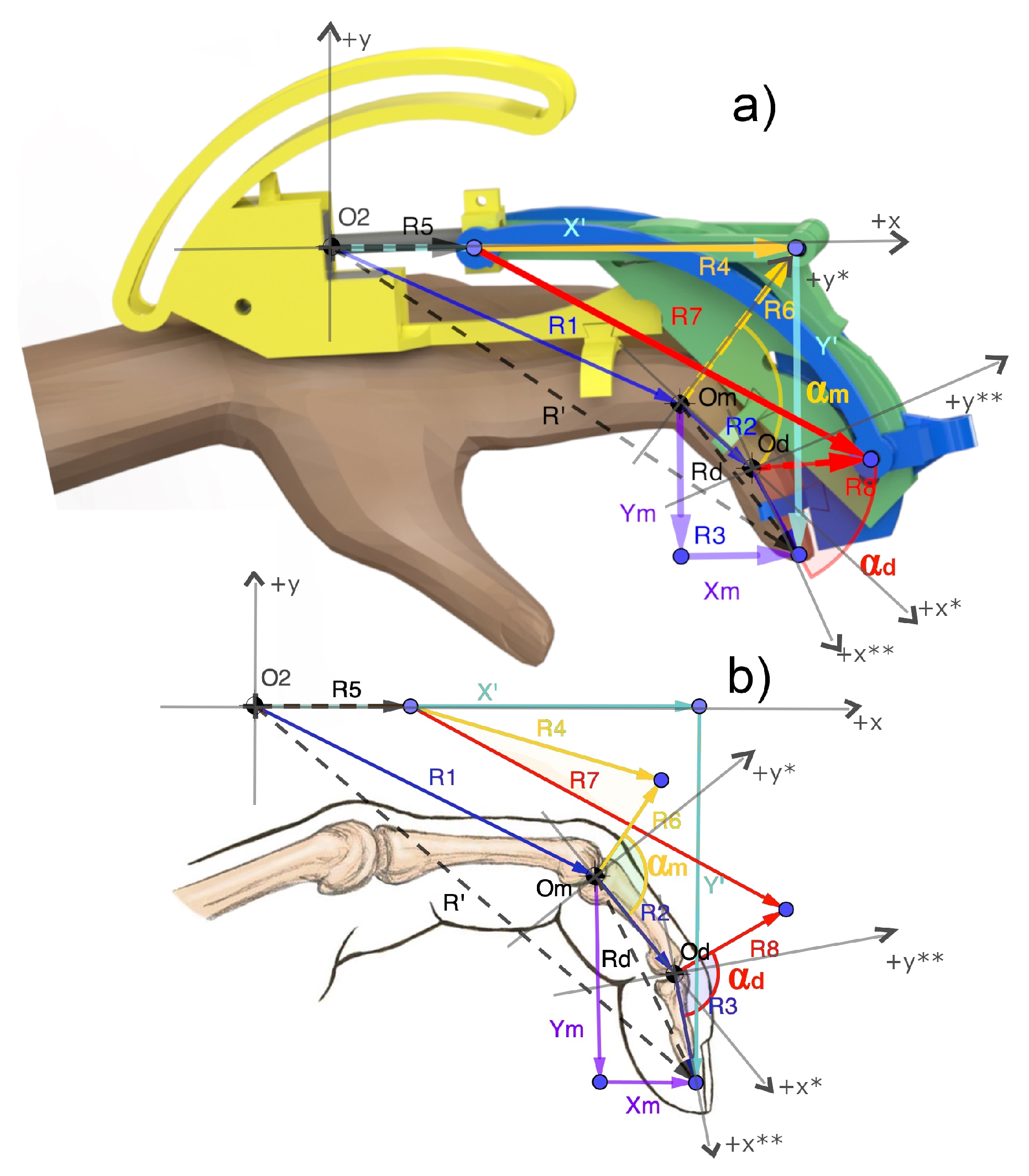
2.3. Kinematic Model
2.3.1. Direct Model
2.3.2. Inverse Model
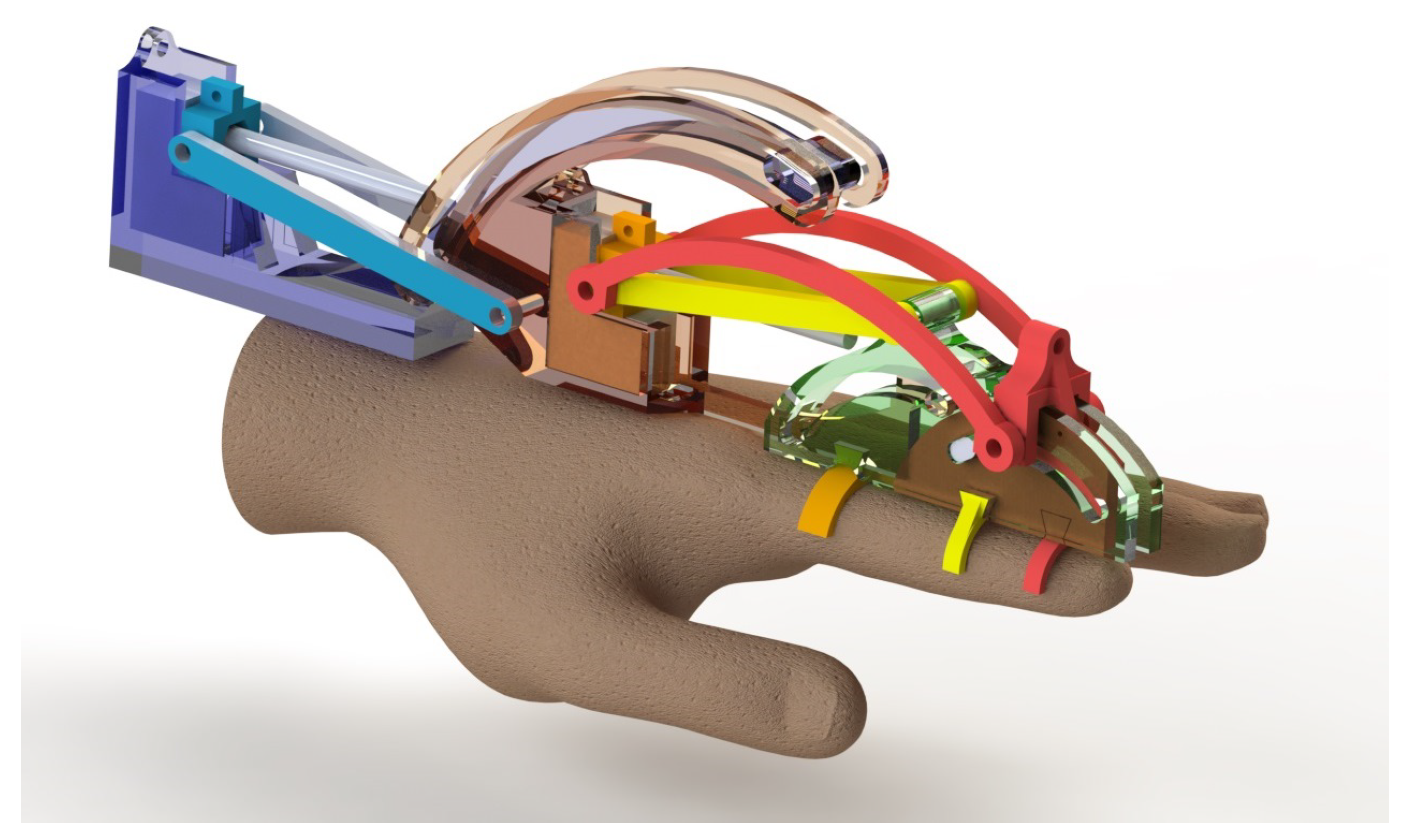
2.4. Prototype Description
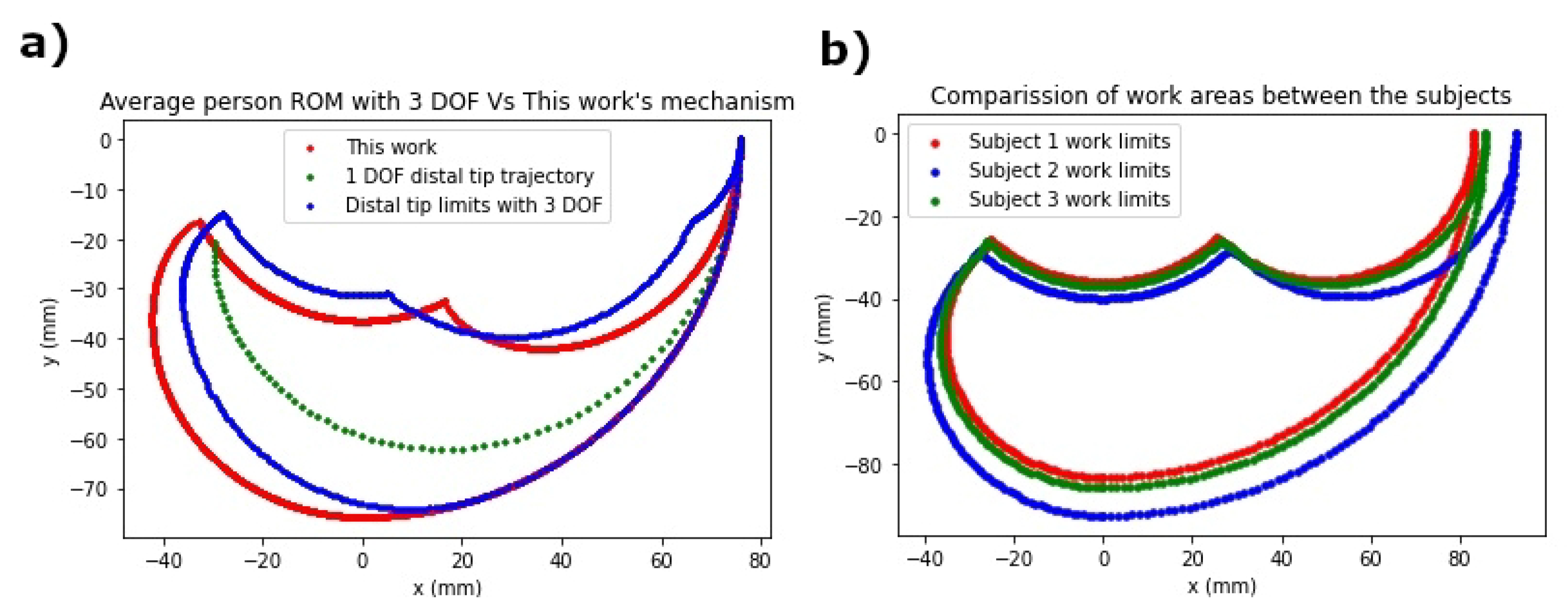
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | Computer-Aided Design |
| ADL | Activities of Daily Living |
| DOF | Degree of Freedom |
| ROM | Range of Motion |
| MCP | Metacarpophalangeal |
| PIP | Proximal Interphalangeal |
| DIP | Distal Interphalangeal |
| MOCAP | MOtion CApture |
| FDM | Fused Deposition Modeling |
Appendix A. Direct Kinematic Model in Detail
Appendix B. Inverse Kinematic Model in Detail
References
- Stilli, A.; Cremoni, A.; Bianchi, M.; Ridolfi, A.; Gerii, F.; Vannetti, F.; Wurdemann, H.A.; Allotta, B.; Althoefer, K. AirExGlove—A novel pneumatic exoskeleton glove for adaptive hand rehabilitation in post-stroke patients. In Proceedings of the 2018 IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018; pp. 579–584. [Google Scholar] [CrossRef]
- Mcconnell, A.; Moioli, R.; Brasil, F.; Vallejo, M.; Corne, D.; Vargas, P.; Stokes, A. Robotic devices and brain-machine interfaces for hand rehabilitation post-stroke. J. Rehabil. Med. 2017, 49, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Zhang, X.; Wang, J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav. Neurol. 2017, 2017, 3908135. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, Q.; Meng, Q.; Li, X.; Yu, H. Design and Development of a Portable Exoskeleton for Hand Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Alnajjar, F.; Gochoo, M.; Renawi, A.; Shimoda, S. Robotic assistive and rehabilitation devices leading to motor recovery in upper limb: A systematic review. Disabil. Rehabil. Assist. Technol. 2021, 1–15. [Google Scholar] [CrossRef]
- Prange, G.; Jannink, M.; Groothuis-Oudshoorn, C.; Hermens, H.; IJzerman, M. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 2006, 43, 171–184. [Google Scholar] [CrossRef]
- Gonzalez, A.; Garcia, L.; Kilby, J.; McNair, P. Robotic devices for paediatric rehabilitation: A review of design features. BioMed. Eng. OnLine 2021, 20, 89. [Google Scholar] [CrossRef]
- Burgar, C.; Lum, P.; Shor, P.; der Loos, H.M.V. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J. Rehabil. Res. Dev. 2000, 376, 663–673. [Google Scholar]
- Colombo, R.; Pisano, F.; Micera, S.; Mazzone, A.; Delconte, C.; Carrozza, M.C.; Dario, P.; Minuco, G. Robotic Techniques for Upper Limb Evaluation and Rehabilitation of Stroke Patients. IEEE Trans. Neural Syst. Rehabil. Eng. A Publ. IEEE Eng. Med. Biol. Soc. 2005, 13, 311–324. [Google Scholar] [CrossRef]
- Qian, Z.; Bi, Z. Recent Development of Rehabilitation Robots. Adv. Mech. Eng. 2014, 7, 563062. [Google Scholar] [CrossRef]
- Ben Abdallah, I.; Bouteraa, Y.; Rekik, C. Design and development of 3D printed myoelectric robotic exoskeleton for hand rehabilitation. Int. J. Smart Sens. Intell. Syst. 2017, 10, 341–366. [Google Scholar] [CrossRef]
- Andriske, L.; Verikios, D.; Hitch, D. Patient and Therapist Experiences of the SaeboFlex: A Pilot Study. Occup. Ther. Int. 2017, 2017, 5462078. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Bitikofer, C.; Sobbi, B.; Perry, J. Design of MobIle Digit Assistive System (MIDAS): A Passive Hand Extension Exoskeleton for Post Stroke Rehabilitation. In Proceedings of the 4th International Symposium on Wearable Robotics, WeRob2018, Pisa, Italy, 16–20 October 2018; pp. 535–539. [Google Scholar] [CrossRef]
- Chiri, A.; Giovacchini, F.; Vitiello, N.; Cattin, E.; Roccella, S.; Vecchi, F.; Carrozza, M.C. HANDEXOS: Towards an exoskeleton device for the rehabilitation of the hand. In Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, St. Louis, MO, USA, 10–15 October 2009; pp. 1106–1111. [Google Scholar] [CrossRef]
- Brokaw, E.; Black, I.; Holley, R.; Lum, P. Hand Spring Operated Movement Enhancer (HandSOME): A Portable, Passive Hand Exoskeleton for Stroke Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Landers, K.; Park, H. Development of a Biomimetic Hand Exotendon Device (BiomHED) for Restoration of Functional Hand Movement Post-Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Peppen, R.; Wagenaar, R.; Wood-Dauphinee, S.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of Augmented Exercise Therapy Time After Stroke: A Meta-Analysis. Stroke 2004, 35, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yi, J.; Chen, X.; Liu, Z.; Wang, Z. BCL-13: A 13-DOF Soft Robotic Hand for Dexterous Grasping and In-hand Manipulation. IEEE Robot. Autom. Lett. 2018, 3, 3379–3386. [Google Scholar] [CrossRef]
- Susanto, E.; Tong, R.K.Y.; Ockenfeld, C.; Ho, N.S. Efficacy of robot-assisted fingers training in chronic stroke survivors: A pilot randomized-controlled trial. J. Neuroeng. Rehabil. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Ho, N.S.; Tong, K.Y.; Hu, X.; Fung, K.; Wei, X.; Rong, W.; Susanto, E. An EMG-driven exoskeleton hand robotic training device on chronic stroke subjects: Task training system for stroke rehabilitation. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar]
- Cafolla, D. A personalized flexible exoskeleton for finger rehabilitation: A conceptual design. In Advances in Mechanism and Machine Science; Uhl, T., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–82. [Google Scholar]
- Case, D.; Ross, A. Sex Determination from Hand and Foot Bone Lengths. J. Forensic Sci. 2007, 52, 264–270. [Google Scholar] [CrossRef]
- Hu, D.; Xiong, C.H.; Liu, M.J. Exploring the existence of better hands for manipulation than the human hand based on hand proportions. J. Theor. Biol. 2018, 440, 100–111. [Google Scholar] [CrossRef]
- Buryanov, A.; Kotiuk, V. Proportions of Hand Segments. Int. J. Morphol. 2010, 28, 755–758. [Google Scholar] [CrossRef]
- Hamilton, R.; Dunsmuir, R. Radiographic Assessment of the Relative Lengths of the Bones of the Fingers of the Human Hand. J. Hand Surg. 2002, 27, 546–548. [Google Scholar] [CrossRef]
- Thompson, D. Biomechanics of the Hand. 2001. Available online: https://ouhsc.edu/bserdac/dthompso/web/namics/hand.htm (accessed on 5 November 2020).
- Becker, J.; Thakor, N. A study of the range of motion of human fingers with application to anthropomorphic designs. IEEE Trans. Biomed. Eng. 1988, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hume, M.; Gellman, H.; Mckellop, H.; Brumfield, R. Functional range of motion of the joints of the hand. J. Hand Surg. 1990, 152, 240–243. [Google Scholar] [CrossRef]
- Littler, J. On The Adaptability of Man’s Hand (with Reference to the Equiangular Curve). Hand 1973, 5, 187–191. [Google Scholar] [CrossRef]
- Johnson, P.W.; Blackstone, J.M. Children and gender—Differences in exposure and how anthropometric differences can be incorporated into the design of computer input devices. Scand. J. Work. Environ. Health 2007, 33, 26–32. [Google Scholar]
- Gillam, L.; McDonald, R.; Ebling, F.; Mayhew, T. Human 2D (index) and 4D (ring) finger lengths and ratios: Cross-sectional data on linear growth patterns, sexual dimorphism and lateral asymmetry from 4 to 60 years of age. J. Anat. 2008, 213, 325–335. [Google Scholar] [CrossRef]
- Singh, B.K. Assessment of stature from hand and phalange length. Int. J. Med. Health Res. 2017, 3, 136–137. [Google Scholar]
- Bataller, A.; Cabrera, J.; Clavijo, M.; Castillo, J.J. Evolutionary synthesis of mechanisms applied to the design of an exoskeleton for finger rehabilitation. Mech. Mach. Theory 2016, 105, 31–43. [Google Scholar] [CrossRef]
- Polygerinos, P.; Lyne, S.; Wang, Z.; Nicolini, L.F.; Mosadegh, B.; Whitesides, G.; Walsh, C. Towards a soft pneumatic glove for hand rehabilitation. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 1512–1517. [Google Scholar]
- Ali, H.F.; Khan, A.M.; Baek, H.; Shin, B.; Kim, Y. Modeling and control of a finger-like mechanism using bending shape memory alloys. Microsyst. Technol. 2021, 27, 2481–2492. [Google Scholar] [CrossRef]




| Variables solved using kinematic chains | ||||||||
| Variables solved by the parametric model | ||||||||
| Input variables | ||||||||
| Constants |
| Exp. Val. (mm) | Theor. Val. (mm) | Abs. Err. (mm) | Rel. Err. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phalanges | Dist. | Mid. | Prox. | Mid. | Prox. | Mid. | Prox. | Mid. | Prox. |
| Subj. 1 | 17 | 22.5 | 42 | 23.8 | 42.5 | −1.3 | −0.5 | −5.77 | 1.19 |
| Subj. 2 | 18.9 | 24.36 | 44.6 | 26.46 | 47.25 | 2.1 | 2.65 | 7.94 | 5.61 |
| Subj. 3 | 17.5 | 24.53 | 41.2 | 24.5 | 43.75 | −0.03 | 2.55 | −0.12 | 5.83 |
| ROM (Degrees) | ||||||
|---|---|---|---|---|---|---|
| Work | Single/Double Action | MCP | PIP | DIP | Transmission Mechanism | DOF |
| [20] | Double | 55 | 65 | N/A | Linkage | 1 |
| [33] | Single | 50.7 | 90 | 61.6 | Linkage | 0 ** |
| [14] | Single *** | 49 | 90 | 50 | Cable | 1 |
| [34] | Single | 250 * | Compliant | 1 | ||
| Present work | Double | 90 | 90 | 85 | Linkage | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Téllez, I.A.; Elias-Espinosa, M.C.; Cervantes-Culebro, H.; Flores-González, A.E. Parametric Design of a Finger Rehabilitation Mechanism with Double Action and Two Degrees of Freedom. Appl. Sci. 2022, 12, 10701. https://doi.org/10.3390/app122110701
Nava-Téllez IA, Elias-Espinosa MC, Cervantes-Culebro H, Flores-González AE. Parametric Design of a Finger Rehabilitation Mechanism with Double Action and Two Degrees of Freedom. Applied Sciences. 2022; 12(21):10701. https://doi.org/10.3390/app122110701
Chicago/Turabian StyleNava-Téllez, Iyari Alejandro, Milton Carlos Elias-Espinosa, Héctor Cervantes-Culebro, and Aldo Elihu Flores-González. 2022. "Parametric Design of a Finger Rehabilitation Mechanism with Double Action and Two Degrees of Freedom" Applied Sciences 12, no. 21: 10701. https://doi.org/10.3390/app122110701
APA StyleNava-Téllez, I. A., Elias-Espinosa, M. C., Cervantes-Culebro, H., & Flores-González, A. E. (2022). Parametric Design of a Finger Rehabilitation Mechanism with Double Action and Two Degrees of Freedom. Applied Sciences, 12(21), 10701. https://doi.org/10.3390/app122110701








