Coordination Energetic Materials—Scientific Curiosity or Future of Energetic Material Applications?
Abstract
1. Introduction
2. CEMs—State of the Art
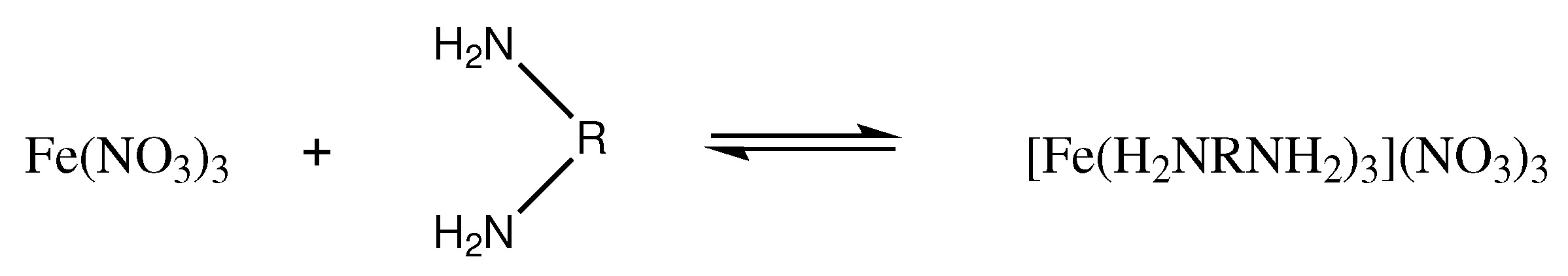
2.1. Synthesis of Coordination Energetic Materials
2.2. Nitrogen-Rich Organic Ligands
2.3. Transition Metal Compounds
2.4. Performance Parameters of Coordination Energetic Materials
3. Discussion
3.1. Criteria for Application of New Energetic Materials
3.2. Practical Considerations
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EM | energetic material |
| CEM | coordination energetic materials |
| Hz | hydrazine |
| OxTANQ | oxidized triaminoguanidine |
| bisTetrAzM | bis(2-tetrazolyl)methane |
| 4A124TrAz | 4-amino-1,2,4-triazole |
| 1A123TrAz | 1-amino,1,2,3-triazole |
| bisTrAz | 4,4’-bi-1,2,4-triazole |
| DNAT | 1,5-di(nitramino)tetrazole |
| DMTetrAz | 1,5-dimethyltetrazole |
| EDA | 1,2-ethylenediamine |
References
- Xu, J.G.; Li, X.Z.; Wu, H.F.; Zheng, F.K.; Chen, J.; Guo, G.C. Substitution of nitrogen-rich linkers with insensitive linkers in azide-based energetic coordination polymers toward safe energetic materials. Cryst. Growth Des. 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- McDonald, K.A.; Seth, S.; Matzger, A.J. Coordination polymers with high energy density: An emerging class of explosives. Cryst. Growth Des. 2015, 15, 5963–5972. [Google Scholar] [CrossRef]
- Nita, M.; Cudziło, S.; Celiński, M. Nowy inicjujący materiał wybuchowy: Chloran (VII)-μ-4-amino-1, 2, 4-triazol-μ-dichloromiedź (II). Biul. Wojsk. Akad. Tech. 2010, 59, 61–69. [Google Scholar]
- Badgujar, D.; Talawar, M.; Asthana, S.; Mahulikar, P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T. Green Energetic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Politzer, P.; Murray, J.S. Some perspectives on estimating detonation properties of C, H, N, O compounds. Cent. Eur. J. Energetic Mater. 2011, 8, 209–220. [Google Scholar]
- Wu, B.D.; Zhou, Z.N.; Li, F.G.; Yang, L.; Zhang, T.L.; Zhang, J.G. Preparation, crystal structures, thermal decompositions and explosive properties of two new high-nitrogen azide ethylenediamine energetic compounds. New J. Chem. 2013, 37, 646–653. [Google Scholar] [CrossRef]
- Hariharanath, B.; Chandrabhanu, K.; Rajendran, A.; Ravindran, M.; Kartha, C. Detonator using nickel hydrazine nitrate as primary explosive. Def. Sci. J. 2006, 56, 383. [Google Scholar] [CrossRef]
- Furlani, C.; Mattogno, G.; Monaci, A.; Tarli, F. Ligand field spectra of hydrazine complexes of NiII, and the spectrochemical position of hydrazine. Inorganica Chim. Acta 1970, 4, 187–191. [Google Scholar] [CrossRef]
- Yan, Q.L.; Cohen, A.; Petrutik, N.; Shlomovich, A.; Zhang, J.G.; Gozin, M. Formation of highly thermostable copper-containing energetic coordination polymers based on oxidized triaminoguanidine. ACS Appl. Mater. Interfaces 2016, 8, 21674–21682. [Google Scholar] [CrossRef] [PubMed]
- Szimhardt, N.; Wurzenberger, M.H.; Zeisel, L.; Gruhne, M.S.; Lommel, M.; Klapötke, T.M.; Stierstorfer, J. 1-AminoTriazole Transition-Metal Complexes as Laser-Ignitable and Lead-Free Primary Explosives. Chem. Eur. J. 2019, 25, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Wurzenberger, M.H.; Gruhne, M.S.; Lommel, M.; Braun, V.; Szimhardt, N.; Stierstorfer, J. Taming the Dragon: Complexation of Silver Fulminate with Nitrogen-Rich Azole Ligands. Inorg. Chem. 2020, 59, 17875–17879. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, T.; Zhang, Y.; Hu, J.; Chen, T.; Wang, Y.; Xu, K. Novel energetic coordination polymers based on 1, 5-di (nitramino) tetrazole with high oxygen content and outstanding properties: Syntheses, crystal structures, and detonation properties. Front. Chem. 2019, 7, 672. [Google Scholar] [CrossRef]
- Manzoor, S.; Yin, X.; Yang, J.Q.; Zhang, J.G.; Zhang, Q.; Chen, D. Synthesis and properties of transition metal coordination energetic materials based on a versatile and multifunctional 1-Aminotetrazol-5-one ligand. Inorganica Chim. Acta 2021, 525, 120468. [Google Scholar] [CrossRef]
- Wurzenberger, M.H.; Gruhne, M.S.; Lommel, M.; Stierstorfer, J. 1-Amino-5-methyltetrazole in Energetic 3 d Transition Metal Complexes–Ligand Design for Future Primary Explosives. Propellants Explos. Pyrotech. 2021, 46, 207–213. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Z.; Qi, S.; Yang, L.; Wu, B.; Huang, H.; Zhang, T. Synthesis, crystal structure, and properties of a novel, highly sensitive energetic, coordination compound: Iron (II) carbohydrazide perchlorate. Cent. Eur. J. Energetic Mater. 2013, 10, 17–36. [Google Scholar]
- Tong, W.C.; Liu, J.C.; Wang, Q.Y.; Yang, L.; Zhang, T.L. Eco-friendly Trifoliate Stable Energetic Zinc Nitrate Coordination Compounds: Synthesis, Structures, Thermal and Explosive Properties. Z. Anorg. Allg. Chem. 2014, 640, 2991–2997. [Google Scholar] [CrossRef]
- Szimhardt, N.; Wurzenberger, M.H.; Klapötke, T.M.; Lechner, J.T.; Reichherzer, H.; Unger, C.C.; Stierstorfer, J. Highly functional energetic complexes: Stability tuning through coordination diversity of isomeric propyl-linked ditetrazoles. J. Mater. Chem. A 2018, 6, 6565–6577. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Li, X.; Lu, M.; Lin, Q. Alkali metals-based energetic coordination polymers as promising primary explosives: Crystal structures, energetic properties, and environmental impact. Chem. Eur. J. 2018, 24, 14213–14219. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M. Energetic materials encyclopedia. In Energetic Materials Encyclopedia; de Gruyter: Berlin, Germany, 2018. [Google Scholar]
- Gruhne, M.S.; Lommel, M.; Wurzenberger, M.H.; Szimhardt, N.; Klapötke, T.M.; Stierstorfer, J. OZM Ball Drop Impact Tester (BIT-132) vs. BAM Standard Method—A Comparative Investigation. Propellants Explos. Pyrotech. 2020, 45, 147–153. [Google Scholar] [CrossRef]
- Borkowski, J.; Nita, M.; Warchoł, R. Dichloran (VII) akwatri (4-amino-1, 2, 4 triazol) disrebranowy inicjujący materiał wybuchowy. Probl. Tech. Uzbroj. 2013, 42, 71–77. [Google Scholar]
- Commission Regulation (EU) No 2021/57 of 25 January 2021 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Lead in Gunshot in or around Wetland. 25 January 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R0057&from=EN (accessed on 10 October 2022).
- Roose, P.; Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines, Aliphatic. Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–55. [Google Scholar]
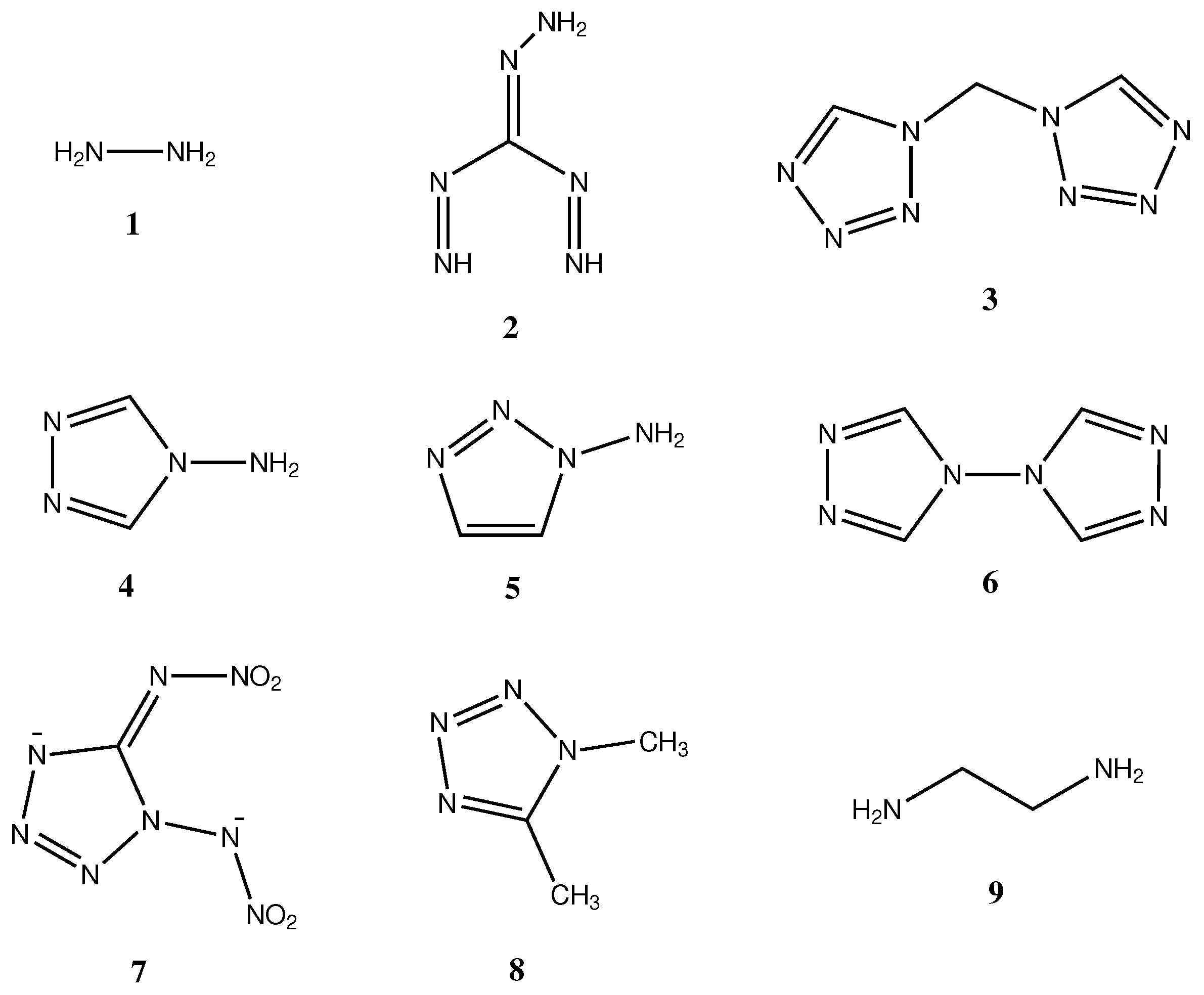
| Chemical Structure | Nitrogen Content [wt. %] | Name Abbreviation | Ref. |
|---|---|---|---|
| 1 | 87.42 | Hz | [7] |
| 2 | 83.97 | OxTANQ | [10] |
| 3 | 73.66 | bisTetrAzM | [12] |
| 4 | 66.64 | 4A124TrAz | [12] |
| 5 | 66.64 | 1A123TrAz | [11] |
| 6 | 61.74 | bisTrAz | [12] |
| 7 | 59.58 | DNAT | [13] |
| 8 | 57.11 | DMTetrAz | [12] |
| 9 | 46.61 | EDA | [8,9] |
| Compound | Sensitivity Parameters | Performance Parameters | Ref. | ||
|---|---|---|---|---|---|
| Friction [N] | Impact [J] | Detonation Velocity [m/s] | Detonation Pressure [GPa] | ||
| Lead(II) azide | 0.1–1.0 | 2.5–4.0 | 5877 | 33.4 | [19,20] |
| 0.196 a | 0.41 b | 4630 | 16.1 | [8] | |
| Lead(II) styphnate | 0.5–1.5 | 2.5–5.0 | - | - | [21] |
| 15.69 a | 0.82 b | 7000 | 20.8 | [8] | |
| 30 | 2 | - | - | [22] | |
| 10 | 5 | - | - | [12] | |
| 64 | 7 | - | - | [12] | |
| >360 | >98 | 8969 | 13.5 | [10] | |
| 3 | 16 | 8147 | 29.8 | [13] | |
| 3 | 16 | 8348 | 31.5 | [13] | |
| 6 | 26 | 8169 | 29.7 | [13] | |
| 5 | 22 | 8187 | 30.3 | [13] | |
| 4 | 18 | 8478 | 32.8 | [13] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlus, K.; Jarosz, T.; Stolarczyk, A. Coordination Energetic Materials—Scientific Curiosity or Future of Energetic Material Applications? Appl. Sci. 2022, 12, 10498. https://doi.org/10.3390/app122010498
Pawlus K, Jarosz T, Stolarczyk A. Coordination Energetic Materials—Scientific Curiosity or Future of Energetic Material Applications? Applied Sciences. 2022; 12(20):10498. https://doi.org/10.3390/app122010498
Chicago/Turabian StylePawlus, Klaudia, Tomasz Jarosz, and Agnieszka Stolarczyk. 2022. "Coordination Energetic Materials—Scientific Curiosity or Future of Energetic Material Applications?" Applied Sciences 12, no. 20: 10498. https://doi.org/10.3390/app122010498
APA StylePawlus, K., Jarosz, T., & Stolarczyk, A. (2022). Coordination Energetic Materials—Scientific Curiosity or Future of Energetic Material Applications? Applied Sciences, 12(20), 10498. https://doi.org/10.3390/app122010498






